Effect of Management System on Fecal Microbiota in Arabian Horses: Preliminary Results
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Animal Research
2.2. Animals
2.3. Fecal Sampling
2.4. 16S rRNA Gene Metabarcoding
2.5. Sequence Analysis
2.6. Statistical Analysis
2.7. Parasitological Analysis
3. Results
3.1. Sequencing Depth and Data Screening
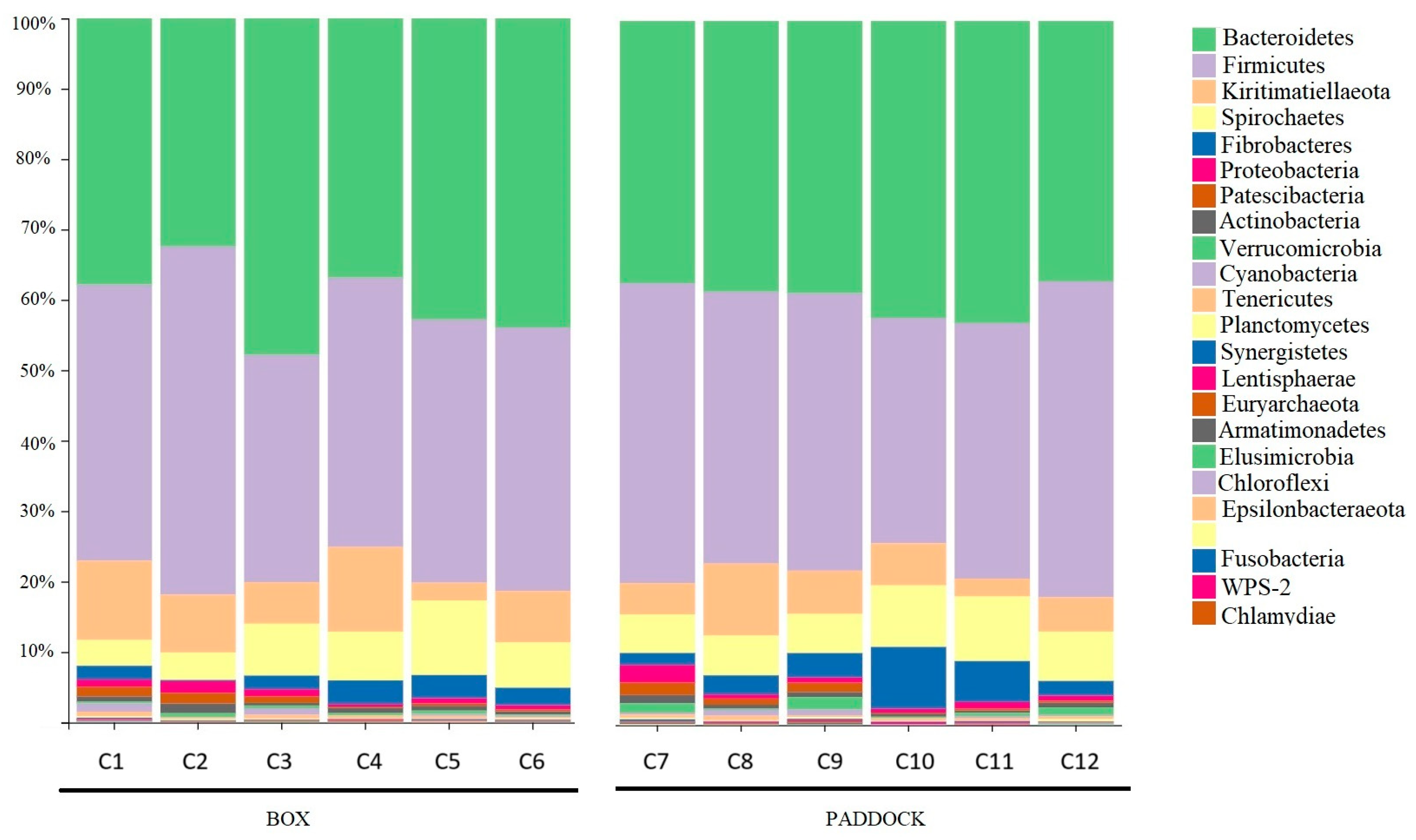
3.2. Microbiota Composition
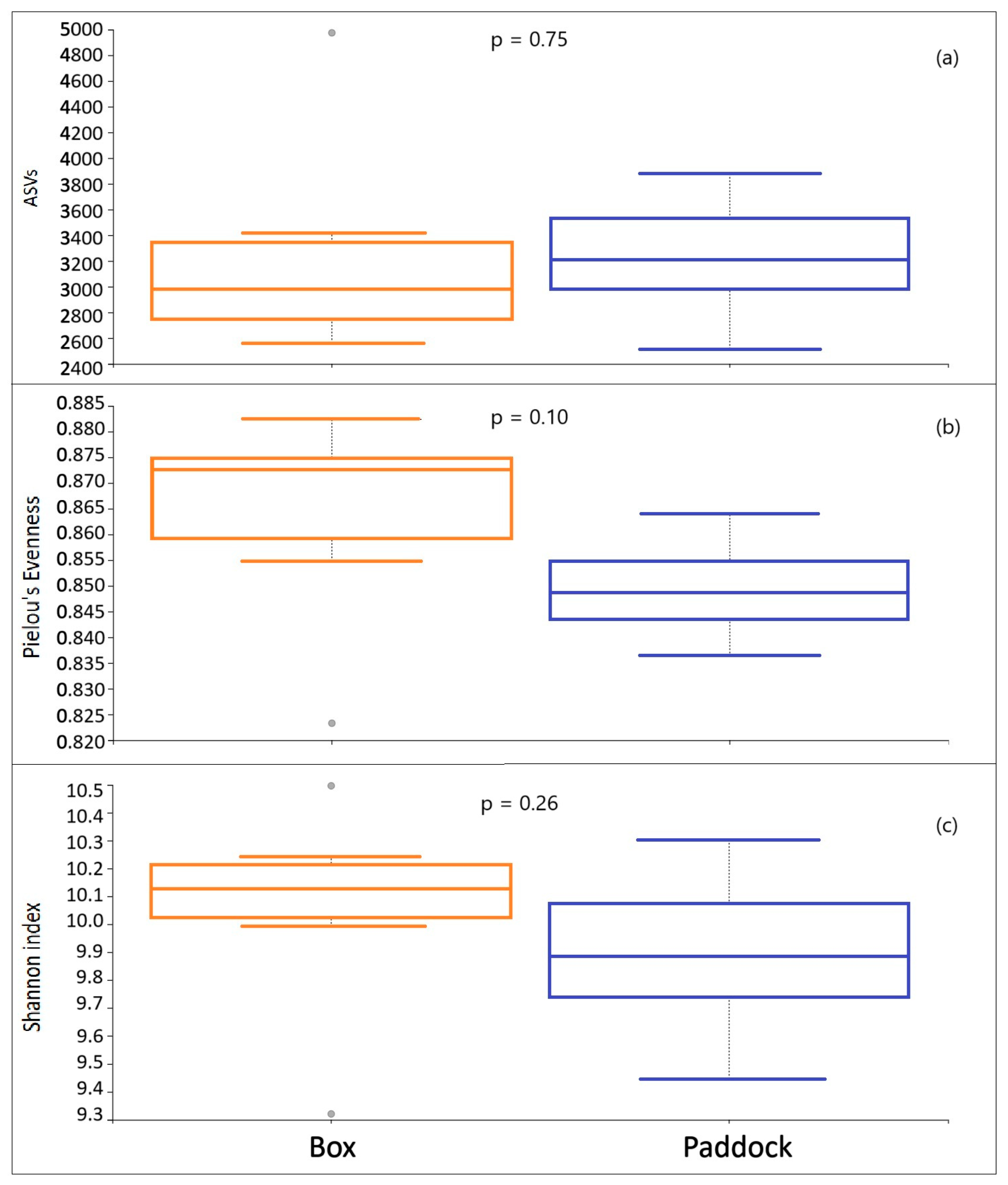
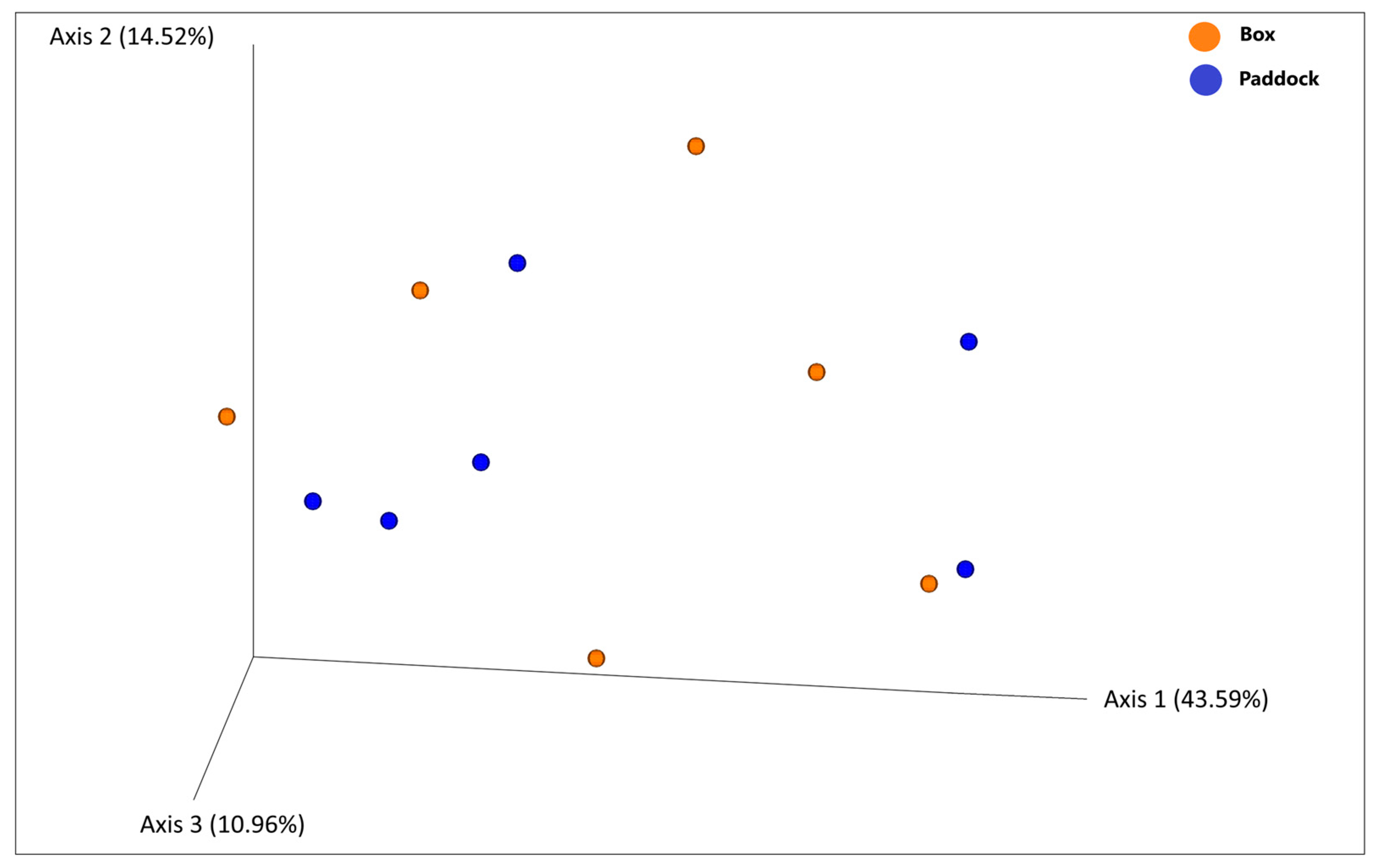
3.3. Diversity Analysis
3.4. Parasitological Examination
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- von Keyserlingk, M.A.G.; Weary, D.M. A 100-Year Review: Animal Welfare in the Journal of Dairy Science—The First 100 Years. J. Dairy. Sci. 2017, 100, 10432–10444. [Google Scholar] [CrossRef]
- Adamaκopoulou, C.; Benedetti, B.; Zappaterra, M.; Felici, M.; Masebo, N.T.; Previti, A.; Passantino, A.; Padalino, B. Cats’ and Dogs’ Welfare: Text Mining and Topics Modeling Analysis of the Scientific Literature. Front. Vet. Sci. 2023, 10, 1268821. [Google Scholar]
- Narayan, E.; Padalino, B. Editorial: Reviews in Animal Welfare. Front. Vet. Sci. 2024, 11, 1485518. [Google Scholar]
- Benedetti, B.; Felici, M.; Nanni Costa, L.; Padalino, B. A Review of Horse Welfare Literature from 1980 to 2023 with a Text Mining and Topic Analysis Approach. Ital. J. Anim. Sci. 2023, 22, 1095–1109. [Google Scholar]
- Mellor, D.J.; Beausoleil, N.J.; Littlewood, K.E.; McLean, A.N.; McGreevy, P.D.; Jones, B.; Wilkins, C. The 2020 Five Domains Model: Including Human–Animal Interactions in Assessments of Animal Welfare. Animals 2020, 10, 1870. [Google Scholar] [CrossRef]
- Keaveney, S.M. Equines and Their Human Companions. J. Bus. Res. 2008, 61, 444–454. [Google Scholar] [CrossRef]
- Luke, K.L.; Rawluk, A.; McAdie, T.; Smith, B.P.; Warren-Smith, A.K. How Equestrians Conceptualise Horse Welfare: Does It Facilitate or Hinder Change? Animal Welfare 2023, 32, e59. [Google Scholar] [CrossRef]
- Wolframm, I.A.; Douglas, J.; Pearson, G. Changing Hearts and Minds in the Equestrian World One Behaviour at a Time. Animals 2023, 13, 748. [Google Scholar] [CrossRef]
- Cellai, S.; Gazzano, A.; Casini, L.; Gazzano, V.; Cecchi, F.; Macchioni, F.; Cozzi, A.; Pageat, L.; Arroub, S.; Fratini, S.; et al. The Memory Abilities of the Elderly Horse. Animals 2024, 14, 3073. [Google Scholar] [CrossRef]
