Validation of Polymorphisms Associated with the Immune Response After Vaccination Against Porcine Reproductive and Respiratory Syndrome Virus in Yorkshire Gilts
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Location and Experimental Units
2.2. PRRSV Vaccination and Blood Sampling
2.3. Genotyping and Quality Control
2.4. Genome-Wide Association Study
2.5. Multiple Testing Correction
2.6. Functional and Enrichment Analysis
2.7. SNP Validation Genotyping
2.8. Statistical Analysis of the Genotype to Phenotype Validation Study
2.9. Power Analysis to Estimate Sample Size
2.10. Functional Validation Using Quantitative RT-PCR
2.11. Cytokine Analysis
3. Results
3.1. Genome-Wide Association Study (GWAS)
3.2. Biological Pathways and Gene Ontology
3.3. SNP Validation Study
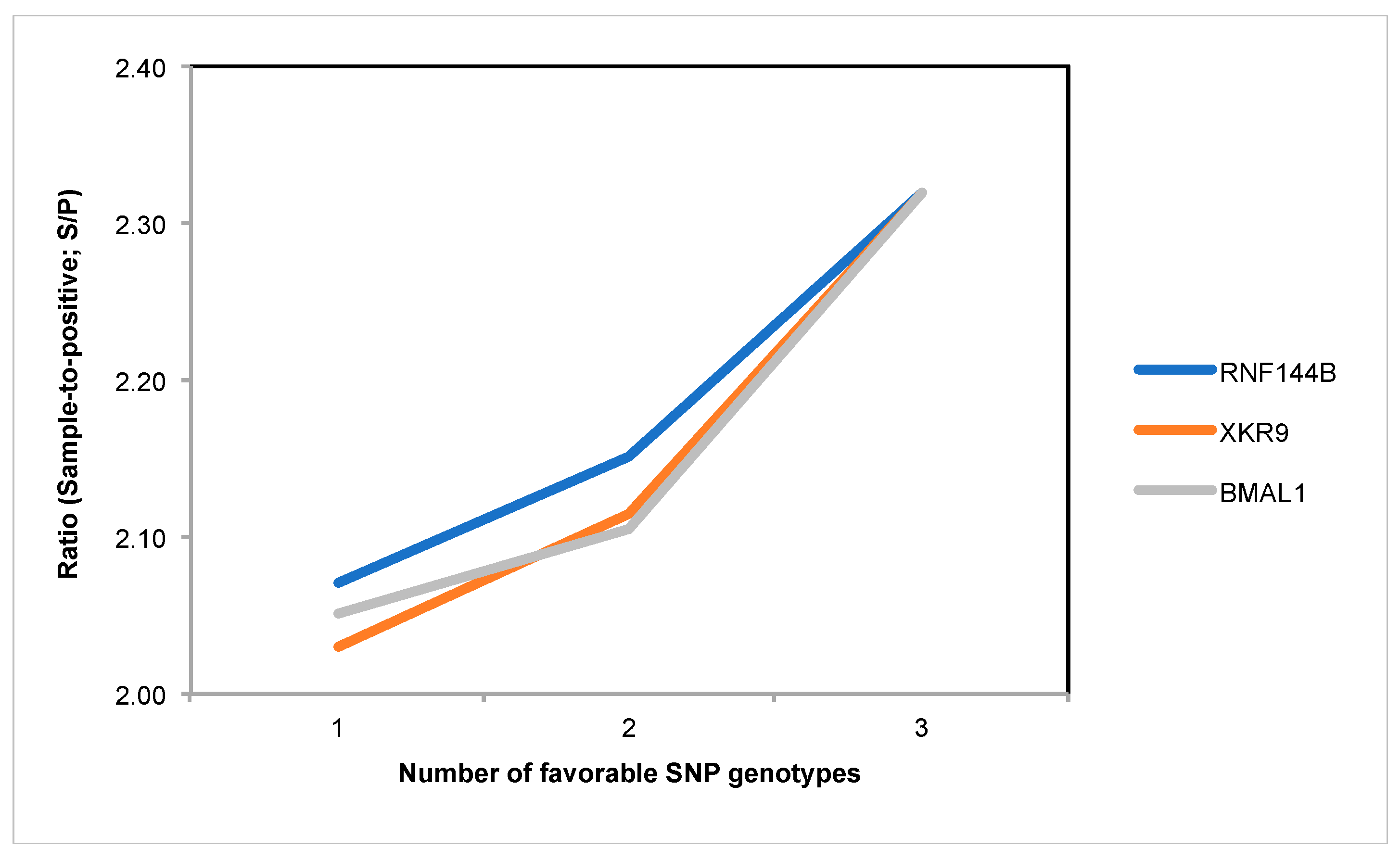
3.4. SNP Genotype Effects
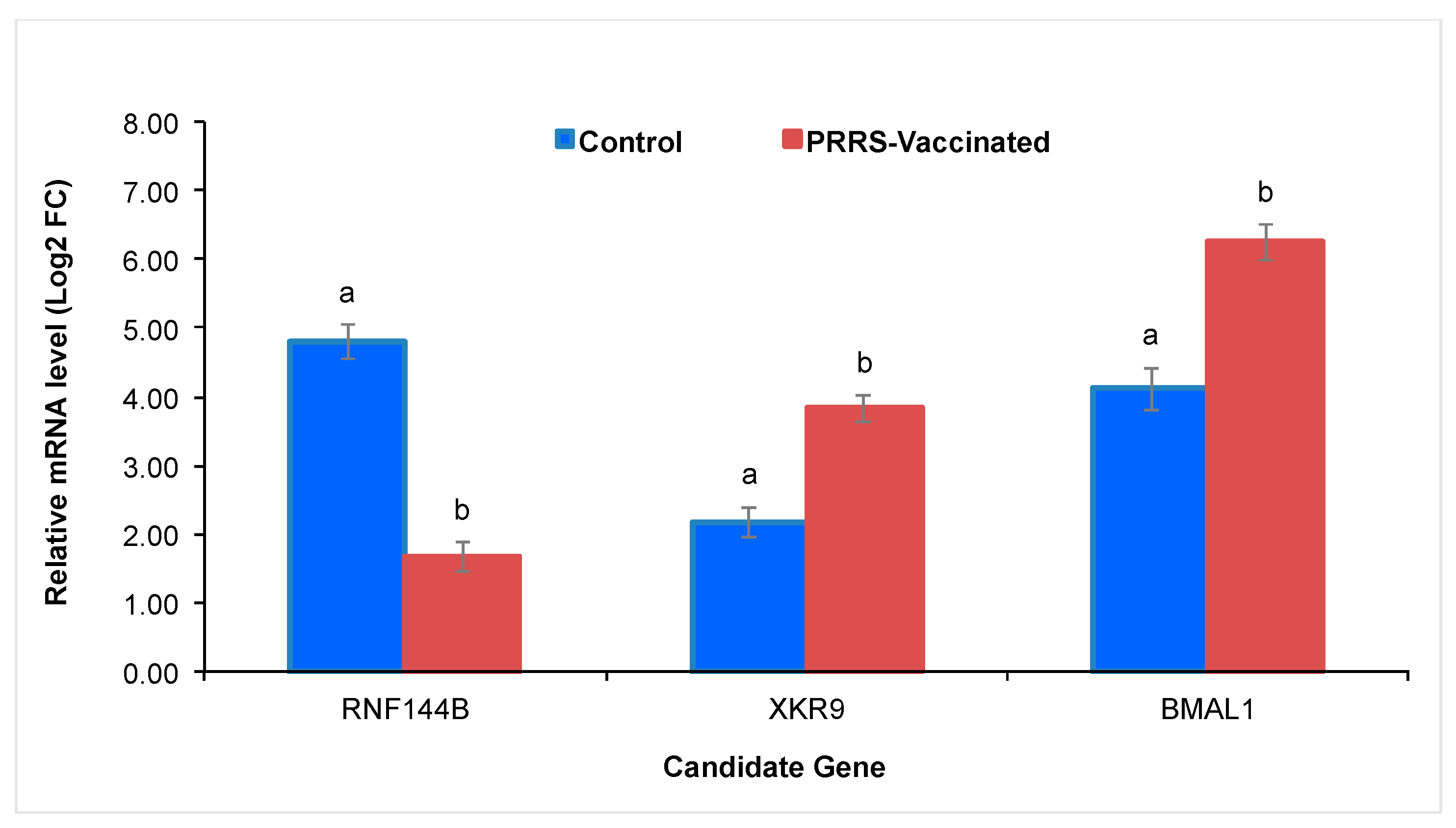
3.5. Quantitative RT-PCR Validation
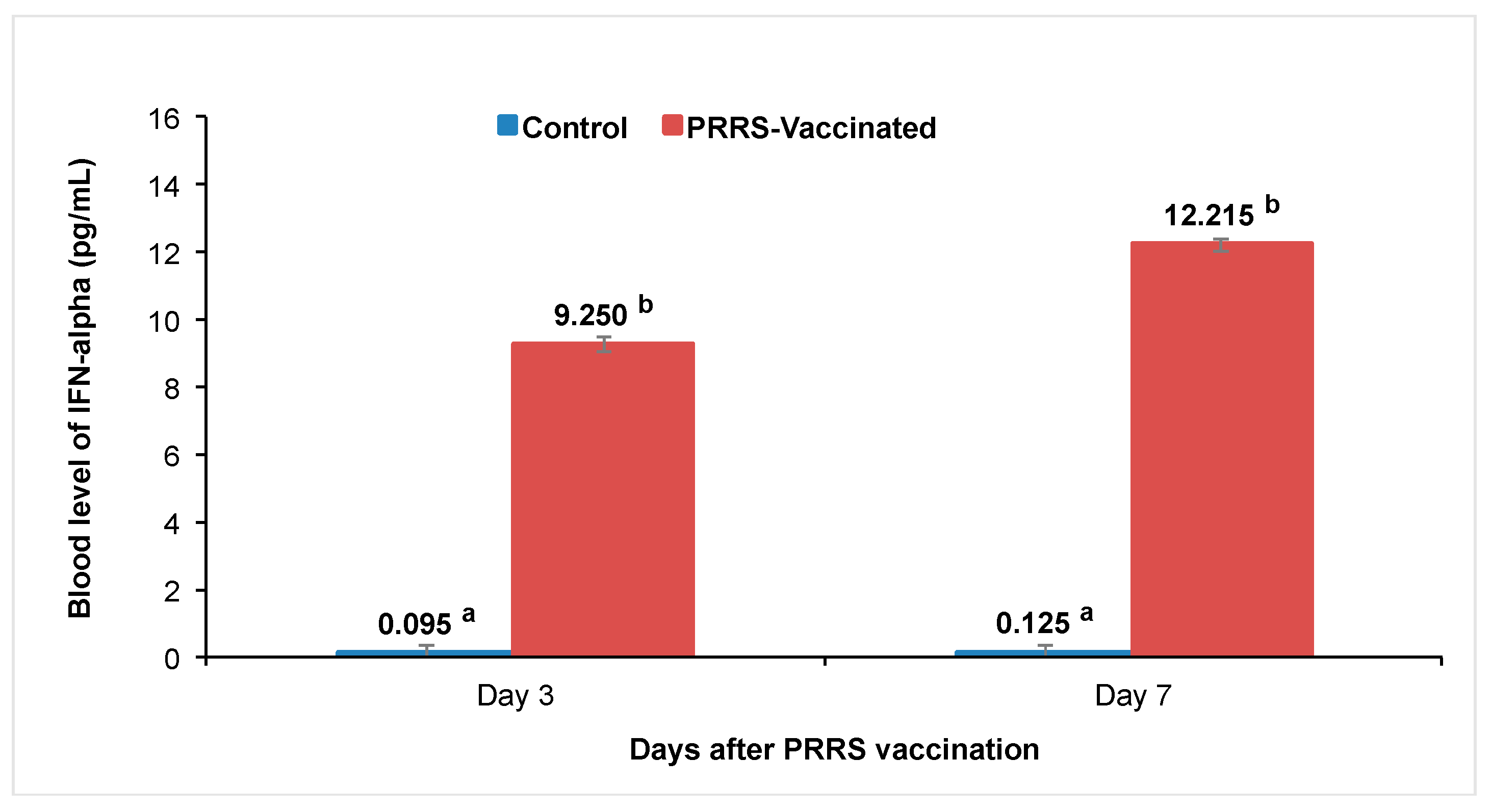
3.6. ELISA to Measure Cytokine IFN-α
3.7. Association Between IFN-α and Gene Expression
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- López-Heydecka, S.M.; Alonso-Morales, R.A.; Mendieta-Zerón, H.; Vázquez-Chagoyán, J.C. Porcine respiratory and reproductive syndrome: Review. Rev. Mex. Cienc. Pecu. 2015, 6, 68–69. [Google Scholar]
- Zimmerman, J.; Locke, A.K.; Ramirez, A.; Schwarts, K.J.; Stevenson, G.W.; Zhang, K. Diseases of Swine, 1st ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2019. [Google Scholar]
- Nan, Y.; Wu, C.; Gu, G.; Sun, W.; Zhang, Y.; Zhou, E.M. Improved vaccine against PRRSV: Current progress and future perspective. Front. Microbiol. 2017, 8, 1635. [Google Scholar]
- Renson, P.; Mahé, S.; Andraud, M.; Le Dimna, M.; Paboeuf, F.; Rose, N.; Bourry, O. Effect of vaccination route (intradermal vs. intramuscular) against porcine reproductive and respiratory syndrome using a modified live vaccine on systemic and mucosal immune response and virus transmission in pigs. BMC Vet. Res. 2024, 20, 5. [Google Scholar]
- Lee, M.A.; Jayaramaiah, U.; You, S.H.; Shin, E.G.; Song, S.M.; Ju, L.; Kang, S.J.; Hyun, B.H.; Lee, H.S. Molecular Characterization of Porcine Reproductive and Respiratory Syndrome Virus in Korea from 2018 to 2022. Pathogens 2023, 12, 757. [Google Scholar] [CrossRef] [PubMed]
- Chae, C. Commercial PRRS modified-live virus vaccines. Vaccines 2021, 9, 185. [Google Scholar] [CrossRef]
- Sanglard, L.P.; Hickmann, F.M.W.; Huang, Y.; Gray, K.A.; Linhares, D.C.L.; Dekkers, J.C.M.; Niederwerder, M.C.; Fernando, R.L.; Braccini Neto, J.; Serão, N.V.L. Genomics of response to porcine reproductive and respiratory syndrome virus in purebred and crossbred sows: Antibody response and performance following natural infection vs. vaccination. J. Anim. Sci. 2021, 99, skab097. [Google Scholar]
