Simple Summary
Getah virus (GETV) is a zoonotic virus transmitted by mosquitoes. Various mammals are naturally infected with GETV, including foxes, pigs, horses, cattle, and sheep. To develop and validate a rapid, sensitive, and portable one-tube assay for the detection of GETV, we employed reverse transcription loop-mediated isothermal amplification (RT-LAMP) in conjunction with Pyrococcus furiosus Argonaute (PfAgo) to provide a straightforward and precise method for detecting porcine GETV in this study. LAMP is used to specifically amplify the target gene of GETV, after which PfAgo is directed to cleave the target gene and the probe, releasing a fluorescent signal. Additionally, the assay results can be observed under UV light or quantified using a fluorescence quantitative instrument. Our experiments demonstrate that this assay is highly specific, capable of detecting extremely low levels of the virus without cross-reacting with other viruses. When tested on real clinical samples, our novel method performed comparably to the gold standard quantitative real-time PCR test. This study provides a rapid, accurate, efficient, portable, and low-cost nucleic acid diagnostic method for the detection of GETV, which could serve as a powerful tool for the prevention and control of GETV.
Abstract
Getah virus (GETV) is a mosquito-borne virus that poses a significant threat to both animal and public health. Traditional diagnostic methods for GETV, such as RT-PCR and RT-qPCR, require expensive equipment and complex procedures, making them unsuitable for rapid, on-site detection. The combination of RT-LAMP and PfAgo offers a novel approach for nucleic acid detection, providing high specificity and effective without the need for sophisticated instruments. Herein, we developed a RT-LAMP combined with PfAgo assay for GETV detection. The RT-LAMP assay was conducted at 60~65 °C, and then the RT-LAMP product was cleaved, together with a fluorescent probe, mediated by PfAgo at 95 °C. After optimizing the primary reaction conditions, the detection limit of the RT-LAMP-PfAgo assay was 100 copies/µL. Importantly, there was no cross-reactivity with other viruses, including PEDV, PDCoV, PoRV, PRRSV, and CSFV. Compared to qPCR, analysis of 86 clinical samples showed that LAMP-PfAgo had a consistent positive rate with the qPCR method. In conclusion, we developed a valuable diagnostic tool for the rapid detection of GETV, enabling timely surveillance and control measures to mitigate the impact of GETV outbreaks.
1. Introduction
Getah virus (GETV) is a neglected mosquito-borne virus belonging to the Alphavirus genus and Togaviridae family. Based on viral antigens, GETV is classified under the Semliki Forest virus complex [1]. GETV is an enveloped, single-stranded, positive-sense RNA virus with a genome of approximately 11.2 kb [2]. The genomic RNA of GETV contains two large open reading frames (ORFs) that encode the four nonstructural proteins (nsP1, nsP2, nsP3, and nsP4) and five structural proteins (capsid [C], E3, E2, 6K, and E1) [3]. The prototype strain MM2021 was the first GETV strain isolated from Culex gelidus collected in Malaysia in 1955. Currently, GETV is widespread in 13 Asian and pan-Pacific countries, including China, South Korea, Japan, Russia, the Philippines, Malaysia, and Australia [4]. Sero-epizootiological studies have demonstrated that GETV has a broad host spectrum, with natural infections in mosquitoes and animals including pigs, horses, goats, cattle, boars, blue foxes, and humans, suggesting a significant expansion in the virus’s host range [5,6,7,8,9,10]. As a multi-host pathogen, GETV is becoming increasingly serious and poses a substantial threat to animal safety and public health. To date, GETV has been found to be pathogenic to horses, pigs, and cattle, causing reproductive disorders, fever, neurological symptoms, diarrhea, and death in mammals, resulting in significant economic losses in the livestock industry [10,11]. Horses infected with GETV often exhibit clinical signs such as fever, rash, and leg edema [12]. Infected piglets display symptoms including arthritis, fever, anorexia, diarrhea, tremors, hind limb paralysis, ataxia, and high mortality. Pregnant sows infected with GETV may experience reproductive disorders such as spontaneous abortion and stillbirth [13]. Therefore, rapid diagnosis is crucial for early surveillance and control to mitigate the risk of GETV outbreaks.
The current major methods of GETV detection include ‘golden standard’ methods such as virus isolation, virus neutralization tests, and indirect fluorescent antibody assay, as well as molecular technologies like RT-PCR, RT-qPCR, loop-mediated isothermal nucleic acid amplification (LAMP), and RT-recombinase aided amplification (RAA). Serological assays like the enzyme-linked immunosorbent assay (ELISA) are also commonly used, along with novel methods such as CRISPR-based technologies [14,15,16,17,18]. However, these processes are not only time-consuming but also demanding on both laboratories and laboratorians. Serological diagnostics and ELISA face similar challenges, including complexity and lack of timeliness [19,20]. With advancements in molecular diagnostic techniques, RT-PCR [21] and RT-qPCR [22] are beginning to be applied to the diagnosis of GETV. Nevertheless, these methods require sophisticated equipment, making them difficult to implement in resource-limited areas. While emerging isothermal amplification techniques like RAA [16] and LAMP [17] show promise in addressing this issue, they still struggle with non-specific amplification. As a result, many studies are turning to CRISPR technology with isothermal amplification to mitigate the false positive results of amplification [23]. However, challenges arise due to constraints in target PAM sequences.
Argonaute (Ago) proteins, widely distributed across many biological systems, have been reported to possess potential in nucleic acid detection [24]. PfAgo, an Argonaute protein derived from Pyrococcus furiosus, can utilize 5′ phosphorylated short ssDNA to specifically cleave both DNA and RNA targets [25]. Unlike CRISPR, PfAgo does not depend on PAM sequences for target recognition. In contrast to CRISPR-associated (Cas) nucleases, which require long RNA guides, PfAgo, as well as most prokaryotic Ago proteins, employs short DNA molecules as a guide DNA (gDNA). Furthermore, short gDNA is more cost-effective to synthesize and easier to store, which promotes the development of Ago-based nucleic acid detection [26]. PfAgo’s systematic cleavage has been exploited for specific detection of various viruses, including SARS-CoV-2, influenza viruses, PEDV, and PDCoV [27,28,29,30].
Previous reports have demonstrated PfAgo’s potential for rapid and on-site detection of viruses in conjunction with RTX-PCR, RAA, and RPA [30,31,32]. In this study, we developed a one-tube method for detecting GETV that combines LAMP with the PfAgo system. This method can accurately detect GETV within 60 min and demonstrates ultra-high sensitivity and strong specificity. It offers a rapid, reliable, convenient, and cost-effective solution for detecting GETV without expensive equipment.
2. Materials and Methods
2.1. Sample Collection and Viruses
A total of 86 clinical samples, including intestinal, liver, and lung tissues, were collected from pig farms in Jiangxi Province, China. The isolated viruses, including GETV, porcine epidemic diarrhea virus (PEDV), porcine deltacoronavirus (PDCoV), porcine rotavirus (PoRV), porcine reproductive and respiratory syndrome virus (PRRSV), and classical swine fever virus (CSFV), were used to establish the LAMP-PfAgo method. The genomic RNAs of these viruses were extracted using RNAiso Plus (Takara, Beijing, China) following the manufacturer’s instructions, and cDNAs were synthesized using the PrimeScript RT Master Mix (Takara, Beijing, China).