- Phelipon, R.; Hennes, N.; Ruet, A.; Bret-Morel, A.; Górecka-Bruzda, A.; Lansade, L. Forage, Freedom of Movement, and Social Interactions Remain Essential Fundamentals for the Welfare of High-Level Sport Horses. Front. Vet. Sci. 2024, 11, 1504116. [Google Scholar] [CrossRef]
- Lesimple, C.; Gautier, E.; Benhajali, H.; Rochais, C.; Lunel, C.; Bensaïd, S.; Khalloufi, A.; Henry, S.; Hausberger, M. Stall Architecture Influences Horses’ Behaviour and the Prevalence and Type of Stereotypies. Appl. Anim. Behav. Sci. 2019, 219, 104833. [Google Scholar] [CrossRef]
- Mazzola, S.M.; Colombani, C.; Pizzamiglio, G.; Cannas, S.; Palestrini, C.; Costa, E.D.; Gazzonis, A.L.; Bionda, A.; Crepaldi, P. Do You Think i Am Living Well? A Four-Season Hair Cortisol Analysis on Leisure Horses in Different Housing and Management Conditions. Animals 2021, 11, 2141. [Google Scholar] [CrossRef]
- Christensen, J.W.; Ladewig, J.; Søndergaard, E.; Malmkvist, J. Effects of Individual versus Group Stabling on Social Behaviour in Domestic Stallions. Appl. Anim. Behav. Sci. 2002, 75, 233–248. [Google Scholar]
- Ruet, A.; Lemarchand, J.; Parias, C.; Mach, N.; Moisan, M.P.; Foury, A.; Briant, C.; Lansade, L. Housing Horses in Individual Boxes Is a Challenge with Regard to Welfare. Animals 2019, 9, 621. [Google Scholar] [CrossRef]
- Dai, F.; Dalla Costa, E.; Minero, M.; Briant, C. Does Housing System Affect Horse Welfare? The AWIN Welfare Assessment Protocol Applied to Horses Kept in an Outdoor Group-Housing System? The ‘Parcours’. Anim. Welfare 2023, 32, e22. [Google Scholar] [CrossRef]
- Bradshaw-Wiley, E.; Randle, H. The Effect of Stabling Routines on Potential Behavioural Indicators of Affective State in Horses and Their Use in Assessing Quality of Life. Animals 2023, 13, 1065. [Google Scholar] [CrossRef]
- Lesimple, C.; Reverchon-Billot, L.; Galloux, P.; Stomp, M.; Boichot, L.; Coste, C.; Henry, S.; Hausberger, M. Free Movement: A Key for Welfare Improvement in Sport Horses? Appl. Anim. Behav. Sci. 2020, 225, 104972. [Google Scholar] [CrossRef]
- König, U.; Visser, E.K.; Hall, C. Indicators of Stress in Equitation. Appl. Anim. Behav. Sci. 2017, 190, 43–56. [Google Scholar] [CrossRef]
- Alexander, T.W.; Plaizier, K.J.C. The Importance of Microbiota in Ruminant Production. Anim. Front. 2016, 6, 4–7. [Google Scholar]
- Blake, A.B.; Suchodolski, J.S. Importance of Gut Microbiota for the Health and Disease of Dogs and Cats. Anim. Front. 2016, 6, 37–42. [Google Scholar] [CrossRef]
- Grond, K.; Sandercock, B.K.; Jumpponen, A.; Zeglin, L.H. The Avian Gut Microbiota: Community, Physiology and Function in Wild Birds. J. Avian Biol. 2018, 49, e01788. [Google Scholar]