- Rowland, R.R.; Lunney, J.; Dekkers, J. Control of porcine reproductive and respiratory syndrome (PRRS) through genetic improvements in disease resistance and tolerance. Front. Genet. 2012, 3, 260. [Google Scholar]
- Aguilar-Trejo, C.M.; Luna-Nevárez, G.; Reyna-Granados, J.R.; Zamorano-Algandar, R.; Romo-Rubio, J.A.; Sánchez-Castro, M.A.; Enns, R.M.; Speidel, S.E.; Thomas, M.G.; Luna-Nevárez, P. Polymorphisms associated with the number of live-born piglets in sows. Rev. Mex. Cienc. Pecu. 2020, 11, 828–847. [Google Scholar] [CrossRef]
- Sanglard, L.P.; Fernando, R.L.; Gray, K.A.; Linhares, D.C.L.; Dekkers, J.C.M.; Niederwerder, M.C.; Serão, N.V.L. Genetic analysis of antibody response to porcine reproductive and respiratory syndrome vaccination as an indicator trait for reproductive performance in commercial sows. Front. Genet. 2020, 11, 1011. [Google Scholar]
- Li, W.; Wang, Z.; Luo, S.; Wu, J.; Zhou, L.; Liu, J. Genome-wide association analysis and genetic parameters for feed efficiency and related traits in Yorkshire and Duroc pigs. Animals 2022, 12, 1902. [Google Scholar] [CrossRef]
- Luan, M.; Ruan, D.; Qiu, Y.; Ye, Y.; Zhou, S.; Yang, J.; Sun, Y.; Ma, F.; Wu, Z.; Yang, J.; et al. Genome-wide association study for loin muscle area of commercial crossbred pigs. Anim. Biosci. 2023, 36, 861–868. [Google Scholar] [CrossRef] [PubMed]
- Ding, R.; Yang, M.; Quan, J.; Li, S.; Zhuang, Z.; Zhou, S.; Zheng, E.; Hong, L.; Li, Z.; Cai, G.; et al. Single-locus and multilocus genome-wide association studies for intramuscular fat in Duroc pigs. Front. Genet. 2019, 10, 619. [Google Scholar] [CrossRef] [PubMed]
- Chang Wu, Z.; Wang, Y.; Huang, X.; Wu, S.; Bao, W. A genome-wide association study of important reproduction traits in Large White pigs. Gene 2022, 838, 146702. [Google Scholar] [CrossRef]
- Óvilo, C.; Trakooljul, N.; Núñez, Y.; Hadlich, F.; Murani, E.; Ayuso, M.; García-Contreras, C.; Vázquez-Gómez, M.; Rey, A.I.; García, F.; et al. SNP discovery and association study for growth, fatness and meat quality traits in Iberian crossbred pigs. Sci. Rep. 2022, 12, 16361. [Google Scholar] [CrossRef]
- Zhou, F.; Quan, J.; Ruan, D.; Qiu, Y.; Ding, R.; Xu, C.; Ye, Y.; Cai, G.; Liu, L.; Zhang, Z.; et al. Identification of candidate genes for economically important carcass cutting in commercial pigs through GWAS. Animals 2023, 13, 3243. [Google Scholar] [CrossRef]
- Boddicker, N.; Waide, E.H.; Rowland, R.R.; Lunney, J.K.; Garrick, D.J.; Reecy, M.; Dekkers, J.C. Evidence for a major QTL associated with host response to porcine reproductive and respiratory syndrome virus challenge. J. Anim. Sci. 2012, 90, 1733–1746. [Google Scholar] [CrossRef]
