Pathological Study on Trigeminal Ganglionitis Among Rabid Dogs in the Philippines
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Clinical Signs
2.2. Histopathological Examination
2.3. Digitization of Tissue Slides
2.4. IHC Analysis
2.5. Indirect Immunofluorescence Double-Staining
3. Results
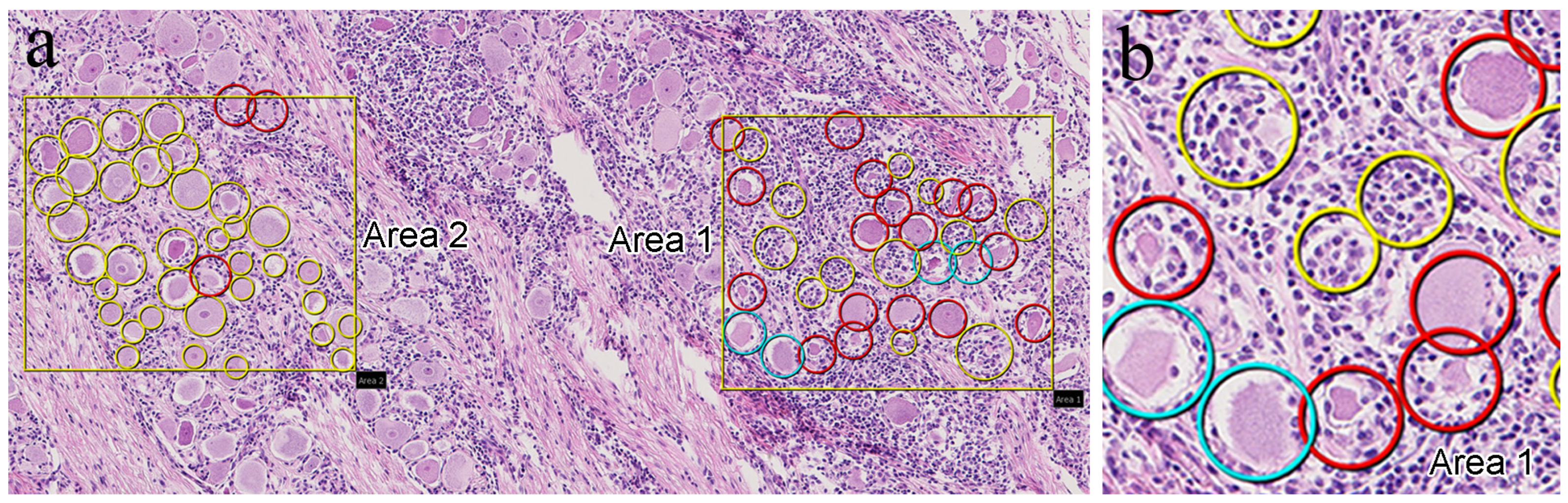
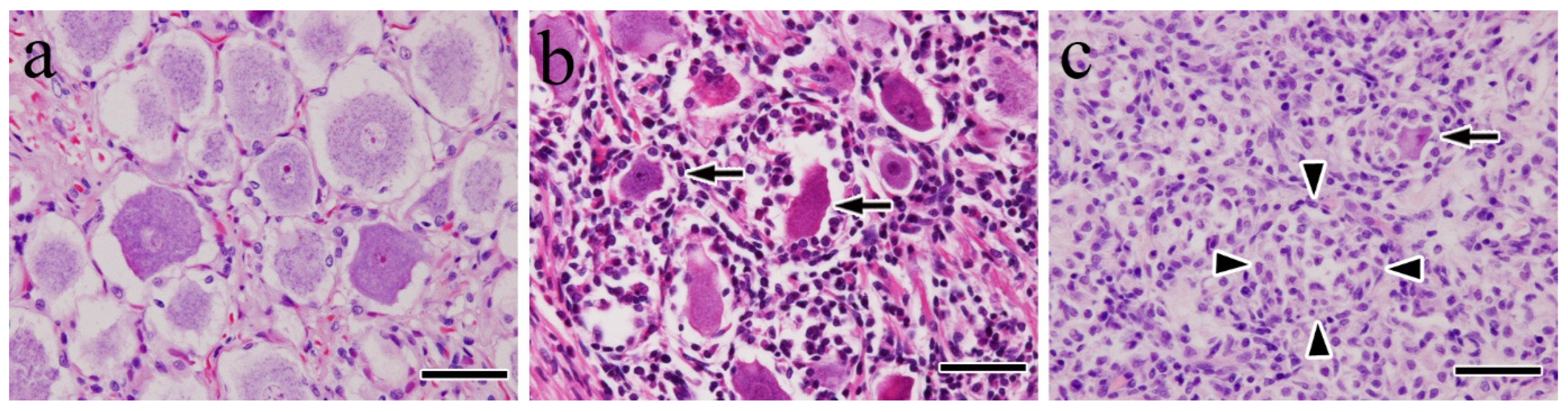
3.1. Histopathological Findings
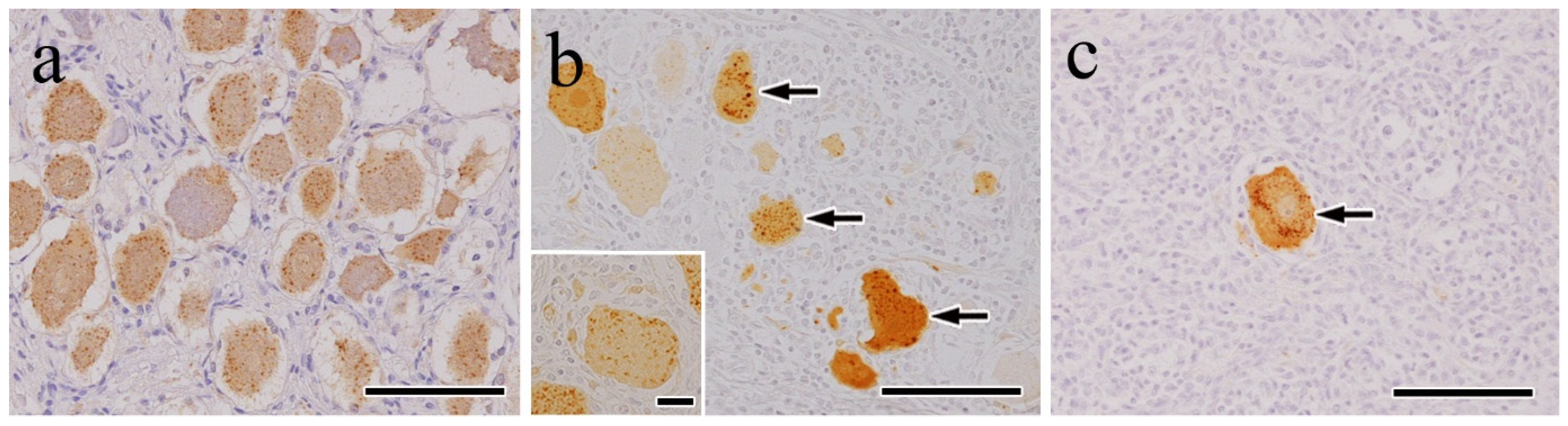
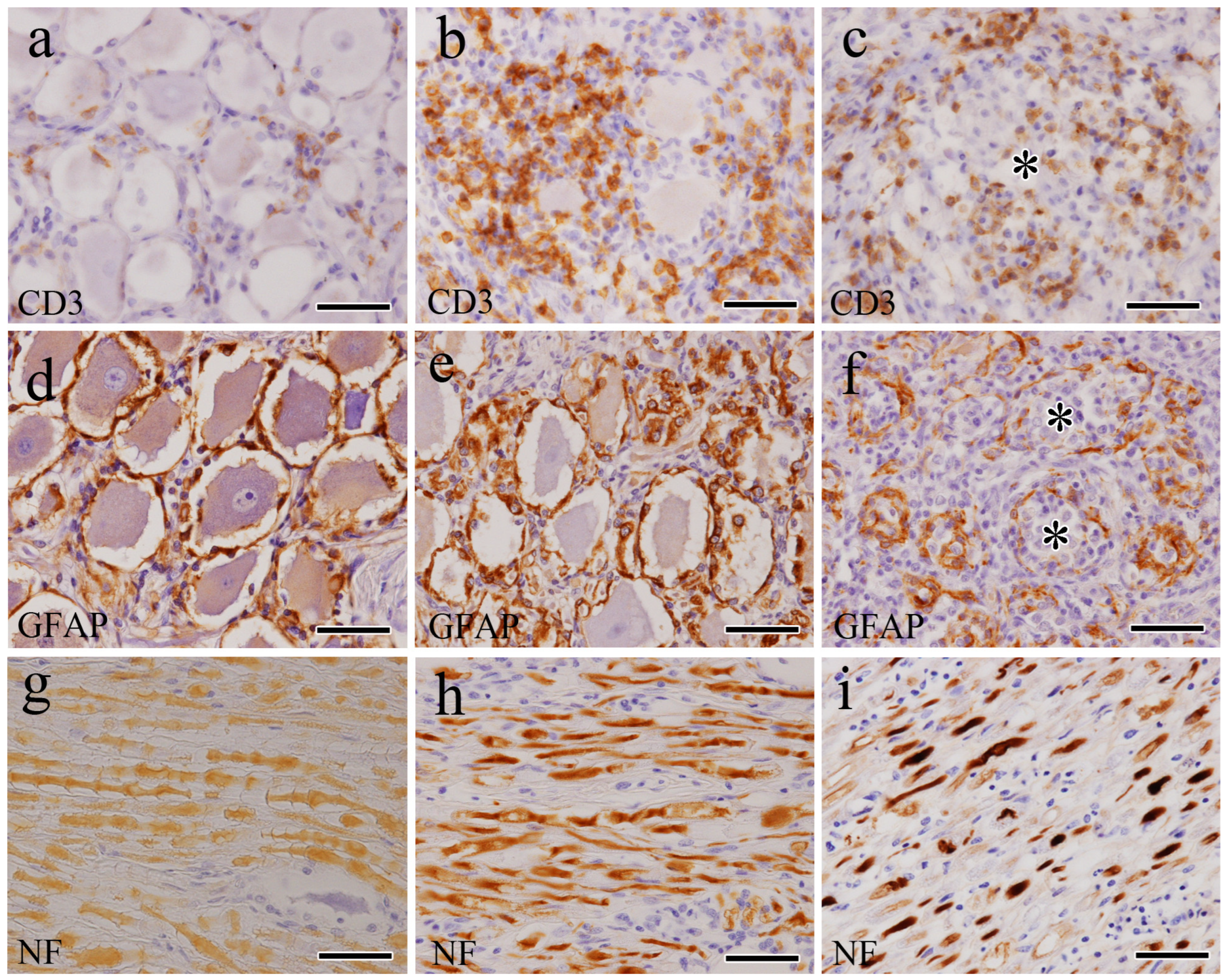
3.2. IHC Findings
3.3. Indirect Immunofluorescence Double-Stain Findings
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wunner, W.H. Rabies Virus. In Rabies; Jackson, A.C., Wunner, W.H., Eds.; Academic Press: Cambridge, MA, USA, 2007; pp. 23–68. [Google Scholar]
- Kumar, A.; Bhatt, S.; Kumar, A.; Rana, T. Canine Rabies: An Epidemiological Significance, Pathogenesis, Diagnosis, Prevention, and Public Health Issues. Comp. Immunol. Microbiol. Infect. Dis. 2023, 97, 101992. [Google Scholar] [CrossRef] [PubMed]
- Hampson, K.; Coudeville, L.; Lembo, T.; Sambo, M.; Kieffer, A.; Attlan, M.; Barrat, J.; Blanton, J.D.; Briggs, D.J.; Cleaveland, S.; et al. Global Alliance for Rabies Control Partners for Rabies Prevention. Estimating the Global Burden of Endemic Canine Rabies. PLOS Negl. Trop. Dis. 2015, 9, e0003709. [Google Scholar]
- Fooks, A.R.; Cliquet, F.; Finke, S.; Freuling, C.; Hemachudha, T.; Mani, R.S.; Müller, T.; Nadin-Davis, S.; Picard-Meyer, E.; Wilde, H.; et al. Rabies. Nat. Rev. Dis. Primers 2017, 3, 17091. [Google Scholar] [CrossRef] [PubMed]
- Knobel, D.L.; Cleaveland, S.; Coleman, P.G.; Fèvre, E.M.; Meltzer, M.I.; Miranda, M.E.G.; Shaw, A.; Zinsstag, J.; Meslin, F.-X. Re-evaluating the Burden of Rabies in Africa and Asia. Bull. World Health Organ. 2005, 83, 360–368. [Google Scholar]