- Trevelline, B.K.; Fontaine, S.S.; Hartup, B.K.; Kohl, K.D. Conservation Biology Needs a Microbial Renaissance: A Call for the Consideration of Host-Associated Microbiota in Wildlife Management Practices. Proc. R. Soc. B Biol. Sci. 2019, 286, 20182448. [Google Scholar]
- Hou, K.; Wu, Z.X.; Chen, X.Y.; Wang, J.Q.; Zhang, D.; Xiao, C.; Zhu, D.; Koya, J.B.; Wei, L.; Li, J.; et al. Microbiota in Health and Diseases. Signal Transduct. Target. Ther. 2022, 7, 135. [Google Scholar] [PubMed]
- Venable, E.B.; Bland, S.D.; McPherson, J.L.; Francis, J. Role of the Gut Microbiota in Equine Health and Disease. Anim. Front. 2016, 6, 43–49. [Google Scholar] [CrossRef]
- Mach, N.; Ruet, A.; Clark, A.; Bars-Cortina, D.; Ramayo-Caldas, Y.; Crisci, E.; Pennarun, S.; Dhorne-Pollet, S.; Foury, A.; Moisan, M.P.; et al. Priming for Welfare: Gut Microbiota Is Associated with Equitation Conditions and Behavior in Horse Athletes. Sci. Rep. 2020, 10, 8311. [Google Scholar] [CrossRef]
- Arias-Esquivel, A.M.; Jeong, K.C.; Fan, P.; Lance, J.; DeNotta, S.; Wickens, C. Gut Microbiome Characteristics of Horses with History of Cribbing Behavior: An Observational Study. J. Vet. Behav. 2024, 72, 40–50. [Google Scholar] [CrossRef]
- Janabis, A.H.D.; Biddle, A.S.; Klein, D.; McKeever, K.H. Exercise Training-Induced Changes in the Gut Microbiota of Standardbred Racehorses. Comp. Exerc. Physiol. 2016, 12, 119–130. [Google Scholar] [CrossRef]
- Mach, N.; Midoux, C.; Leclercq, S.; Pennarun, S.; Le Moyec, L.; Rué, O.; Robert, C.; Sallé, G.; Barrey, E. Mining the Equine Gut Metagenome: Poorly-Characterized Taxa Associated with Cardiovascular Fitness in Endurance Athletes. Commun. Biol. 2022, 5, 1032. [Google Scholar] [CrossRef]
- Park, T.; Yoon, J.; Yun, Y.M.; Unno, T. Comparison of the Fecal Microbiota with High- and Low Performance Race Horses. J. Anim. Sci. Technol. 2024, 62, 425–437. [Google Scholar] [CrossRef]
- Garber, A.; Hastie, P.; Murray, J.A. Factors Influencing Equine Gut Microbiota: Current Knowledge. J. Equine Vet. Sci. 2020, 88, 102943. [Google Scholar]
- Weinert-Nelson, J.R.; Biddle, A.S.; Sampath, H.; Williams, C.A. Fecal Microbiota, Forage Nutrients, and Metabolic Responses of Horses Grazing Warm- and Cool-Season Grass Pastures. Animals 2023, 13, 790. [Google Scholar] [CrossRef] [PubMed]
- Massacci, F.R.; Clark, A.; Ruet, A.; Lansade, L.; Costa, M.; Mach, N. Inter-Breed Diversity and Temporal Dynamics of the Faecal Microbiota in Healthy Horses. J. Anim. Breed. Genet. 2020, 137, 103–120. [Google Scholar] [CrossRef] [PubMed]