- Sanglard, L.P.; Huang, Y.; Gray, K.A.; Linhares, D.; Dekkers, J.; Niederwerder, M.C.; Fernando, R.L.; Serão, N. Further host-genomic characterization of total antibody response to PRRSV vaccination and its relationship with reproductive performance in commercial sows: Genome-wide haplotype and zygosity analyses. Genet. Sel. Evol. 2021, 53, 91. [Google Scholar] [CrossRef]
- Dunkelberger, J.R.; Serão, N.V.L.; Weng, Z.; Waide, E.H.; Niederwerder, M.C.; Kerrigan, M.A.; Lunney, J.K.; Rowland, R.R.R.; Dekkers, J.C.M. Genomic regions associated with host response to porcine reproductive and respiratory syndrome vaccination and coinfection in nursery pigs. BMC Genom. 2017, 18, 865. [Google Scholar] [CrossRef]
- Torricelli, M.; Fratto, A.; Ciullo, M.; Sebastiani, C.; Arcangeli, C.; Felici, A.; Giovannini, S.; Sarti, F.M.; Sensi, M.; Biagetti, M. Porcine reproductive and respiratory ryndrome (PRRS) and CD163 resistance polymorphic markers: What is the scenario in naturally infected pig livestock in central Italy? Animals 2023, 13, 2477. [Google Scholar] [CrossRef]
- Walker, L.R.; Jobman, E.E.; Sutton, K.M.; Wittler, J.; Johnson, R.K.; Ciobanu, D.C. Genome-wide association analysis for porcine reproductive and respiratory syndrome virus susceptibility traits in two genetic populations of pigs1. J. Anim. Sci. 2019, 97, 3253–3261. [Google Scholar] [CrossRef]
- He, Y.; Zhou, X.; Zheng, R.; Jiang, Y.; Yao, Z.; Wang, X.; Zhang, Z.; Zhang, H.; Li, J.; Yuan, X. The Association of an SNP in the EXOC4 gene and reproductive traits suggests its use as a breeding marker in pigs. Animals 2021, 11, 521. [Google Scholar] [CrossRef]
- Liu, Z.; Shan, B.; Ni, C.; Feng, S.; Liu, W.; Wang, X.; Wu, H.; Yang, Z.; Liu, J.; Wei, S.; et al. Optimized protocol for double vaccine immunization against classical swine fever and porcine reproductive and respiratory syndrome. BMC Vet. Res. 2023, 19, 14. [Google Scholar]
- Serão, N.V.; Kemp, R.A.; Mote, B.E.; Willson, P.; Harding, J.C.; Bishop, S.C.; Plastow, G.S.; Dekkers, J.C. Genetic and genomic basis of antibody response to porcine reproductive and respiratory syndrome (PRRS) in gilts and sows. Genet. Sel. Evol. 2016, 48, 51. [Google Scholar] [CrossRef]
- Serão, N.V.; Matika, O.; Kemp, R.A.; Harding, J.C.; Bishop, S.C.; Plastow, G.S.; Dekkers, J.C. Genetic analysis of reproductive traits and antibody response in a PRRS outbreak herd. J. Anim. Sci. 2014, 92, 2905–2921. [Google Scholar] [PubMed]
- Mader, T.L.; Johnson, L.J.; Gaughan, J.B. A comprehensive index for assessing environmental stress in animals. J. Anim Sci. 2010, 88, 2153–2165. [Google Scholar]
- Purcell, S.; Neale, B.; Todd-Brown, K.; Thomas, L.; Ferreira, M.A.; Bender, D.; Maller, J.; Sklar, P.; de Bakker, P.I.; Daly, M.J.; et al. PLINK: A tool set for whole-genome association and population-based linkage analyses. Am. J. Hum. Genet. 2007, 81, 559–575. [Google Scholar] [CrossRef] [PubMed]