2.2. RT-LAMP Assay
To identify the conserved regions, the complete genome sequences of GETV currently available in GenBank were analyzed by MEGA11 (https://www.megasoftware.net/, accessed on 10 March 2024). The conserved sequence fragment of the NSP1 gene of GETV was selected as the targeted region, and used to design the GETV LAMP primers with PrimerExplore (http://primerexplorer.jp/elamp4.0.0/index.html, accessed on March 12 March 2024). GETV-F3/B3 were the forward outer/reverse outer primers, GETV-FIP/BIP were the forward inner/reverse inner primers, and GETV-LB was the loop primer used for LAMP amplification. The gDNA design was based on PfAgo’s ability to cleave DNA complementary to its bases, mediated by ssDNA with a phosphate group at the 5′-end. Moreover, the probe was designed as an ssDNA sequence complementary to the newly generated gDNA, flanked by a carboxyfluorescein (FAM) at the 5′-end and a quencher BHQ1 at the 3′-end termini. The qPCR-F/R was utilized for quantitative real-time PCR target GETV (Table 1).

Table 1.
Sequence of primers, gDNAs, and probes used in this study.
In order to construct the standards of GETV NSP1 gene, the targeted cDNA of GETV underwent PCR-based amplification with the primer GETV-NSP1-F/R. The PCR parameters were as follows: 98 °C for 5 min, 35 cycles of 98 °C for 10 s, 58 °C for 20 s, 72 °C for 30 s, and a final extension at 72 °C for 5 min. The resulting product was purified with the E.Z.N.A™ Gel Extraction Kit (Omega, Suzhou, China), ligated into pMD™ 19-T (Takara, Beijing, China) to form a standard plasmid, and then cloned into E. coli. The recombinant plasmid was extracted using the TIANprep Mini Plasmid Kit (TIANGEN, Beijing, China) following the protocol of the manufacturer. The concentration of the plasmid DNA was determined by the NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA), and the plasmid copy number was calculated based on the previous method [33].
The LAMP reaction was carried out in a final volume of 25 μL. To optimize the reaction conditions, the reactions contained different concentrations of MgSO4 (at 2, 4, 6, 8, and 10 mM, Sigma-Aldrich, St. Louis, MO, USA), dNTPs mix (at 0.2, 0.4, 0.6, 1.0, and 1.4 mM, Sangon, Shanghai, China), each of inner primer GETV-FIP and GETV-BIP (at 0.2, 0.4, 0.8, 1.2, 1.6, 2.0 μM), each of outer primer GETV-F3 and B3 (0.2 μM), loop primer GETV-LB 0.4 μM, 2.5 μL 10× Isothermal Amplification Buffer, Bst 2.0 DNA Polymerase (8U, New England Biolabs, Ipswich, MA, USA), and 1 μL template DNA from standard plasmid or cDNA from clinical samples. Furthermore, the temperature of the LAMP reaction was determined by incubating the reaction systems at 53, 57, 61, 65, and 70 °C for 50 min in a water bath, respectively. The reaction time was then determined at 10, 20, 30, 40, and 50 min, respectively. The LAMP products were electrophoresed on 1.5% agarose gel in 1× TAE buffer, or directly visualized by adding 1× SYBR Green I in the reaction tube for diction by a color change.
2.3. PfAgo Cleavage
The PfAgo endonuclease was purchased from Jiaohong Biotech (Shanghai, China). The LAMP primers, gDNAs, and probes were synthesized by Sangon Biotech (Shanghai, China) and are listed in Table 1. To integrate the PfAgo system into the RT-LAMP assay, 3 μL of the abovementioned LAMP reaction product was transferred to PfAgo reaction with 20 μL volume containing 2 μL of 10× reaction buffer, 1.5 mM Mn2+, 0.75 μM of each gDNA (total three gDNAs), 4 μL PfAgo (200 U/μL), and 0.25 μM probe with FAM at the 5′ end and BHQ1 at the 3′ end on the top. The reaction was carried out in a real-time fluorescence quantitative PCR instrument (CFX96TM, BioRad, Hercules, CA, USA) at 95 °C for 30 min for the cleavage and the fluorescence signal following the PfAgo-mediated cleavage reaction was observed under UV light.
2.4. One-Tube RT-LAMP-PfAgo Assay Development
To combine the RT-LAMP and PfAgo reactions into a single tube, the LAMP reaction was conducted as follows: 2.5 μL 10× Isothermal Amplification Buffer, 1.0 mM dNTP mix, 6 mM MgSO4, 1 μL Bst 2.0 DNA Polymerase, a primer mixture (1.6 μM FIP, 1.6 μM BIP, 0.2 μM F3, 0.2 μM B3, and 0.4 μM LB), and 2 μL sample cDNA. The LAMP reaction mixture was introduced to the bottom of the tube, followed by 10 μL of the PfAgo system consisting of 2 μL of 10× reaction buffer, 1.0 mM Mn2+, 2.0 μM of each gDNA, 3 μL PfAgo (200 U/μL), and then 0.25 μM probe wasplaced in a filter paper disc attached to the tube cap. After incubating for 30 min at 65 °C, the mixture was centrifuged at 5000× g for 1 min, allowing the filter paper disc to fall into the bottom of the tube, where it was thoroughly mixed and incubated for an additional 30 min at 95 °C. The reaction results were observed in a fluorescence detector or under UV light.
To test the sensitivity, specificity, and detection limit of the established assay, the recombinant standard plasmid of GETV NSP1 gene was serially diluted from 1 × 106 copies/μL to 1 × 100 copies/μL and used as templates to assess the sensitivity of the LAMP-PfAgo assay, and the genomic cDNA of PEDV, PDCoV, PoRV, PRRSV, and CSFV was utilized to evaluate the specificity of the LAMP-PfAgo system. The results were observed under UV light after 30 min of the LAMP-PfAgo reaction at 95 °C.
2.5. Validation and Field Testing
Total RNA from clinical samples was extracted using RNAiso Plus (Takara, Beijing, China) and cDNAs were synthesized using the Reverse Transcriptase M-MLV (Takara, Beijing, China). The cDNAs from these samples were then used for GETV screening both using RT-LAMP-PfAgo and RT-qPCR [10]. The clinical application was evaluated by comparing the detection results of RT-LAMP-PfAgo with those of RT-qPCR.
2.6. Statistical Analysis
Each of the experiments was carried out in triplicate and GraphPad Prism was used to statistically evaluate the data. A students’ t test was employed to analyze the data, with p-values of less than 0.05 considered statistically significant.