- Lentz, T.L.; Burrage, T.G.; Smith, A.L.; Crick, J.; Tignor, G.H. Is the Acetylcholine Receptor a Rabies Virus Receptor? Science 1982, 215, 182–184. [Google Scholar] [CrossRef]
- Ajayi, B.B.; Rabo, J.S.; Baba, S.S. Rabies in Apparently Healthy Dogs: Histological and Immunohistochemical Studies. Niger. Postgrad. Med. J. 2006, 13, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Balachandran, A.; Charlton, K. Experimental Rabies Infection of Non-nervous Tissues in Skunks (Mephitis mephitis) and Foxes (Vulpes vulpes). Vet. Pathol. 1994, 31, 93–102. [Google Scholar] [CrossRef]
- Jackson, A.C.; Ye, H.; Phelan, C.C.; Ridaura-Sanz, C.; Zheng, Q.; Li, Z.; Wan, X.; Lopez-Corella, E. Extraneural Organ Involvement in Human Rabies. Lab. Investig. 1999, 79, 945–951. [Google Scholar]
- Jogai, S.; Radotra, B.D.; Banerjee, A.K. Rabies Viral Antigen in Extracranial Organs: A Post-Mortem Study. Neuropathol. Appl. Neurobiol. 2002, 28, 334–338. [Google Scholar] [CrossRef]
- de Morais, C.F.; de Assis, R.V. Cardiac Involvement in Human Rabies. Case Report. Rev. Inst. Med. Trop. Sao Paulo 1985, 27, 145–149. [Google Scholar] [CrossRef]
- Tobiume, M.; Sato, Y.; Katano, H.; Nakajima, N.; Tanaka, K.; Noguchi, A.; Inoue, S.; Hasegawa, H.; Iwasa, Y.; Tanaka, J.; et al. Rabies Virus Dissemination in Neural Tissues of Autopsy Cases Due to Rabies Imported into Japan from the Philippines: Immunohistochemistry. Pathol. Int. 2009, 59, 555–566. [Google Scholar] [CrossRef]
- Lackay, S.N.; Kuang, Y.; Fu, Z.F. Rabies in Small Animals. Vet. Clin. N. Am. Small Anim. Pract. 2008, 38, 851–861. [Google Scholar] [CrossRef]
- Whalen, L.R.; Kitchell, R.L. Electrophysiologic Studies of the Cutaneous Nerves of the Head of the Dog. Am. J. Vet. Res. 1983, 44, 615–627. [Google Scholar] [CrossRef]