- Grinde, K.E.; Browning, B.L.; Reiner, A.P.; Thornton, T.A.; Browning, S.R. Adjusting for principal components can induce collider bias in genome-wide association studies. PLoS Genet. 2024, 20, e1011242. [Google Scholar]
- Kaler, A.S.; Purcell, L.C. Estimation of a significance threshold for genome-wide association studies. BMC Genom. 2019, 20, 618. [Google Scholar]
- Weir, B.S. Forensics. In Handbook of Statistical Genetics, 1st ed.; Balding, D.J., Bishop, M., Cannings, C., Eds.; John Wiley and Sons, Ltd.: New York, NY, USA, 2001; p. 275. [Google Scholar]
- Falconer, D.S.; Mackay, T.F.C. Introduction to Quantitative Genetics, 4th ed.; Longman Scientific and Technical: New York, NY, USA, 1996. [Google Scholar]
- Montaner-Tarbes, S.; Del Portillo, H.A.; Montoya, M.; Fraile, L. Key gaps in the knowledge of the porcine respiratory and reproductive syndrome virus (PRRSV). Front. Vet. Sci. 2019, 6, 38. [Google Scholar]
- Ko, H.; Sammons, J.; Pasternak, J.A.; Hamonic, G.; Starrak, G.; MacPhee, D.J.; Detmer, S.E.; Plastow, G.S.; Harding, J.C.S. Phenotypic effect of a single nucleotide polymorphism on SSC7 on fetal outcomes in PRRSV-2 infected gilts. Liv. Sci. 2022, 255, 104800. [Google Scholar]
- Hong, Y.; Tan, C.; He, X.; Wu, D.; Zhang, Y.; Song, C.; Wu, Z. Genome-wide association study of reproductive traits in Large White pigs. Animals 2024, 14, 2874. [Google Scholar] [CrossRef] [PubMed]
- Luna-Nevárez, G.; Kelly, A.C.; Camacho, L.E.; Limesand, S.W.; Reyna-Granados, J.R.; Luna-Nevárez, P. Discovery and validation of candidate SNP markers associated to heat stress response in pregnant ewes managed inside a climate-controlled chamber. Trop. Anim. Health Prod. 2020, 52, 3457–3466. [Google Scholar] [CrossRef] [PubMed]
- Luna-Nevárez, G.; Pendleton, A.L.; Luna-Ramirez, R.I.; Limesand, S.W.; Reyna-Granados, J.R.; Luna-Nevárez, P. Genome-wide association study of a thermo-tolerance indicator in pregnant ewes exposed to an artificial heat-stressed environment. J. Therm. Biol. 2021, 101, 103095. [Google Scholar] [CrossRef]
- Zamorano-Algandar, R.; Medrano, J.F.; Thomas, M.G.; Enns, R.M.; Speidel, S.E.; Sánchez-Castro, M.A.; Luna-Nevárez, G.; Leyva-Corona, J.C.; Luna-Nevárez, P. Genetic markers associated with milk production and thermotolerance in Holstein dairy cows managed in a heat-stressed environment. Biology 2023, 12, 679. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, E.K.; Baranowska, I.; Wade, C.M.; Salmon Hillbertz, N.H.; Zody, M.C.; Anderson, N.; Biagi, T.M.; Patterson, N.; Pielberg, G.R.; Kulbokas, E.J.; et al. Efficient mapping of Mendelian traits in dogs through genome-wide association. Nat. Genet. 2007, 39, 1321–1328. [Google Scholar] [CrossRef]