3. Results
3.1. Principle of the LAMP-PfAgo Method
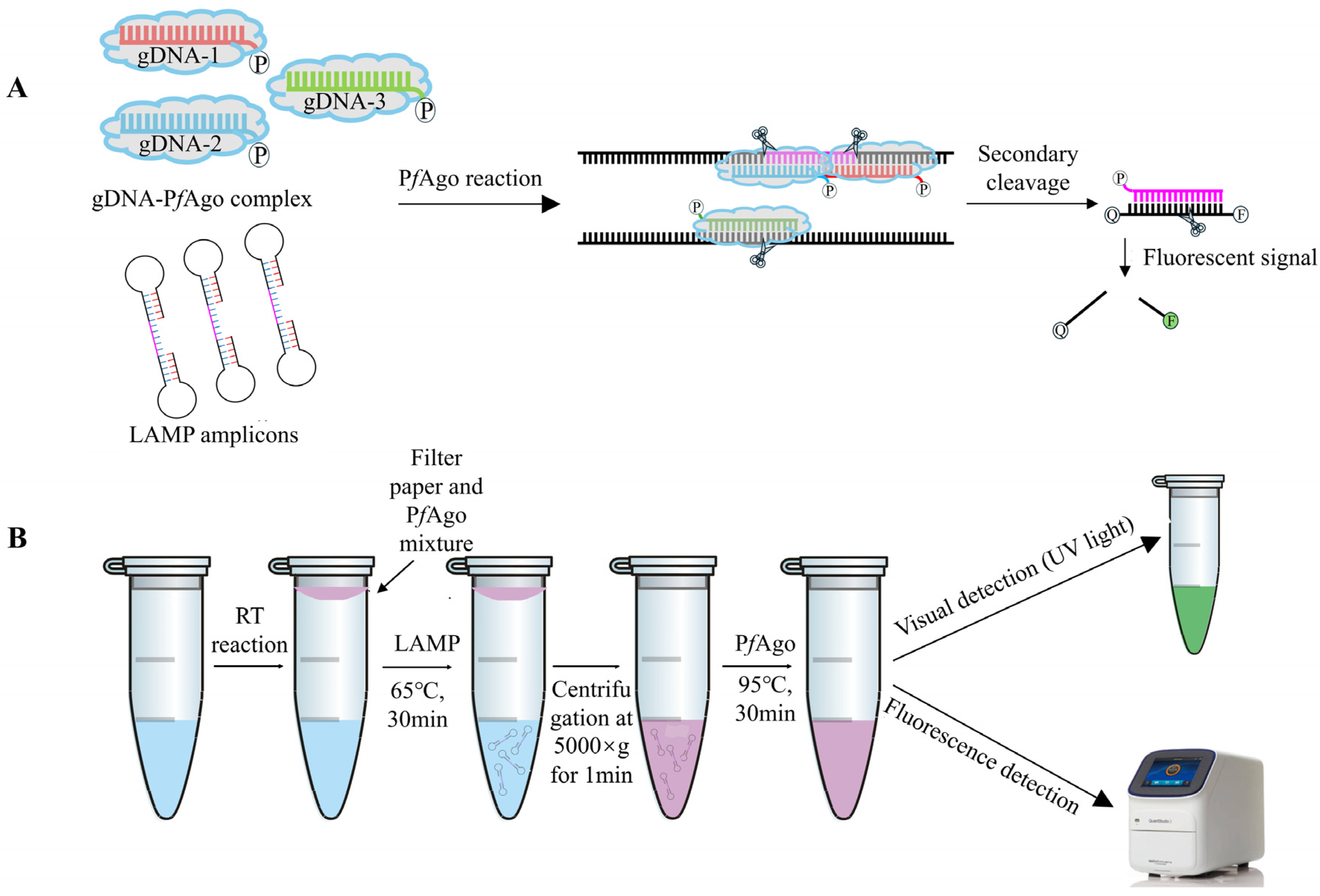
The mechanism underlying the LAMP-PfAgo assay for GETV is illustrated in Figure 1. Briefly, LAMP-amplified fragments are specifically identified by the PfAgo protein guided by 5′-phosporylated gDNA. PfAgo then cleaves the phosphodiester bonds between the 10th and 11th bases of the target DNA from the 5′ end upon base pairing between gDNA and one strand of the LAMP amplicons, resulting in a new 5′-phosporylated ssDNA. This newly formatted ssDNA can act as new gDNA, specifically identifying the synthetically designed single-stranded DNA probe labeled with a fluorophore (FAM) and a quencher (BHQ1) at its termini. PfAgo then cleaves the DNA probe to release fluorescent signals (Figure 1A). For one-tube LAMP-PfAgo detection, viral RNA is extracted and cDNA is synthesized, serving as the template for the LAMP reaction. The PfAgo mixture is placed in a filter paper disc attached to the inside of the tube cap. The LAMP reaction is carried out at 65 °C for 30 min. Subsequently, the reaction tube is centrifuged at 5000× g for 1 min, allowing the filter paper disc to fall to the bottom of the tube. The PfAgo reaction mixture is then thoroughly mixed with the LAMP products and incubated at 95 °C for 30 min. Fluorescent signals can be detected using a fluorescence detector or visualized with the naked eye under UV light (Figure 1B).
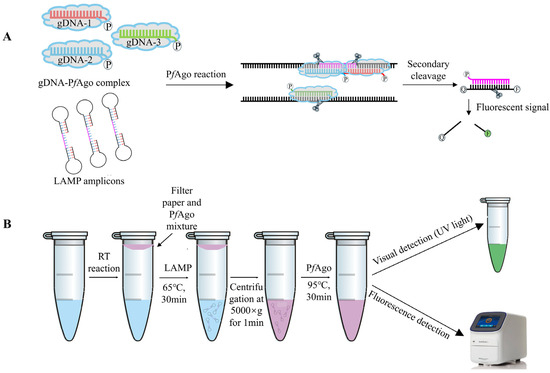
Figure 1.
RT-LAMP-PfAgo-based GETV Detection Methods. (A) Schematic diagram of the LAMP-PfAgo assay designed to produce sequence-specific fluorescent signals. (B) Schematic workflow of the one-tube LAMP-PfAgo process for detecting GETV.
3.2. Optimization of LAMP Assay for Detection of GETV
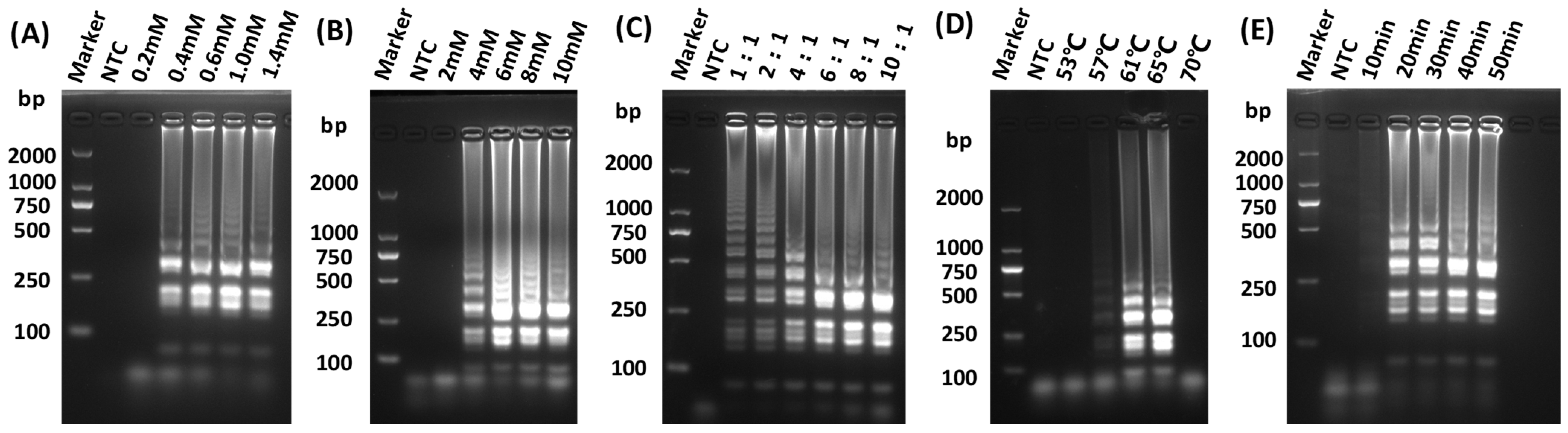
The LAMP assay for detecting GETV was successfully developed. To determine the optimal amplification efficiency for isothermal amplification, reactions were prepared with varying concentrations of dNTPs mix, MgSO4, and each of inner primers FIP and BIP. Furthermore, the temperature of the LAMP reaction was determined by incubating the reaction systems at 53, 57, 61, 65, and 70 °C for 10, 20, 30, 40, and 50 min in a water bath, respectively. The optimized parameters of the reaction system were as follows: 1 mM dNTPs, 6 mM MgSO4, and an internal to external primer ratio of 8:1 (Figure 2A–C), with an incubation condition at 65 °C for 30 min in a water bath (Figure 2D,E).