- Bathla, G.; Hegde, A.N. The Trigeminal Nerve: An Illustrated Review of Its Imaging Anatomy and Pathology. Clin. Radiol. 2013, 68, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Kent, M.; Song, R.B.; Glass, E.N.; de Lahunta, A.D. A Salivation Abnormality with Trigeminal Nerve Dysfunction in Dogs. J. Vet. Dent. 2019, 36, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Mayhew, P.D.; Bush, W.W.; Glass, E.N. Trigeminal Neuropathy in Dogs: A Retrospective Study of 29 Cases (1991–2000). J. Am. Anim. Hosp. Assoc. 2002, 38, 262–270. [Google Scholar] [CrossRef] [PubMed]
- Swain, C.E.; Cherubini, G.B.; Mantis, P. Low Field MRI Measurements of the Normal Canine Trigeminal Nerve. Front. Vet. Sci. 2020, 7, 274. [Google Scholar] [CrossRef]
- Laast, V.A.; Pardo, C.A.; Tarwater, P.M.; Queen, S.E.; Reinhart, T.A.; Ghosh, M.; Adams, R.J.; Zink, M.C.; Mankowski, J.L. Pathogenesis of Simian Immunodeficiency Virus-Induced Alterations in Macaque Trigeminal Ganglia. J. Neuropathol. Exp. Neurol. 2007, 66, 26–34. [Google Scholar] [CrossRef]
- Boonsriroj, H.; Manalo, D.L.; Kimitsuki, K.; Shimatsu, T.; Shiwa, N.; Shinozaki, H.; Takahashi, Y.; Tanaka, N.; Inoue, S.; Park, C.-H. A Pathological Study of the Salivary Glands of Rabid Dogs in the Philippines. J. Vet. Med. Sci. 2016, 78, 35–42. [Google Scholar] [CrossRef]
- Jackson, A.C. Pathogenesis. In Rabies; Jackson, A.C., Wunner, W.H., Eds.; Academic Press: Cambridge, MA, USA, 2007; pp. 341–381. [Google Scholar]
- Murphy, F.A.; Harrison, A.K.; Winn, W.C.; Bauer, S.P. Comparative Pathogenesis of Rabies and Rabies-Like Viruses: Infection of the Central Nervous System and Centrifugal Spread of Virus to Peripheral Tissues. Lab. Investig. 1973, 29, 1–16. [Google Scholar]