- Baraille, M.; Buttet, M.; Grimm, P.; Milojevic, V.; Julliand, S.; Julliand, V. Changes of Faecal Bacterial Communities and Microbial Fibrolytic Activity in Horses Aged from 6 to 30 Years Old. PLoS ONE 2024, 19, e0303029. [Google Scholar] [CrossRef] [PubMed]
- Antwis, R.E.; Lea, J.M.D.; Unwin, B.; Shultz, S. Gut Microbiome Composition Is Associated with Spatial Structuring and Social Interactions in Semi-Feral Welsh Mountain Ponies. Microbiome 2018, 6, 207. [Google Scholar] [CrossRef]
- Ang, L.; Vinderola, G.; Endo, A.; Kantanen, J.; Jingfeng, C.; Binetti, A.; Burns, P.; Qingmiao, S.; Suying, D.; Zujiang, Y.; et al. Gut Microbiome Characteristics in Feral and Domesticated Horses from Different Geographic Locations. Commun. Biol. 2022, 5, 172. [Google Scholar] [CrossRef]
- Metcalf, J.L.; Song, S.J.; Morton, J.T.; Weiss, S.; Seguin-Orlando, A.; Joly, F.; Feh, C.; Taberlet, P.; Coissac, E.; Amir, A.; et al. Evaluating the Impact of Domestication and Captivity on the Horse Gut Microbiome. Sci. Rep. 2017, 7, 15497. [Google Scholar] [CrossRef]
- Górniak, W.; Cholewińska, P.; Szeligowska, N.; Wołoszyńska, M.; Soroko, M.; Czyż, K. Effect of Intense Exercise on the Level of Bacteroidetes and Firmicutes Phyla in the Digestive System of Thoroughbred Racehorses. Animals 2021, 11, 290. [Google Scholar] [CrossRef]
- Garrett, L.A.; Brown, R.; Poxton, I.R. A Comparative Study of the Intestinal Microbiota of Healthy Horses and Those Suffering from Equine Grass Sickness. Veter. Microbiol. 2002, 87, 81–88. [Google Scholar]
- Kauter, A.; Epping, L.; Semmler, T.; Antao, E.M.; Kannapin, D.; Stoeckle, S.D.; Gehlen, H.; Lübke-Becker, A.; Günther, S.; Wieler, L.H.; et al. The Gut Microbiome of Horses: Current Research on Equine Enteral Microbiota and Future Perspectives. Anim. Microbiome 2019, 1, 14. [Google Scholar]
- Theelen, M.J.P.; Luiken, R.E.C.; Wagenaar, J.A.; Sloet van Oldruitenborgh-Oosterbaan, M.M.; Rossen, J.W.A.; Zomer, A.L. The Equine Faecal Microbiota of Healthy Horses and Ponies in the Netherlands: Impact of Host and Environmental Factors. Animals 2021, 11, 1762. [Google Scholar] [CrossRef]
- Chaucheyras-Durand, F.; Sacy, A.; Karges, K.; Apper, E. Gastro-Intestinal Microbiota in Equines and Its Role in Health and Disease: The Black Box Opens. Microorganisms 2022, 10, 2517. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.C.; Arroyo, L.G.; Allen-Vercoe, E.; Stämpfli, H.R.; Kim, P.T.; Sturgeon, A.; Weese, J.S. Comparison of the Fecal Microbiota of Healthy Horses and Horses with Colitis by High Throughput Sequencing of the V3-V5 Region of the 16S RRNA Gene. PLoS ONE 2012, 7, e41484. [Google Scholar] [CrossRef]