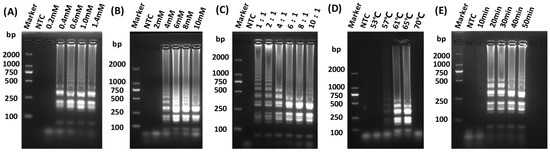
Figure 2.
Optimization of LAMP reaction conditions. (A) Screening for optimal concentration of dNTPs for LAMP; (B) Screening for optimal concentration of MgSO4 for LAMP; (C) Screening for optimal reaction internal and external primer concentration ratio for LAMP; (D) Screening for optimal reaction temperature for LAMP; (E) Screening for optimal reaction times for LAMP.
3.3. Optimization of LAMP-PfAgo Reaction
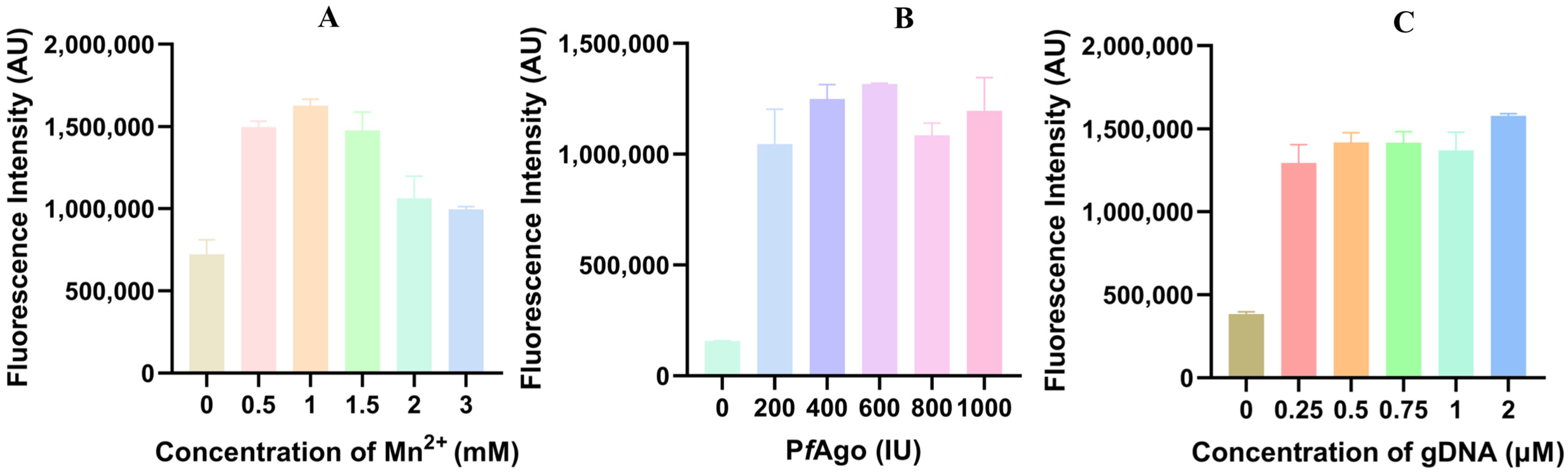
To improve the efficiency of the LAMP-PfAgo reaction, the concentrations of Mn2+ (at 0, 0.5, 1.0, 1.5, 2.0, 3.0 mM), PfAgo (200 U/μL, at 0, 1.0, 2.0, 3.0, 4.0, 5.0 μL), and gDNA (at 0.25, 0.5, 0.75, 1.0, 2.0 μM), were optimized. The results indicated that the highest fluorescence value was achieved with 1.0 mM Mn2+, 600 U PfAgo, and 2 μM gDNA, respectively (Figure 3A–C).
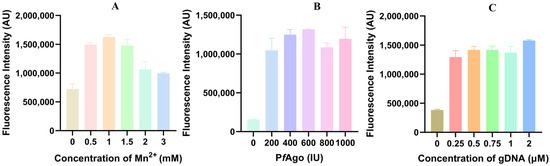
Figure 3.
Optimization of primary reaction components for LAMP-PfAgo. (A) Fluorescence intensity measured by LAMP-PfAgo reaction at different Mn2+ concentrations; (B) Fluorescence intensity measured by LAMP-PfAgo reaction at different PfAgo concentrations; (C) Fluorescence intensity measured by LAMP-PfAgo reaction at different gDNA concentrations.