- Visscher, P.M. Sizing up human height variation. Nat. Genet. 2008, 40, 89–90. [Google Scholar] [CrossRef]
- Hickmann, F.M.W.; Braccini-Neto, J.; Kramer, L.M.; Huang, Y.; Gray, K.; Dekkers, J.C.M.; Sanglard, L.P.; Serão, N.V.L. Host genetics of response to porcine reproductive and respiratory syndrome in sows: Antibody response as an indicator trait for improved reproductive performance. Front. Gen. 2021, 12, 707873. [Google Scholar] [CrossRef]
- Liu, X.; Wang, Q.; Chen, W.; Wang, C. Dynamic regulation of innate immunity by ubiquitin and ubiquitin-like proteins. Cytokine Growth Factor Rev. 2013, 24, 559–570. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, B.; Zhu, X.; Zhao, L.; Chu, C.; Guo, Q.; Wei, R.; Yin, X.; Zhang, Y.; Li, X. RNF144B inhibits LPS-induced inflammatory responses by binding TBK1. J. Leukoc. Biol. 2019, 106, 1303–1311. [Google Scholar] [CrossRef]
- Crow, M.K. Type I interferon in organ-targeted autoimmune and inflammatory diseases. Arthritis Res. Ther. 2010, 12 (Suppl. 1), S5. [Google Scholar] [CrossRef]
- Li, J.; Miller, L.C.; Sang, Y. Current status of vaccines for porcine reproductive and respiratory syndrome: Interferon response, immunological overview, and future prospects. Vaccines 2024, 12, 606. [Google Scholar] [CrossRef] [PubMed]
- Budronim, V.; Versteeg, G.A. Negative regulation of the innate immune response through proteasomal degradation and deubiquitination. Viruses 2021, 13, 584. [Google Scholar] [CrossRef] [PubMed]
- Ke, H.; Yoo, D. The viral innate immune antagonism and an alternative vaccine design for PRRS virus. Vet. Microbiol. 2017, 209, 75–89. [Google Scholar] [PubMed]
- Han, J.; Wu, M.; Liu, Z. Dysregulation in IFN-γ signaling and response: The barricade to tumor immunotherapy. Front. Immunol. 2023, 14, 1190333. [Google Scholar]
- Cheon, H.; Wang, Y.; Wightman, S.M.; Jackson, M.W.; Stark, G.R. How cancer cells make and respond to interferon-I. Trends Cancer 2023, 9, 83–92. [Google Scholar]
- Ivashkiv, L.B.; Donlin, L.T. Regulation of type I interferon responses. Nat. Rev. Immunol. 2014, 14, 36–49. [Google Scholar] [CrossRef]
- Li, G.; Zhang, J.; Zhao, Z.; Wang, J.; Li, J.; Xu, W.; Cui, Z.; Sun, P.; Yuan, H.; Wang, T.; et al. RNF144B negatively regulates antiviral immunity by targeting MDA5 for autophagic degradation. EMBO Rep. 2024, 25, 4594–4624. [Google Scholar]
- Zhang, X.; Feng, W.H. Porcine reproductive and respiratory syndrome virus evades antiviral innate immunity via MicroRNAs regulation. Front. Microbiol. 2021, 12, 804264. [Google Scholar]
- Zhang, L.; Zhang, L.; Pan, Y.; Gao, J.; Xu, Y.; Li, X.; Tian, Z.; Chen, H.; Wang, Y. Downregulation of miR-218 by porcine reproductive and respiratory syndrome virus facilitates viral replication via inhibition of type I interferon responses. J. Biol. Chem. 2021, 296, 100683. [Google Scholar] [CrossRef]