- Elzinga, S.E.; Weese, J.S.; Adams, A.A. Comparison of the Fecal Microbiota in Horses With Equine Metabolic Syndrome and Metabolically Normal Controls Fed a Similar All-Forage Diet. J. Equine Vet. Sci. 2016, 44, 9–16. [Google Scholar] [CrossRef]
- Dougal, K.; De La Fuente, G.; Harris, P.A.; Girdwood, S.E.; Pinloche, E.; Geor, R.J.; Nielsen, B.D.; Schott, H.C.; Elzinga, S.; Jamie Newbold, C. Characterisation of the Faecal Bacterial Community in Adult and Elderly Horses Fed a High Fibre, High Oil or High Starch Diet Using 454 Pyrosequencing. PLoS ONE 2014, 9, e87424. [Google Scholar] [CrossRef]
- Den Besten, G.; Van Eunen, K.; Groen, A.K.; Venema, K.; Reijngoud, D.J.; Bakker, B.M. The Role of Short-Chain Fatty Acids in the Interplay between Diet, Gut Microbiota, and Host Energy Metabolism. J. Lipid Res. 2013, 54, 2325–2340. [Google Scholar]
- Fusco, W.; Lorenzo, M.B.; Cintoni, M.; Porcari, S.; Rinninella, E.; Kaitsas, F.; Lener, E.; Mele, M.C.; Gasbarrini, A.; Collado, M.C.; et al. Short-Chain Fatty-Acid-Producing Bacteria: Key Components of the Human Gut Microbiota. Nutrients 2023, 15, 2211. [Google Scholar] [CrossRef]
- Stojanov, S.; Berlec, A.; Štrukelj, B. The Influence of Probiotics on the Firmicutes/Bacteroidetes Ratio in the Treatment of Obesity and Inflammatory Bowel Disease. Microorganisms 2020, 8, 1715. [Google Scholar] [CrossRef]
- Costa, M.C.; Silva, G.; Ramos, R.V.; Staempfli, H.R.; Arroyo, L.G.; Kim, P.; Weese, J.S. Characterization and Comparison of the Bacterial Microbiota in Different Gastrointestinal Tract Compartments in Horses. Vet. J. 2015, 205, 74–80. [Google Scholar] [CrossRef]
- Fernandes, J.; Su, W.; Rahat-Rozenbloom, S.; Wolever, T.M.S.; Comelli, E.M. Adiposity, Gut Microbiota and Faecal Short Chain Fatty Acids Are Linked in Adult Humans. Nutr. Diabetes 2014, 4, e121. [Google Scholar] [CrossRef]
- Boucher, L.; Leduc, L.; Leclère, M.; Costa, M.C. Current Understanding of Equine Gut Dysbiosis and Microbiota Manipulation Techniques: Comparison with Current Knowledge in Other Species. Animals 2024, 14, 758. [Google Scholar] [CrossRef]
- Magne, F.; Gotteland, M.; Gauthier, L.; Zazueta, A.; Pesoa, S.; Navarrete, P.; Balamurugan, R. The Firmicutes/Bacteroidetes Ratio: A Relevant Marker of Gut Dysbiosis in Obese Patients? Nutrients 2020, 12, 1474. [Google Scholar] [CrossRef] [PubMed]
