Development of Multiple Real-Time Fluorescent Quantitative PCR for Vibrio Pathogen Detection in Aquaculture
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Bacteria, Clinical, and Environmental Samples
2.3. DNA Extraction
2.4. Standard Recombinant Plasmids Construction
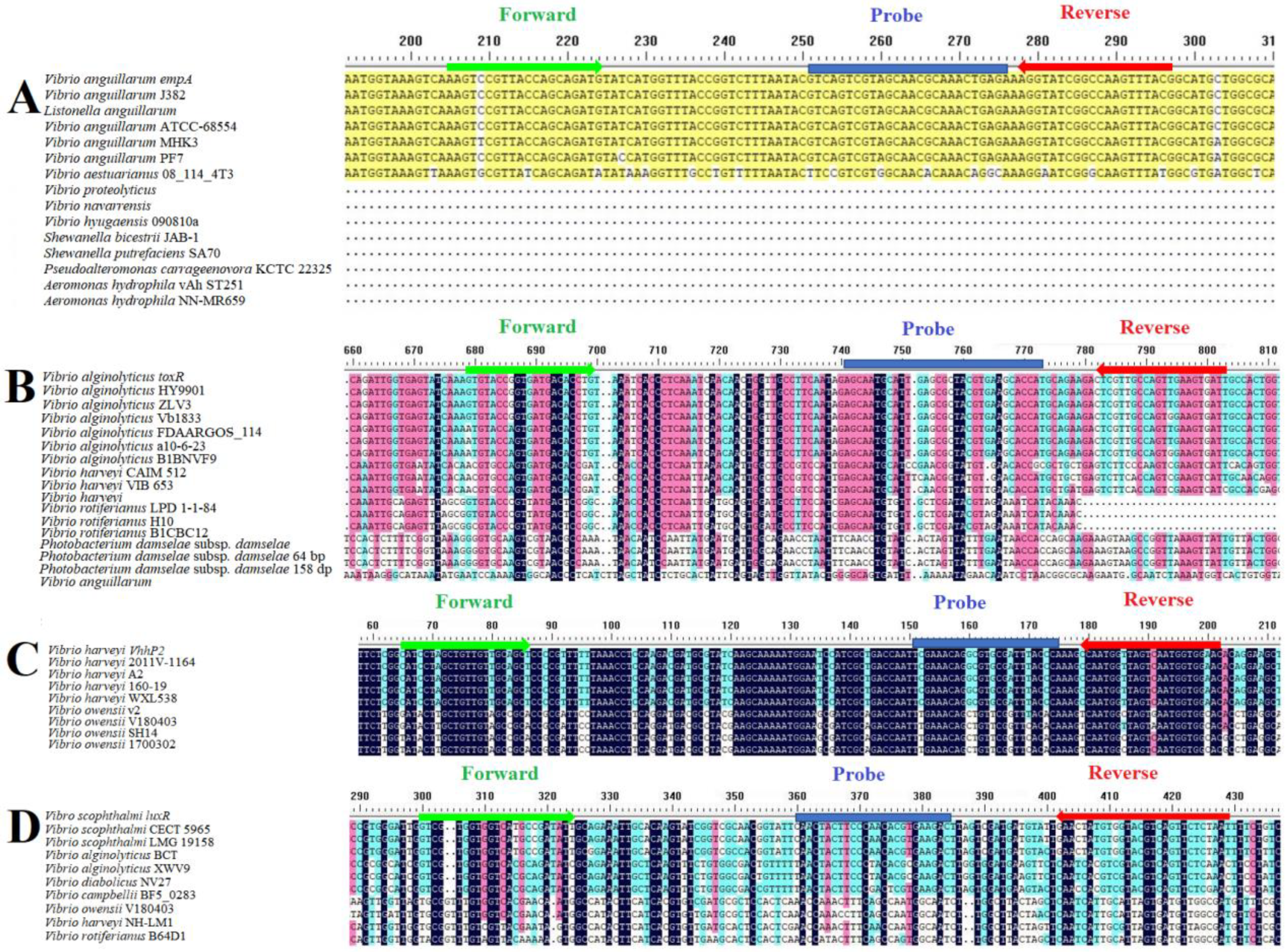