- Cai, H.; Zhang, H.; Cheng, H.; Liu, M.; Wen, S.; Ren, J. Progress in PRRSV infection and adaptive immune response mechanisms. Viruses 2023, 15, 1442. [Google Scholar] [CrossRef]
- Du, T.; Nan, Y.; Xiao, S.; Zhao, Q.; Zhou, E.M. Antiviral strategies against PRRSV infection. Trends Microbiol. 2017, 25, 968–979. [Google Scholar] [PubMed]
- Suzuki, J.; Imanishi, E.; Nagata, S. Exposure of phosphatidylserine by Xk-related protein family members during apoptosis. J. Biol. Chem. 2014, 289, 30257–30267. [Google Scholar] [PubMed]
- Wei, X.; Li, R.; Qiao, S.; Chen, X.; Xing, G.; Zhanga, G. Porcine reproductive and respiratory syndrome virus utilizes porcine reproductive and respiratory syndrome virus utilizes host cells. J. Virol. 2020, 94, e00709-20. [Google Scholar]
- Straub, M.S.; Alvadia, C.; Sawicka, M.; Dutzler, R. Cryo-EM structures of the caspase-activated protein XKR9 involved in apoptotic lipid scrambling. Elife 2021, 10, e69800. [Google Scholar] [PubMed]
- Hernández, J.; Li, Y.; Mateu, E. Swine dendritic cell response to porcine reproductive and respiratory syndrome virus: An update. Front. Immunol. 2021, 12, 712109. [Google Scholar] [CrossRef]
- Li, H.; Li, K.; Zhang, K.; Li, Y.; Gu, H.; Liu, H.; Yang, Z.; Cai, D. The circadian physiology: Implications in livestock health. Int. J. Mol. Sci. 2021, 22, 2111. [Google Scholar] [CrossRef]
- Mazzoccoli, G.; Vinciguerra, M.; Carbone, A.; Relógio, A. The circadian clock, the immune system, and viral infections: The intricate relationship between biological time and host–virus interaction. Pathogens 2020, 9, 83. [Google Scholar] [CrossRef]
- Nguyen, K.D.; Fentress, S.; Qiu, Y.; Yun, K.; Cox, J.S.; Chawla, A. Circadian gene Bmal1 regulates diurnal oscillations of Ly6C(hi) inflammatory monocytes. Science 2013, 341, 1483–1488. [Google Scholar]
- Fairbairn, L.; Kapetanovic, R.; Beraldi, D.; Sester, D.P.; Tuggle, C.K.; Archibald, A.L.; Hume, D.A. Comparative analysis of monocyte subsets in the pig. J. Immunol. 2013, 190, 6389–6396. [Google Scholar]
- Silver, A.C.; Buckley, S.M.; Hughes, M.E.; Hastings, A.K.; Nitabach, M.N.; Fikrig, E. Daily oscillations in expression and responsiveness of Toll-like receptors in splenic immune cells. Heliyon 2018, 4, e00579. [Google Scholar]
- Borrmann, H.; McKeating, J.A.; Zhuang, X. The Circadian Clock and Viral Infections. J. Biol. Rhythm. 2021, 36, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Guo, Z.; Wu, M.; Chen, F.; Chen, L. Circadian rhythm regulates the function of immune cells and participates in the development of tumors. Cell Death Discov. 2024, 10, 199. [Google Scholar] [CrossRef]