- Destrez, A.; Grimm, P.; Julliand, V. Dietary-Induced Modulation of the Hindgut Microbiota Is Related to Behavioral Responses during Stressful Events in Horses. Physiol. Behav. 2019, 202, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Klindworth, A.; Pruesse, E.; Schweer, T.; Peplies, J.; Quast, C.; Horn, M.; Glöckner, F.O. Evaluation of General 16S Ribosomal RNA Gene PCR Primers for Classical and Next-Generation Sequencing-Based Diversity Studies. Nucleic Acids Res. 2013, 41, e1. [Google Scholar] [CrossRef] [PubMed]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, Interactive, Scalable and Extensible Microbiome Data Science Using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-Resolution Sample Inference from Illumina Amplicon Data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Katoh, K.; Standley, D.M. MAFFT Multiple Sequence Alignment Software Version 7: Improvements in Performance and Usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef]
- Price, M.N.; Dehal, P.S.; Arkin, A.P. FastTree 2—Approximately Maximum-Likelihood Trees for Large Alignments. PLoS ONE 2010, 5, e9490. [Google Scholar] [CrossRef]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA Ribosomal RNA Gene Database Project: Improved Data Processing and Web-Based Tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef]
- Werner, J.J.; Koren, O.; Hugenholtz, P.; Desantis, T.Z.; Walters, W.A.; Caporaso, J.G.; Angenent, L.T.; Knight, R.; Ley, R.E. Impact of Training Sets on Classification of High-Throughput Bacterial 16S RRNA Gene Surveys. ISME J. 2012, 6, 94–103. [Google Scholar]
- Cringoli, G.; Maurelli, M.P.; Levecke, B.; Bosco, A.; Vercruysse, J.; Utzinger, J.; Rinaldi, L. The Mini-FLOTAC Technique for the Diagnosis of Helminth and Protozoan Infections in Humans and Animals. Nat. Protoc. 2017, 12, 1723–1732. [Google Scholar] [CrossRef]
- Ayoub, C.; Arroyo, L.G.; MacNicol, J.L.; Renaud, D.; Weese, J.S.; Gomez, D.E. Fecal Microbiota of Horses with Colitis and Its Association with Laminitis and Survival during Hospitalization. J. Vet. Intern. Med. 2022, 36, 2213–2223. [Google Scholar] [CrossRef] [PubMed]
- Thomson, P.; Garrido, D.; Santibáñez, R.; Lara, F. Preliminary Functional Analysis of the Gut Microbiome in Colic Horses. Animals 2024, 14, 3222. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, M.L.; Swecker, W.S.; Jensen, R.V.; Ponder, M.A. Characterization of the Fecal Bacteria Communities of Forage-Fed Horses by Pyrosequencing of 16S RRNA V4 Gene Amplicons. FEMS Microbiol. Lett. 2012, 326, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Salem, S.E.; Maddox, T.W.; Berg, A.; Antczak, P.; Ketley, J.M.; Williams, N.J.; Archer, D.C. Variation in Faecal Microbiota in a Group of Horses Managed at Pasture over a 12-Month Period. Sci. Rep. 2018, 8, 8510. [Google Scholar] [CrossRef]