- Tsiang, H.; Ceccaldi, P.E.; Lycke, E.J. Rabies Virus Infection and Transport in Human Sensory Dorsal Root Ganglia Neurons. J. Gen. Virol. 1991, 72, 1191–1194. [Google Scholar] [CrossRef] [PubMed]
- Rupprecht, C.E.; Niezgoda, M.; Hanlon, C. Rabies in Terrestrial Animals. In Rabies; Jackson, A.C., Wunner, W.H., Eds.; Aca-Demic Press: Cambridge, MA, USA, 2007; pp. 217–220. [Google Scholar]
- Cummings, J.F.; de Lahunta, A.D.; Mitchell, W.J. Ganglioradiculitis in the Dog. A Clinical, Light- and Electron-Microscopic Study. Acta Neuropathol. 1983, 60, 29–39. [Google Scholar] [CrossRef]
- Funamoto, M.; Nibe, K.; Morozumi, M.; Edamura, K.; Uchida, K. Pathological Features of Ganglioradiculitis (Sensory Neuropathy) in Two Dogs. J. Vet. Med. Sci. 2007, 69, 1247–1253. [Google Scholar] [CrossRef] [PubMed]
- Porter, B.; Schatzberg, S.; McDonough, S.; Mertens, D.; de Lahunta, A.D. Ganglioradiculitis (Sensory Neuronopathy) in a Dog: Clinical, Morphologic, and Immunohistochemical Findings. Vet. Pathol. 2002, 39, 598–602. [Google Scholar] [CrossRef] [PubMed]
- Summers, B.A.; Cummings, J.F.; de Lahunta, A. Diseases of the Peripheral Nervous System. In Veterinary Neuropathology; Mosby: St. Louis, MO, USA, 1995; pp. 412–430. [Google Scholar]
- Mitrabhakdi, E.; Shuangshoti, S.; Wannakrairot, P.; Lewis, R.A.; Susuki, K.; Laothamatas, J.; Hemachudha, T. Difference in Neuropathogenetic Mechanisms in Human Furious and Paralytic Rabies. J. Neurol. Sci. 2005, 238, 3–10. [Google Scholar] [CrossRef]
- Tangchai, P.; Vejjajiva, A. Pathology of the Peripheral Nervous System in Human Rabies. A Study of Nine Autopsy Cases. Brain 1971, 94, 299–306. [Google Scholar] [CrossRef]
- O’Toole, D.; Mills, K.; Ellis, J.; Welch, V.; Fillerup, M. Poliomyelomalacia and Ganglioneuritis in a Horse with Paralytic Rabies. J. Vet. Diagn. Invest. 1993, 5, 94–97. [Google Scholar] [CrossRef]