- Chen, X.X.; Qiao, S.; Li, R.; Wang, J.; Li, X.; Zhang, G. Evasion strategies of porcine reproductive and respiratory syndrome virus. Front. Microbiol. 2023, 14, 1140449. [Google Scholar] [CrossRef]
- Cortey, M.; Arocena, G.; Ait-Ali, T. Analysis of the genetic diversity and mRNA expression level in porcine reproductive and respiratory syndrome virus vaccinated pigs that developed short or long viremias after challenge. Vet. Res. 2018, 49, 19. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Bailey, S.D.; Lupien, M. Laying a solid foundation for Manhattan—‘Setting the functional basis for the post-GWAS era’. Trends Genet. 2014, 30, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Deng, N.; Zhou, H.; Fan, H.; Yuan, Y. Single nucleotide polymorphisms and cancer susceptibility. Oncotarget 2017, 8, 110635–110649. [Google Scholar] [CrossRef]
- Statello, L.; Guo, C.J.; Chen, L.L. Gene regulation by long non-coding RNAs and its biological functions. Nat. Rev. Mol. Cell Biol. 2021, 22, 96–118. [Google Scholar] [CrossRef]


| SNP ID 1 | Variant 2 | SSC 3 | Position 4 | Gene 5 | Alleles 6 | p-Value 7 | FDR 8 |
|---|---|---|---|---|---|---|---|
| rs81358818 | Intronic | 2 | 45,943,026 | BMAL1 | C/T | 1.77 × 10−8 | 0.0001 |
| rs705026086 | Intronic | 18 | 31,655,798 | FOXP2 | A/G | 2.25 × 10−8 | 0.0009 |
| rs343308278 | 3’UTR | 13 | 71,562,721 | GP9 | C/T | 1.12 × 10−6 | 0.0030 |
| rs708860811 | Intronic | 4 | 64,697,573 | XKR9 | A/G | 1.40 × 10−6 | 0.0025 |
| rs80844350 | Intronic | 4 | 39,499,547 | CPQ | A/G | 1.46 × 10−6 | 0.0026 |
| rs331531082 | Intergenic | 18 | 32,235,504 | -------- | C/T | 1.81 × 10−6 | 0.0030 |
| rs80969120 | Intergenic | 4 | 64,456,714 | -------- | C/T | 2.67 × 10−6 | 0.0032 |
| rs707264998 | Intronic | 7 | 14,111,503 | RNF144B | A/G | 4.36 × 10−6 | 0.0041 |
| rs707607708 | Intronic | 3 | 3,156,851 | SDK1 | C/T | 4.73 × 10−6 | 0.0045 |
| rs3475576322 | Non-coding | 12 | 19,663,911 | -------- | C/G | 5.63 × 10−6 | 0.0047 |
| Canonical Pathway 1 | p-Value 2 | Key Genes 3 |
|---|---|---|
| Immune system | 0.027 | RNF144B/XKR9 |
| Cytokine–cytokine receptor interaction | 0.043 | RNF144B/BMAL1 |
| SNP ID 1 | Gene 2 | Allele Frequency 3 | HWE Test 4 | HWE p-Value 5 | |
|---|---|---|---|---|---|
| A | G | ||||
| rs707264998 | RNF144B | 0.69 | 0.31 | 0.28 | 0.79 |
| rs708860811 | XKR9 | 0.46 | 0.54 | 1.15 | 0.46 |
| rs80844350 | CPQ | 0.51 | 0.49 | 0.86 | 0.58 |
| rs705026086 | FOXP2 | 0.23 | 0.77 | 0.47 | 0.71 |
| C | T | ||||
| rs81358818 | BMAL1 | 0.47 | 0.53 | 0.75 | 0.64 |
| rs343308278 | GP9 | 0.38 | 0.62 | 24.19 | < 0.01 |
| rs707607708 | SDK1 | 0.97 | 0.03 | 18.73 | < 0.01 |
| SNP ID 1 | Gene 2 | Least-Square Means by Genotype ± SE 3 | p-Value 4 | ||
|---|---|---|---|---|---|
| AA | AG | GG | |||
| rs707264998 | RNF144B | 1.90 ± 0.03 a | 2.36 ± 0.10 b | 2.51 ± 0.09 b | 0.0009 |