- McKinney, C.A.; Bedenice, D.; Pacheco, A.P.; Oliveira, B.C.M.; Paradis, M.R.; Mazan, M.; Widmer, G. Assessment of Clinical and Microbiota Responses to Fecal Microbial Transplantation in Adult Horses with Diarrhea. PLoS ONE 2021, 16, e0244381. [Google Scholar] [CrossRef]
- Van den Berg, M.; Hoskin, S.O.; Rogers, C.W.; Grinberg, A. Fecal PH and Microbial Populations in Thoroughbred Horses During Transition from Pasture to Concentrate Feeding. J. Equine Vet. Sci. 2013, 33, 215–222. [Google Scholar] [CrossRef]
- Morrison, P.K.; Newbold, C.J.; Jones, E.; Worgan, H.J.; Grove-White, D.H.; Dugdale, A.H.; Barfoot, C.; Harris, P.A.; Argo, C.M.G. The Equine Gastrointestinal Microbiome: Impacts of Age and Obesity. Front. Microbiol. 2018, 9, 3017. [Google Scholar] [CrossRef]
- Lara, F.; Castro, R.; Thomson, P. Changes in the Gut Microbiome and Colic in Horses: Are They Causes or Consequences? Open Vet. J. 2022, 12, 242–249. [Google Scholar] [CrossRef]
- Mariat, D.; Firmesse, O.; Levenez, F.; Guimarǎes, V.D.; Sokol, H.; Doré, J.; Corthier, G.; Furet, J.P. The Firmicutes/Bacteroidetes Ratio of the Human Microbiota Changes with Age. BMC Microbiol. 2009, 9, 123. [Google Scholar] [CrossRef]
- Ayoub, C.; Arroyo, L.G.; Renaud, D.; Weese, J.S.; Gomez, D.E. Fecal Microbiota Comparison Between Healthy Teaching Horses and Client-Owned Horses. J. Equine Vet. Sci. 2022, 118, 104105. [Google Scholar] [CrossRef]
- Mach, N.; Lansade, L.; Bars-Cortina, D.; Dhorne-Pollet, S.; Foury, A.; Moisan, M.P.; Ruet, A. Gut Microbiota Resilience in Horse Athletes Following Holidays out to Pasture. Sci. Rep. 2021, 11, 5007. [Google Scholar] [CrossRef]
- Lesimple, C.; Poissonnet, A.; Hausberger, M. How to Keep Your Horse Safe? An Epidemiological Study about Management Practices. Appl. Anim. Behav. Sci. 2016; 181, 105–114. [Google Scholar] [CrossRef]
- Walshe, N.; Mulcahy, G.; Crispie, F.; Cabrera-Rubio, R.; Cotter, P.; Jahns, H.; Duggan, V. Outbreak of Acute Larval Cyathostominosis—A “Perfect Storm” of Inflammation and Dysbiosis. Equine Vet. J. 2021, 53, 727–739. [Google Scholar] [CrossRef] [PubMed]
- Peachey, L.E.; Jenkins, T.P.; Cantacessi, C. This Gut Ain’t Big Enough for Both of Us. Or Is It? Helminth–Microbiota Interactions in Veterinary Species. Trends Parasitol. 2017, 33, 619–632. [Google Scholar] [PubMed]