- Abreu, C.C.; Nakayama, P.A.; Nogueira, C.I.; Mesquita, L.P.; Lopes, P.F.R.; Wouters, F.; Varaschin, M.S., Jr.; Bezerra, P.S. Histopathology and Immunohistochemistry of Tissues Outside Central Nervous System in Bovine Rabies. J. Neurovirol. 2014, 20, 388–397. [Google Scholar] [CrossRef]
- Rossiter, J.P.; Hsu, L.; Jackson, A.C. Selective Vulnerability of Dorsal Root Ganglia Neurons in Experimental Rabies after Peripheral Inoculation of CVS-11 in Adult Mice. Acta Neuropathol. 2009, 118, 249–259. [Google Scholar] [CrossRef]
- Hamir, A.N.; Moser, G.; Rupprecht, C.E. Clinicopathologic Variation in Raccoons Infected with Different Street Rabies Virus Isolates. J. Vet. Diagn. Investig. 1996, 8, 31–37. [Google Scholar] [CrossRef]
- Pardo, C.A.; McArthur, J.C.; Griffin, J.W. HIV Neuropathy: Insights in the Pathology of HIV Peripheral Nerve Disease. J. Peripher. Nerv. Syst. 2001, 6, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Burdo, T.H.; Orzechowski, K.; Knight, H.L.; Miller, A.D.; Williams, K. Dorsal Root Ganglia Damage in SIV-Infected Rhesus Macaques: An Animal Model of HIV-Induced Sensory Neuropathy. Am. J. Pathol. 2012, 180, 1362–1369. [Google Scholar] [CrossRef] [PubMed]
- Lakritz, J.R.; Bodair, A.; Shah, N.; O’Donnell, R.; Polydefkis, M.J.; Miller, A.D.; Burdo, T.H. Monocyte Traffic, Dorsal Root Ganglion Histopathology, and Loss of Intraepidermal Nerve Fiber Density in SIV Peripheral Neuropathy. Am. J. Pathol. 2015, 185, 1912–1923. [Google Scholar] [CrossRef]
- Rizzo, S.; Basso, C.; Troost, D.; Aronica, E.; Frigo, A.C.; Driessen, A.H.G.; Thiene, G.; Wilde, A.A.M.; van der Wal, A.C.V. T-Cell-Mediated Inflammatory Activity in the Stellate Ganglia of Patients with Ion-Channel Disease and Severe Ventricular Arrhythmias. Circ. Arrhythm. Electrophysiol. 2014, 7, 224–229. [Google Scholar] [CrossRef]