2.5. TaqMan Real-Time Fluorescent Quantitative PCR (qPCR) Primer Design
2.6. Optimization of Reaction Conditions for Multiplex qPCR
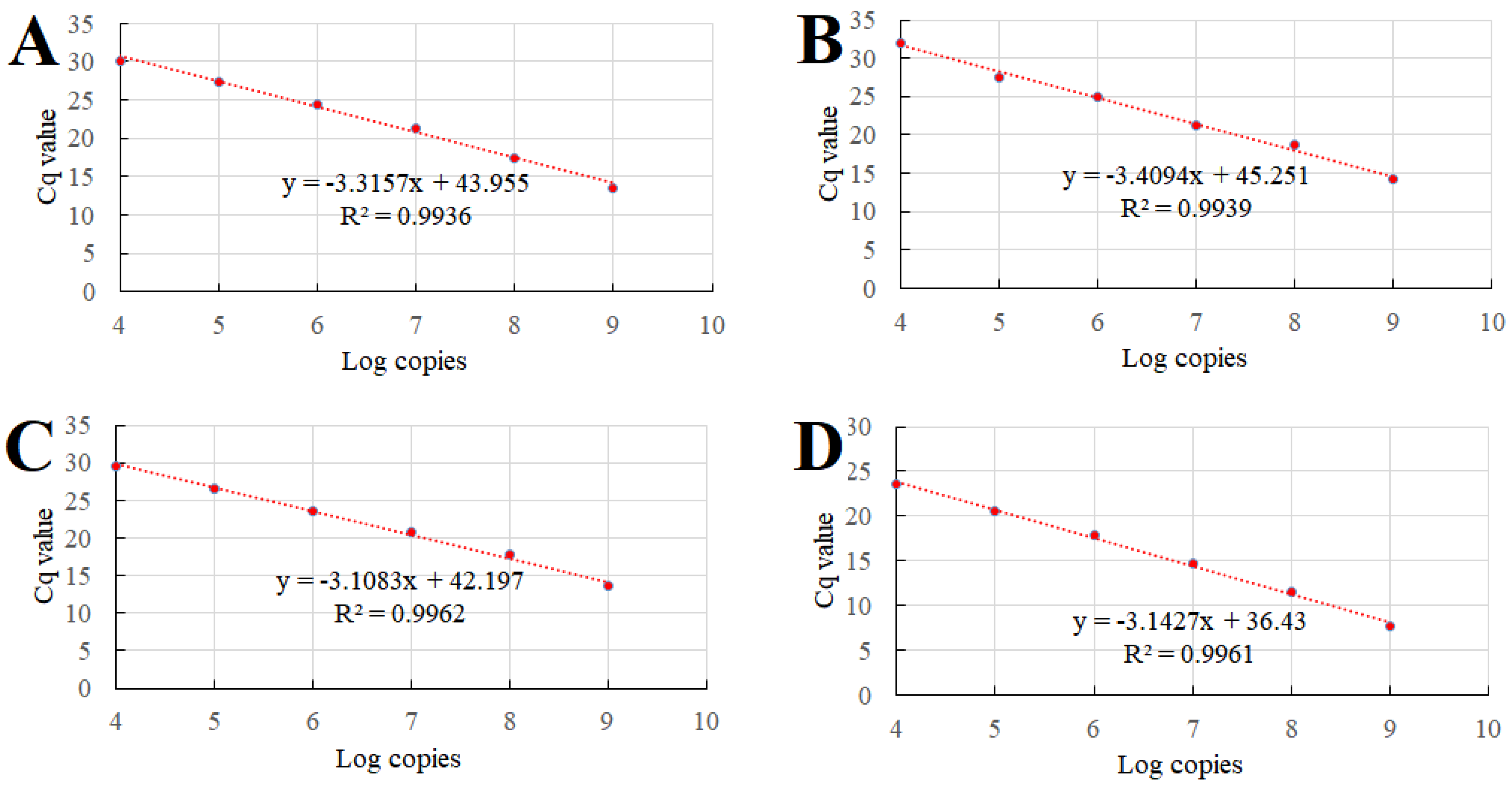
2.7. Establishment of Standard Curves of the qPCR
2.8. Specificity Test
2.9. Sensitivity Test
2.10. Repeatability Test