3.4. Sensitivity and Specificity of the LAMP-PfAgo
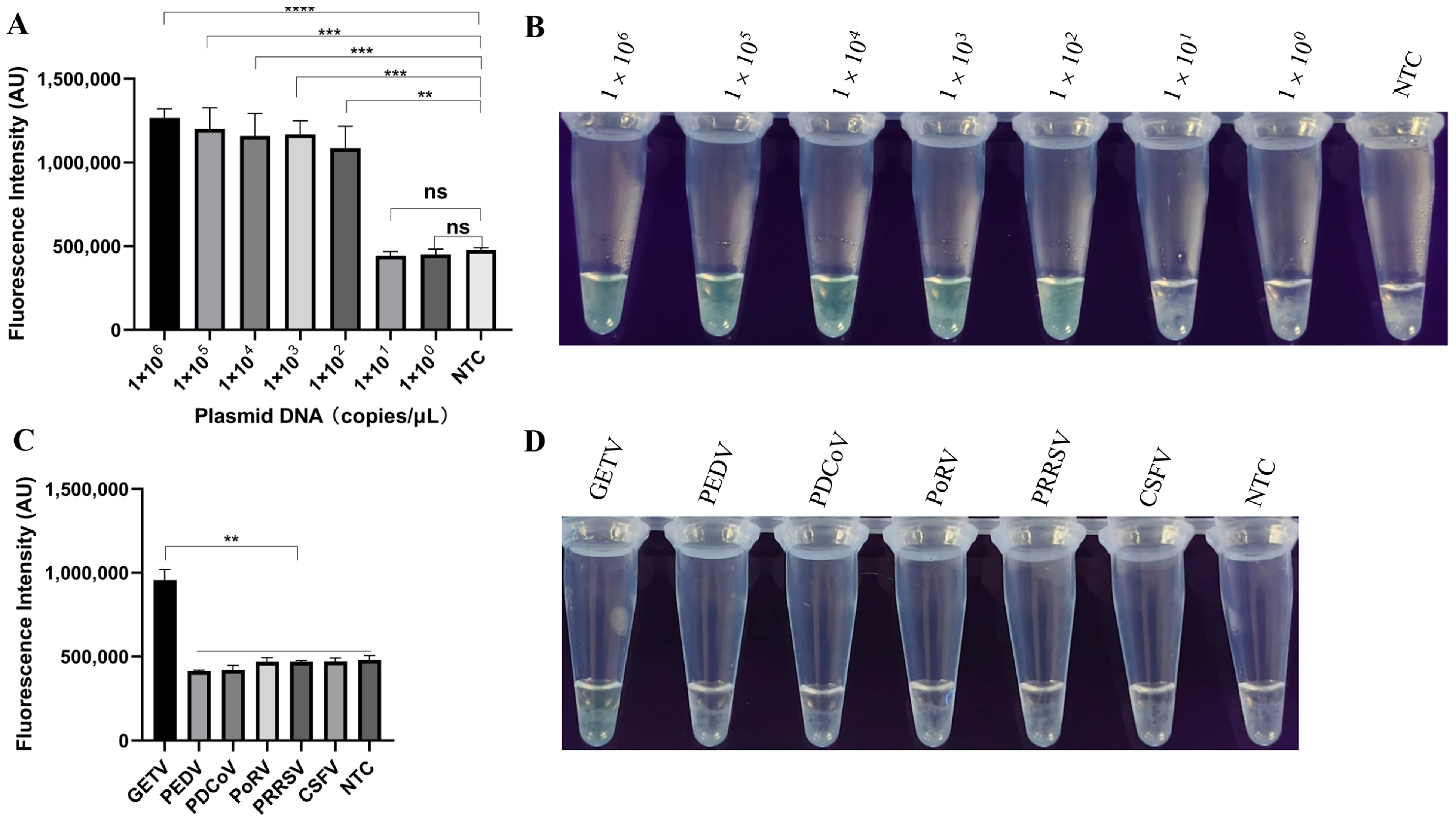
The recombinant plasmid was diluted tenfold (from 1 × 106 to 1 × 102 copies) and used to determine the limit of detection of the LAMP-PfAgo. As shown in Figure 4A, the lowest detection limit of LAMP-PfAgo was 1 × 102 copies/μL and the results were validated under UV light (Figure 4B). Using the LAMP-PfAgo method constructed in this study, we detected several associated swine pathogens, including PEDV, PDCoV, PoRV, PRRSV, and CSFV. The results indicated that only GETV showed a significant increase in fluorescence during amplification (Figure 4C). The same results were visually confirmed under UV light (Figure 4D).
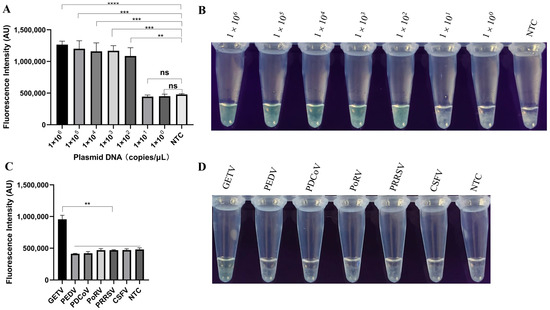
Figure 4.
Evaluation of the sensitivity and specificity of LAMP-PfAgo for GETV detection. (A) End-point fluorescence intensity of LAMP-PfAgo sensitivity; (B) Visualization of LAMP-PfAgo sensitivity under UV light; (C) Fluorescence intensity during the specificity test for detecting GETV using LAMP-PfAgo; (D) Visualization results of LAMP-PfAgo specificity under UV light. Each experiment was performed in triplicate and statistical analysis was conducted using a student’s t-test in GraphPad Prism 8. NTC indicates negative control; ** indicates p < 0.01; *** indicates p < 0.001; **** indicates p < 0.0001; ns indicates no significance.
3.5. Validation and Clinical Sample Detection
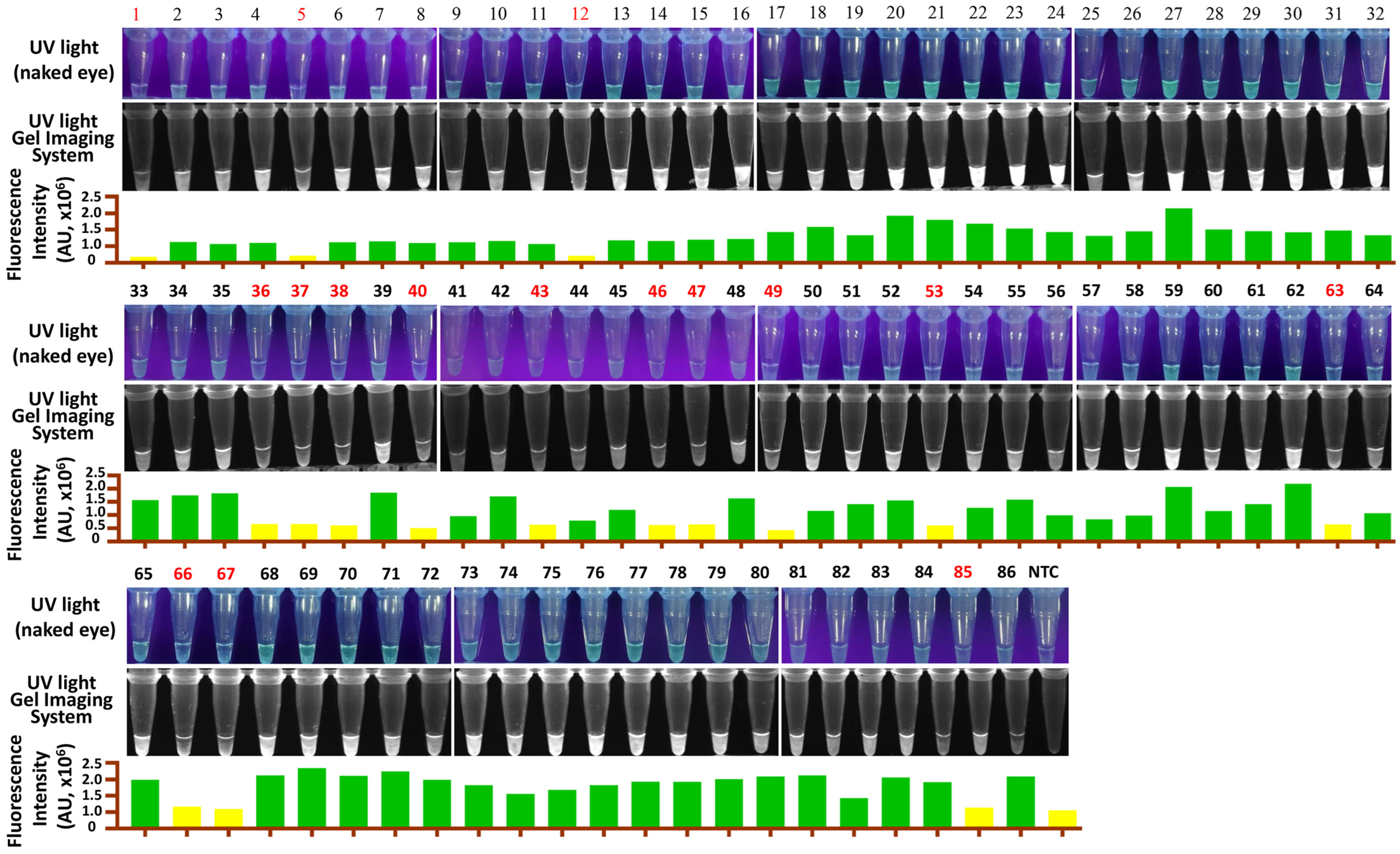
RT-LAMP-PfAgo and RT-qPCR were used to analyze 86 samples from pigs suspected of GETV infection. The results showed that RT-LAMP-PfAgo detected 70 positive samples out of 86 (Figure 5) with a 100% consistency with RT-qPCR results (Table 2). Our findings indicate that the RT-LAMP-PfAgo method developed in this study is suitable for clinical diagnosis.