- Davis, B.M.; Rall, G.F.; Schnell, M.J. Everything You Always Wanted to Know About Rabies Virus (but Were Afraid to Ask). Annu. Rev. Virol. 2015, 2, 451–471. [Google Scholar] [CrossRef]
- Zhang, H.; Huang, J.; Song, Y.; Liu, X.; Qian, M.; Huang, P.; Li, Y.; Zhao, L.; Wang, H. Regulation of Innate Immune Responses by Rabies Virus. Animal Model. Exp. Med. 2022, 5, 418–429. [Google Scholar] [CrossRef] [PubMed]
- Jager, S.E.; Pallesen, L.T.; Richner, M.; Harley, P.; Hore, Z.; McMahon, S.; Denk, F.; Vaegter, C.B. Changes in the Transcriptional Fingerprint of Satellite Glial Cells Following Peripheral Nerve Injury. Glia 2020, 68, 1375–1395. [Google Scholar] [CrossRef]
- Donegan, M.; Kernisant, M.; Cua, C.; Jasmin, L.; Ohara, P.T. Satellite Glial Cell Proliferation in the Trigeminal Ganglia After Chronic Constriction Injury of the Infraorbital Nerve. Glia 2013, 61, 2000–2008. [Google Scholar] [CrossRef]
- Chen, P.; Piao, X.; Bonaldo, P. Role of Macrophages in Wallerian Degeneration and Axonal Regeneration After Peripheral Nerve Injury. Acta Neuropathol. 2015, 130, 605–618. [Google Scholar] [CrossRef]
- de Lanunta, A.; Glass, E. Lower Motor Neuron: General Somatic Efferent, Cranial Nerve. In Veterinary Neuroanatomy and Clinical Neurology, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2009; pp. 139–144. [Google Scholar]

| Antibody (a) | Host | Clone | Dilution | Antigen Retrieval (b) | Source | Target Cells |
|---|---|---|---|---|---|---|
| CD3 | Rabbit | polyclonal | Prediluted | MW, 170 W, 10 min | Agilent (Dako) | T cells |
| CD20 | Rabbit | polyclonal | 1:400 | MW, 170 W, 10 min | Thermo | B cells |