| rs708860811 | XKR9 | 2.24 ± 0.11 a | 1.88 ± 0.07 b | 1.76 ± 0.08 b | 0.0065 |
| rs80844350 | CPQ | 1.96 ± 0.12 a | 1.79 ± 0.10 a | 1.68 ± 0.14 a | 0.4238 |
| rs705026086 | FOXP2B | 1.74 ± 0.06 a | 2.01 ± 0.06 a | 2.18 ± 0.08 a | 0.1875 |
| CC | CT | TT | |||
| rs81358818 | BMAL1 | 2.31 ± 0.08 a | 2.14 ± 0.06 b | 1.87 ± 0.07 c | <0.0001 |
| SNP ID 1 | Gene 2 | Allele Substitution Effects | Fixed Estimates Effects | ||||
|---|---|---|---|---|---|---|---|
| F. Allele 3 | p-Value 4 | Estimate ± SE 5 | p-Value 6 | AddE 7 | DomE 8 | ||
| rs707264998 | RNF144B | G | <0.0010 | 0.301 ± 0.016 | <0.0010 | 0.305 | 0.155 |
| rs708860811 | XKR9 | A | <0.0100 | 0.230 ± 0.010 | <0.0080 | 0.240 | 0.120 |
| rs81358818 | BMAL1 | C | <0.0001 | 0.216 ± 0.012 | <0.0001 | 0.220 | 0.050 |
| Treatment | Candidate Genes | ||
|---|---|---|---|
| RNF144B | XKR9 | BMAL1 | |
| Control | −0.6581 ** | 0.4738 * | 0.3816 * |
| PRRSV-vaccinated | −0.7221 ** | 0.5420 * | 0.4933 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Icedo-Nuñez, S.; Luna-Ramirez, R.I.; Enns, R.M.; Speidel, S.E.; Hernández, J.; Zeng, X.; Sánchez-Castro, M.A.; Aguilar-Trejo, C.M.; Luna-Nevárez, G.; López-González, M.C.; et al. Validation of Polymorphisms Associated with the Immune Response After Vaccination Against Porcine Reproductive and Respiratory Syndrome Virus in Yorkshire Gilts. Vet. Sci. 2025, 12, 295. https://doi.org/10.3390/vetsci12040295
Icedo-Nuñez S, Luna-Ramirez RI, Enns RM, Speidel SE, Hernández J, Zeng X, Sánchez-Castro MA, Aguilar-Trejo CM, Luna-Nevárez G, López-González MC, et al. Validation of Polymorphisms Associated with the Immune Response After Vaccination Against Porcine Reproductive and Respiratory Syndrome Virus in Yorkshire Gilts. Veterinary Sciences. 2025; 12(4):295. https://doi.org/10.3390/vetsci12040295
Chicago/Turabian StyleIcedo-Nuñez, Salvador, Rosa I. Luna-Ramirez, R. Mark Enns, Scott E. Speidel, Jesús Hernández, Xi Zeng, Miguel A. Sánchez-Castro, Carlos M. Aguilar-Trejo, Guillermo Luna-Nevárez, Martha C. López-González, and et al. 2025. "Validation of Polymorphisms Associated with the Immune Response After Vaccination Against Porcine Reproductive and Respiratory Syndrome Virus in Yorkshire Gilts" Veterinary Sciences 12, no. 4: 295. https://doi.org/10.3390/vetsci12040295
APA StyleIcedo-Nuñez, S., Luna-Ramirez, R. I., Enns, R. M., Speidel, S. E., Hernández, J., Zeng, X., Sánchez-Castro, M. A., Aguilar-Trejo, C. M., Luna-Nevárez, G., López-González, M. C., Reyna-Granados, J. R., & Luna-Nevárez, P. (2025). Validation of Polymorphisms Associated with the Immune Response After Vaccination Against Porcine Reproductive and Respiratory Syndrome Virus in Yorkshire Gilts. Veterinary Sciences, 12(4), 295. https://doi.org/10.3390/vetsci12040295