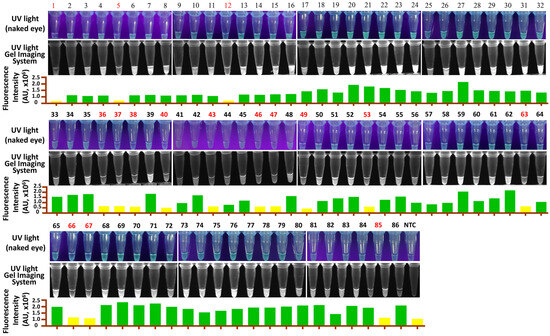
Figure 5.
Detection results of clinical samples for GETV using the RT-LAMP-PfAgo assay. Samples in red indicate a negative result. NTC, negative control.

Table 2.
Comparison of clinical sample detection results between RT-LAMP-PfAgo and RT-qPCR methods.
4. Discussion
Since its discovery in Hainan province, China, in 1964, GETV has spread to 17 provinces [34]. As an arbovirus, GETV can infect various mammals, including blue foxes, pigs, horses, cows, and sheep [16]. Notably, antibodies against GETV have also been found in the blood serum of febrile patients [35]. Research has shown that GETV can infect human and primate cells as well [10,36]. These findings suggest that GETV poses a potential threat to public health. In pigs, GETV has been found to infect pigs in different stages. Piglets infected with GETV display symptoms such as arthritis, fever, anorexia, diarrhea, tremors, hind limb paralysis, ataxia, and high mortality. Pregnant sows infected with GETV can experience reproductive disorders, including spontaneous abortion and stillbirth [13]. Since 2015, GETV outbreaks have occurred continuously in various provinces of China, mainly causing death and/or reproductive disorders [10,11]. Sero-epidemiology investigations indicated frequent infections of GETV in swine in China [18]. However, the lack of effective vaccines or treatments results in significant economic losses to the pig industry caused by GETV infections. Enhancing early detection and surveillance of the pathogen and improving the sensitivity of detection methods are essential for the diagnosis, prevention, and control of the disease.
To date, several tools have been developed for the detection of GETV, such as RT-PCR, RT-qPCR, LAMP, RAA, and ELISA [14,15,16,17,18]. RT-qPCR is regarded as the gold standard for viral diagnosis. Although RT-PCR and RT-qPCR assays are widely used in GETV diagnosis, they are limited by requiring expensive instruments and complex reaction processes, and are thus not suitable for rapid portable clinical detection. Therefore, there is an urgent need to develop a rapid, accurate, sensitive, portable, and low-cost GETV nucleic acid diagnostic tool to meet the current demand for on-site screening and clinical diagnosis.
The PfAgo system offers a novel perspective for the development of nucleic acid molecular diagnostics due to its exceptional ability to recognize and cleave target DNA. Similar to the CRISPR system, PfAgo requires guidance from gDNA (phosphorylated short single-stranded DNA) to effectively cleave target DNA sequences [25]. Previous studies have developed a series of diagnostic methods in combination with isothermal amplification techniques, such as RAA-PfAgo [29], RPA-PfAgo [37], and LAMP-PfAgo [38]. The integration of the PfAgo system into the RT-LAMP assay can effectively prevent false positive results caused by the amplification process, thereby ensuring the accuracy of the diagnostic outcomes due to its step-by-step cleavage specificity [39,40,41]. Unlike RT-PCR and RT-qPCR, the entire reaction of RT-LAMP-PfAgo is maintained at two consecutive temperatures, significantly reducing the reliance on costly instruments.
In the LAMP-PfAgo assay developed in this study, the gDNA and probe sequences were specifically designed between FIP and BIP of the LAMP primer set. This design ensures that all the sequences recognized by PfAgo are derived from the amplification of target sequences, rather than from non-specific amplification in the LAMP reaction. The entire reaction is maintained at two consecutive temperatures and the results can be visualized under UV light, with a minimum detection of 102 copies/µL. There was no cross-reactivity with other pig-related pathogens, demonstrating the potential of this method for detecting GETV in the field. Furthermore, the design of the single-tube operating method not only minimizes the risk of open-cap contamination but also enhances the portability and ease of use of the one-tube assay, making it suitable for deployment in resource-limited settings.
Overall, this study provides a valuable diagnostic tool for the rapid and sensitive detection of GETV, enabling timely surveillance and control measures to mitigate the impact of GETV outbreaks. The one-tube assay’s portability and ease of use make it suitable for deployment in resource-limited settings, contributing to improved public and animal health.
5. Conclusions
Our study underscores the significant threat posed by GETV to both animals and public health. Since its first discovery in Hainan province, China, in 1964, GETV has spread to 17 provinces and can infect various mammals, including blue foxes, pigs, horses, cows, and sheep. The detection of antibodies against GETV in febrile patients and its ability to infect human and primate cells highlight its potential public health risk. In pigs, GETV infections can cause severe symptoms such as arthritis, fever, anorexia, diarrhea, tremors, hind limb paralysis, ataxia, and high mortality in piglets, as well as reproductive disorders in pregnant sows. Continuous outbreaks since 2015 have led to significant economic losses in the pig industry due to the lack of effective vaccines or treatments. Enhancing early detection and surveillance of GETV is crucial for the diagnosis, prevention, and control of the disease.
Several detection tools, including RT-PCR, RT-qPCR, LAMP, RAA, and ELISA, have been developed for GETV. However, these methods are often limited by expensive instruments and complex processes, making them unsuitable for rapid, portable clinical detection. The PfAgo system offers a novel approach for nucleic acid molecular diagnostics, providing very fast, high specificity and accuracy by preventing false positive results through step-by-step cleavage specificity. The LAMP-PfAgo assay developed in this study has a minimum detection limit of 102 copies/µL and no cross-reactivity with other swine-associated pathogens. The results can be observed directly with the naked eye, greatly simplifying the detection procedures. This method provides a rapid, accurate, efficient, portable, and low-cost diagnostic tool for GETV, which could be a valuable and powerful tool for the field detection and control of the virus.