| GFAP | Rabbit | polyclonal | Prediluted | No treatment | Nichirei | Satellite cells |
| Iba-1 | Rabbit | polyclonal | 1:500 | No treatment | Wako | Macrophages |
| HLA-DR | Mouse | TAL.1B5 | 1:500 | MW, 170 W, 10 min | Agilent (Dako) | Macrophages |
| NF | Mouse | 2F11 | Prediluted | Pro-K, 37 °C, 10 min | Agilent (Dako) | Ganglions, axons |
| S-100 | Mouse | 4C4.9 | Prediluted | MW, 170 W, 10 min | Thermo | Schwann cells |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iamohbhars, N.; Cabic, A.G.B.; Markbordee, B.; Shiina, R.; Tamura, N.; Shiwa-Sudo, N.; Kimitsuki, K.; Espino, M.J.M.; Manalo, D.L.; Inoue, S.; et al. Pathological Study on Trigeminal Ganglionitis Among Rabid Dogs in the Philippines. Vet. Sci. 2025, 12, 299. https://doi.org/10.3390/vetsci12040299
Iamohbhars N, Cabic AGB, Markbordee B, Shiina R, Tamura N, Shiwa-Sudo N, Kimitsuki K, Espino MJM, Manalo DL, Inoue S, et al. Pathological Study on Trigeminal Ganglionitis Among Rabid Dogs in the Philippines. Veterinary Sciences. 2025; 12(4):299. https://doi.org/10.3390/vetsci12040299
Chicago/Turabian StyleIamohbhars, Nuttipa, Alpha Grace B. Cabic, Boonkanit Markbordee, Ryota Shiina, Natsumi Tamura, Nozomi Shiwa-Sudo, Kazunori Kimitsuki, Mark Joseph M. Espino, Daria Llenaresas Manalo, Satoshi Inoue, and et al. 2025. "Pathological Study on Trigeminal Ganglionitis Among Rabid Dogs in the Philippines" Veterinary Sciences 12, no. 4: 299. https://doi.org/10.3390/vetsci12040299
APA StyleIamohbhars, N., Cabic, A. G. B., Markbordee, B., Shiina, R., Tamura, N., Shiwa-Sudo, N., Kimitsuki, K., Espino, M. J. M., Manalo, D. L., Inoue, S., & Park, C.-H. (2025). Pathological Study on Trigeminal Ganglionitis Among Rabid Dogs in the Philippines. Veterinary Sciences, 12(4), 299. https://doi.org/10.3390/vetsci12040299