- Midha, A.; Schlosser, J.; Hartmann, S. Reciprocal Interactions between Nematodes and Their Microbial Environments. Front. Cell Infect. Microbiol. 2017, 7, 144. [Google Scholar] [CrossRef]
- Cortés, A.; Peachey, L.E.; Jenkins, T.P.; Scotti, R.; Cantacessi, C. Helminths and Microbes within the Vertebrate Gut—Not All Studies Are Created Equal. Parasitology 2019, 146, 1371–1378. [Google Scholar] [CrossRef]
- Daniels, S.P.; Leng, J.; Swann, J.R.; Proudman, C.J. Bugs and Drugs: A Systems Biology Approach to Characterising the Effect of Moxidectin on the Horse’s Faecal Microbiome. Anim. Microbiome 2020, 2, 38. [Google Scholar] [CrossRef]
- A Guide to the Treatment and Control of Equine Gastrointestinal Parasite Infections 8; ESCCAP: Worcestershire, UK, 2018; ISBN 9781907259753.
| Code | F/B Ratio | Code | F/B Ratio | ||
|---|---|---|---|---|---|
| Box | C1 | 1.03 | Paddock | C7 | 1.14 |
| C2 | 1.52 | C8 | 1.00 | ||
| C3 | 0.67 | C9 | 1.02 | ||
| C4 | 1.03 | C10 | 0.76 | ||
| C5 | 0.87 | C11 | 0.84 | ||
| C6 | 0.85 | C12 | 1.21 |
| Code | Strongyles (EPG) | Code | Strongyles (EPG) | ||
|---|---|---|---|---|---|
| Box | C1 | 10 | Paddock | C7 | 245 |
| C2 | 10 | C8 | Not detected | ||
| C3 | Not detected | C9 | Not detected | ||
| C4 | Not detected | C10 | Not detected | ||
| C5 | 5 | C11 | Not detected | ||
| C6 | 25 | C12 | 5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Curadi, M.C.; Vallone, F.; Tenuzzo, M.; Gazzano, A.; Gazzano, V.; Macchioni, F.; Vannini, C. Effect of Management System on Fecal Microbiota in Arabian Horses: Preliminary Results. Vet. Sci. 2025, 12, 309. https://doi.org/10.3390/vetsci12040309
Curadi MC, Vallone F, Tenuzzo M, Gazzano A, Gazzano V, Macchioni F, Vannini C. Effect of Management System on Fecal Microbiota in Arabian Horses: Preliminary Results. Veterinary Sciences. 2025; 12(4):309. https://doi.org/10.3390/vetsci12040309
Chicago/Turabian StyleCuradi, Maria Claudia, Flavio Vallone, Martina Tenuzzo, Angelo Gazzano, Valentina Gazzano, Fabio Macchioni, and Claudia Vannini. 2025. "Effect of Management System on Fecal Microbiota in Arabian Horses: Preliminary Results" Veterinary Sciences 12, no. 4: 309. https://doi.org/10.3390/vetsci12040309
APA StyleCuradi, M. C., Vallone, F., Tenuzzo, M., Gazzano, A., Gazzano, V., Macchioni, F., & Vannini, C. (2025). Effect of Management System on Fecal Microbiota in Arabian Horses: Preliminary Results. Veterinary Sciences, 12(4), 309. https://doi.org/10.3390/vetsci12040309