Author Contributions
Conceptualization, D.S. and Y.Y.; methodology, Z.L. and D.S.; validation, Z.L., F.Y. and M.F.; formal analysis, Q.W. and K.F., investigation, Z.L., F.Y. and M.F.; resources, Z.L.; data curation, D.H. and G.W.; writing—original draft preparation, Z.L. and M.F.; writing—review and editing, D.S.; project administration, D.S.; funding acquisition, D.S. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the National Natural Science Foundation of China (31960711) and the Natural Science Foundation of Jiangxi Province (20232ACB205014). The founders had no role in the study design, sample collection, detection, sequencing, analysis, interpretation, or manuscript preparation.
Institutional Review Board Statement
The Jiangxi Agricultural University Institutional Animal Care and Use Committee approved the animal use protocol for this study (protocol number JXAULL-2022-10-35). All the procedures were carried out in accordance with The Care and Use Guidelines of Experimental Animals established by the Ministry of Agriculture of China. The specimens utilized in this study were collected from diseased pigs.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data is contained within the article.
Acknowledgments
We are grateful to all those who helped with the field sampling.
Conflicts of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Li, Y.Y.; Liu, H.; Fu, S.H.; Li, X.L.; Guo, X.F.; Li, M.H.; Feng, Y.; Chen, W.X.; Wang, L.H.; Lei, W.W.; et al. From discovery to spread: The evolution and phylogeny of Getah virus. Infect. Genet. Evol. 2017, 55, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Mukhopadhyay, S.; Merits, A.; Bolling, B.; Nasar, F.; Coffey, L.L.; Powers, A.; Weaver, S.C. Ictv Report Consortium. ICTV Virus Taxonomy Profile: Togaviridae. J. Gen. Virol. 2018, 99, 761–762. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Zhai, X.; Li, X.; Wang, Y.; He, W.T.; Jiang, Z.; Veit, M.; Su, S. Attenuation of Getah Virus by a Single Amino Acid Substitution at Residue 253 of the E2 Protein that Might Be Part of a New Heparan Sulfate Binding Site on Alphaviruses. J. Virol. 2022, 96, e0175121. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Wang, H.; Liang, G. Getah Virus (Alphavirus): An Emerging, Spreading Zoonotic Virus. Pathogens 2022, 11, 945. [Google Scholar] [CrossRef] [PubMed]
- Kanamitsu, M.; Taniguchi, K.; Urasawa, S.; Ogata, T.; Wada, Y.; Wada, Y.; Saroso, J.S. Geographic distribution of arbovirus antibodies in indigenous human populations in the Indo-Australian archipelago. Am. J. Trop. Med. Hyg. 1979, 28, 351–363. [Google Scholar] [CrossRef]
- Sanderson, C.J. A serologic survey of Queensland cattle for evidence of arbovirus infections. Am. J. Trop. Med. Hyg. 1969, 18, 433–439. [Google Scholar] [CrossRef] [PubMed]
- Shortridge, K.F.; Mason, D.K.; Watkins, K.L.; Aaskov, J.G. Serological evidence for the transmission of Getah virus in Hong Kong. Vet. Rec. 1994, 134, 527–528. [Google Scholar] [CrossRef]
- Sugiyama, I.; Shimizu, E.; Nogami, S.; Suzuki, K.; Miura, Y.; Sentsui, H. Serological survey of arthropod-borne viruses among wild boars in Japan. J. Vet. Med. Sci. 2009, 71, 1059–1061. [Google Scholar] [CrossRef]
- Li, Y.; Fu, S.; Guo, X.; Li, X.; Li, M.; Wang, L.; Gao, X.; Lei, W.; Cao, L.; Lu, Z.; et al. Serological Survey of Getah Virus in Domestic Animals in Yunnan Province, China. Vector Borne Zoonotic Dis. 2019, 19, 59–61. [Google Scholar] [CrossRef]
- Zhao, J.; Dellicour, S.; Yan, Z.; Veit, M.; Gill, M.S.; He, W.T.; Zhai, X.; Ji, X.; Suchard, M.A.; Lemey, P.; et al. Early Genomic Surveillance and Phylogeographic Analysis of Getah Virus, a Reemerging Arbovirus, in Livestock in China. J. Virol. 2023, 97, e0109122. [Google Scholar] [CrossRef]
- Shi, N.; Zhu, X.; Qiu, X.; Cao, X.; Jiang, Z.; Lu, H.; Jin, N. Origin, genetic diversity, adaptive evolution and transmission dynamics of Getah virus. Transbound. Emerg. Dis. 2022, 69, e1037–e1050. [Google Scholar] [CrossRef] [PubMed]
- Kamada, M.; Wada, R.; Kumanomido, T.; Imagawa, H.; Sugiura, T.; Fukunaga, Y. Effect of viral inoculum size on appearance of clinical signs in equine Getah virus infection. J. Vet. Med. Sci. 1991, 53, 803–806. [Google Scholar] [CrossRef][Green Version]
- Yang, T.; Li, R.; Hu, Y.; Yang, L.; Zhao, D.; Du, L.; Li, J.; Ge, M.; Yu, X. An outbreak of Getah virus infection among pigs in China, 2017. Transbound. Emerg. Dis. 2018, 65, 632–637. [Google Scholar] [CrossRef] [PubMed]
- Shibata, I.; Hatano, Y.; Nishimura, M.; Suzuki, G.; Inaba, Y. Isolation of Getah virus from dead fetuses extracted from a naturally infected sow in Japan. Vet. Microbiol. 1991, 27, 385–391. [Google Scholar] [CrossRef]
- Shi, N.; Liu, H.; Li, L.X.; Hu, B.; Zhang, L.; Zhao, C.F.; Deng, X.Y.; Li, X.T.; Xue, X.H.; Bai, X.; et al. Development of a TaqMan probe-based quantitative reverse transcription PCR assay for detection of Getah virus RNA. Arch. Virol. 2018, 163, 2877–2881. [Google Scholar] [CrossRef] [PubMed]
- Nie, M.; Deng, H.; Zhou, Y.; Sun, X.; Huang, Y.; Zhu, L.; Xu, Z. Development of a reverse transcription recombinase-aided amplification assay for detection of Getah virus. Sci. Rep. 2021, 11, 20060. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Li, L.X.; Bu, Y.P.; Liu, Y.; Sun, X.T.; Shi, N.; Deng, X.Y.; Lu, R.G.; Hu, B.; Jin, N.Y.; et al. Rapid Visual Detection of Getah Virus Using a Loop-Mediated Isothermal Amplification Method. Vector Borne Zoonotic Dis. 2019, 19, 741–746. [Google Scholar] [CrossRef]
- Sun, Q.; Xie, Y.; Guan, Z.; Zhang, Y.; Li, Y.; Yang, Y.; Zhang, J.; Li, Z.; Qiu, Y.; Li, B.; et al. Seroprevalence of Getah virus in Pigs in Eastern China Determined with a Recombinant E2 Protein-Based Indirect ELISA. Viruses 2022, 14, 2173. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhang, X.; Li, L.X.; Shi, N.; Sun, X.T.; Liu, Q.; Jin, N.Y.; Si, X.K. First isolation and characterization of Getah virus from cattle in northeastern China. BMC Vet. Res. 2019, 15, 320. [Google Scholar] [CrossRef]
- Qiu, X.; Cao, X.; Shi, N.; Zhang, H.; Zhu, X.; Gao, Y.; Mai, Z.; Jin, N.; Lu, H. Development and application of an indirect ELISA for detecting equine IgG antibodies against Getah virus with recombinant E2 domain protein. Front. Microbiol. 2022, 13, 1029444. [Google Scholar] [CrossRef]
- Liu, H.; Hu, J.; Li, L.X.; Lu, Z.S.; Sun, X.T.; Lu, H.J.; Jin, N.Y.; Zhang, L.; Zhang, L.N. Seroepidemiological investigation of Getah virus in the China-Myanmar border area from 2022–2023. Front. Microbiol. 2023, 14, 1309650. [Google Scholar] [CrossRef]
- Cao, X.; Qiu, X.; Shi, N.; Ha, Z.; Zhang, H.; Xie, Y.; Wang, P.; Zhu, X.; Zhao, W.; Zhao, G.; et al. Establishment of a reverse transcription real-time quantitative PCR method for Getah virus detection and its application for epidemiological investigation in Shandong, China. Front. Microbiol. 2022, 13, 1009610. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Chen, H.; Hu, A.; Cui, X.; Shi, C.; Lu, Z.; Meng, F.; Lv, F.; Zhao, H.; Bie, X. Establishment of LAMP-CRISPR/Cas12a for rapid detection of Escherichia coli O157:H7 and one-pot detection. Food Microbiol. 2024, 124, 104622. [Google Scholar] [CrossRef]
- Kropocheva, E.V.; Lisitskaya, L.A.; Agapov, A.A.; Musabirov, A.A.; Kulbachinskiy, A.V.; Esyunina, D.M. Prokaryotic Argonaute Proteins as a Tool for Biotechnology. Mol. Biol. 2022, 56, 854–873. [Google Scholar] [CrossRef] [PubMed]
- Swarts, D.C.; Hegge, J.W.; Hinojo, I.; Shiimori, M.; Ellis, M.A.; Dumrongkulraksa, J.; Terns, R.M.; Terns, M.P.; van der Oost, J. Argonaute of the archaeon Pyrococcus furiosus is a DNA-guided nuclease that targets cognate DNA. Nucleic Acids Res. 2015, 43, 5120–5129. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Jung, Y.; Lim, D. Argonaute system of Kordia jejudonensis is a heterodimeric nucleic acid-guided nuclease. Biochem. Biophys. Res. Commun. 2020, 525, 755–758. [Google Scholar] [CrossRef] [PubMed]
- He, R.; Wang, L.; Wang, F.; Li, W.; Liu, Y.; Li, A.; Wang, Y.; Mao, W.; Zhai, C.; Ma, L. Pyrococcus furiosus Argonaute-mediated nucleic acid detection. Chem. Commun. 2019, 55, 13219–13222. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Zhou, H.; Guo, X.; Liu, D.; Li, Z.; Sun, J.; Huang, J.; Liu, T.; Zhao, P.; Xu, H.; et al. Argonaute-integrated isothermal amplification for rapid, portable, multiplex detection of SARS-CoV-2 and influenza viruses. Biosens. Bioelectron. 2022, 207, 114169. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhou, C.; Guo, B.; Yang, X.; Wang, H. Pyrococcus furiosus Argonaute-mediated porcine epidemic diarrhea virus detection. Appl. Microbiol. Biotechnol. 2024, 108, 137. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, T.; Zhou, C.; Guo, B.; Wang, H. Pyrococcus furiosus Argonaute Based Detection Assays for Porcine Deltacoronavirus. ACS Synth. Biol. 2024, 13, 1323–1331. [Google Scholar] [CrossRef] [PubMed]
- Han, R.; Wang, F.; Chen, W.; Ma, L. A Fast and Sensitive One-Tube SARS-CoV-2 Detection Platform Based on RTX-PCR and Pyrococcus furiosus Argonaute. Biosensors 2024, 14, 245. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xia, W.; Zhao, W.; Hao, P.; Wang, Z.; Yu, X.; Shentu, X.; Sun, K. RT-RPA-PfAgo System: A Rapid, Sensitive, and Specific Multiplex Detection Method for Rice-Infecting Viruses. Biosensors 2023, 13, 941. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, T.; Song, D.; Huang, T.; Peng, Q.; Chen, Y.; Li, A.; Zhang, F.; Wu, Q.; Ye, Y.; et al. Comparison and evaluation of conventional RT-PCR, SYBR green I and TaqMan real-time RT-PCR assays for the detection of porcine epidemic diarrhea virus. Mol. Cell Probes. 2017, 33, 36–41. [Google Scholar] [CrossRef]
- Rattanatumhi, K.; Prasertsincharoen, N.; Naimon, N.; Kuwata, R.; Shimoda, H.; Ishijima, K.; Yonemitsu, K.; Minami, S.; Supriyono; Tran, N.T.B.; et al. A serological survey and characterization of Getah virus in domestic pigs in Thailand, 2017–2018. Transbound. Emerg. Dis. 2022, 69, 913–918. [Google Scholar] [CrossRef]
- Lu, G.; Ou, J.; Ji, J.; Ren, Z.; Hu, X.; Wang, C.; Li, S. Emergence of Getah Virus Infection in Horse with Fever in China, 2018. Front. Microbiol. 2019, 10, 1416. [Google Scholar]
- Guth, S.; Hanley, K.A.; Althouse, B.M.; Boots, M. Ecological processes underlying the emergence of novel enzootic cycles: Arboviruses in the neotropics as a case study. PLoS Negl. Trop. Dis. 2020, 14, e0008338. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chen, L.; Zhang, Z.; Zhang, R.; Xu, J.; Yang, P.; Sun, Y.; Chen, Y.; Xie, C.; Lin, M.; et al. Development and application of a novel recombinase polymerase amplification-Pyrococcus furiosus argonaute system for rapid detection of goose parvovirus. Poult. Sci. 2024, 103, 104141. [Google Scholar] [CrossRef]
- Yu, Z.; Shi, D.; Dong, Y.; Shao, Y.; Chen, Z.; Cheng, F.; Zhang, Y.; Wang, Z.; Tu, J.; Song, X. Pyrococcus furiosus argonaute combined with loop-mediated isothermal amplification for rapid, ultrasensitive, and visual detection of fowl adenovirus serotype 4. Poult. Sci. 2024, 103, 103729. [Google Scholar] [CrossRef] [PubMed]
- Xun, G.; Liu, Q.; Chong, Y.; Guo, X.; Li, Z.; Li, Y.; Fei, H.; Li, K.; Feng, Y. Argonaute with stepwise endonuclease activity promotes specific and multiplex nucleic acid detection. Bioresour. Bioprocess. 2021, 8, 46. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Yang, L.; Zhang, X.; Tang, Y.; Wang, Y.; Shao, X.; Gao, S.; Liu, X.; Wang, P. Rapid and Sensitive Genotyping of SARS-CoV-2 Key Mutation L452R with an RPA-PfAgo Method. Anal. Chem. 2022, 94, 17151–17159. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, Y.; Wu, W.; Kang, T.; Sun, J.; Jiang, H. Rapid and sensitive detection of Mycoplasma synoviae using RPA combined with Pyrococcus furiosus Argonaute. Poult. Sci. 2024, 103, 103244. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).