2.11. Clinical Sample Testing
2.12. Statistical Analysis
3. Results
3.1. Primers and Probe Designed for qPCR Assay
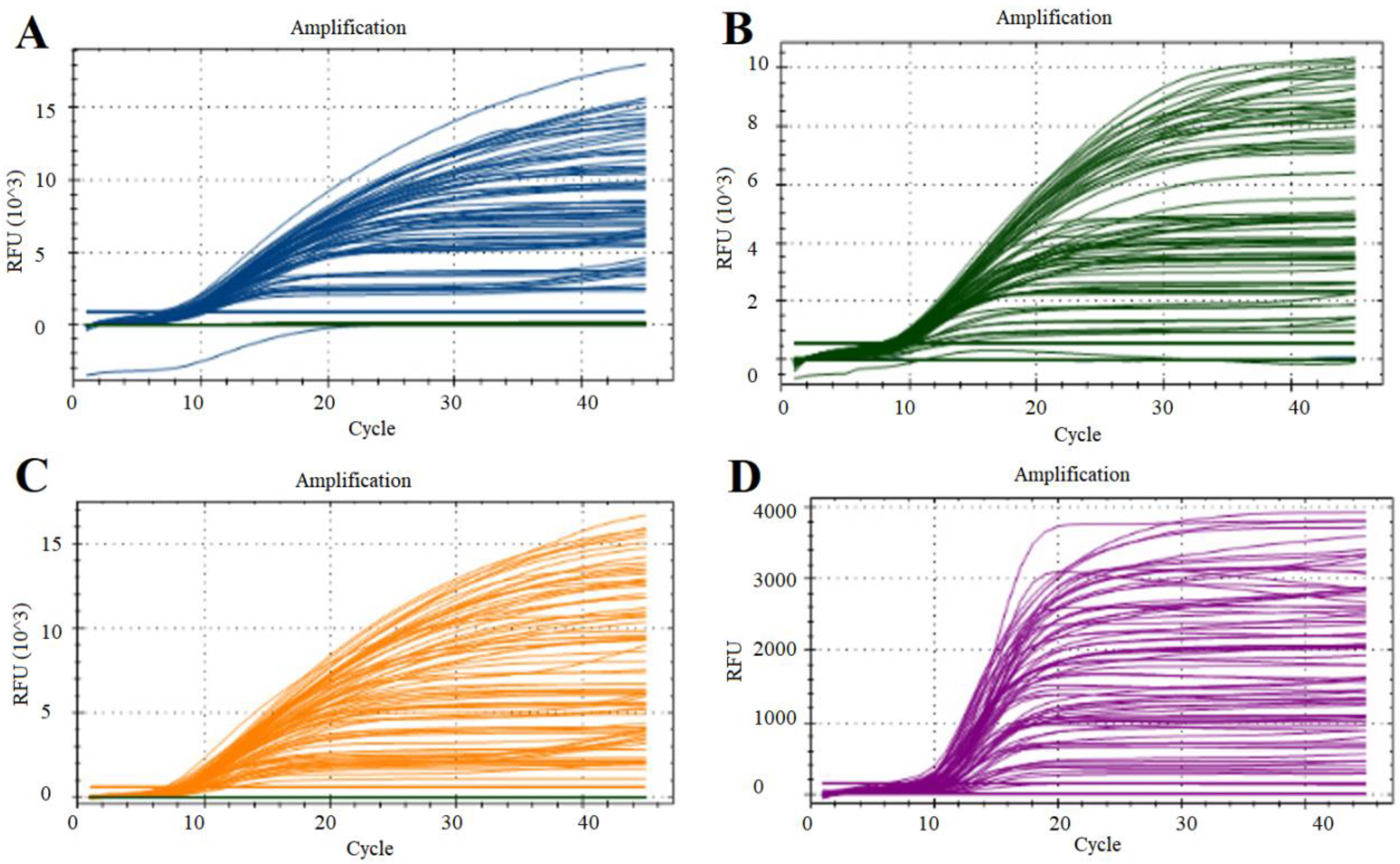
3.2. Optimization of qPCR Reaction Conditions
3.3. Standard Plasmid Construction and Standard Curve Establishment
3.4. Specificity of the Multiplex qPCR
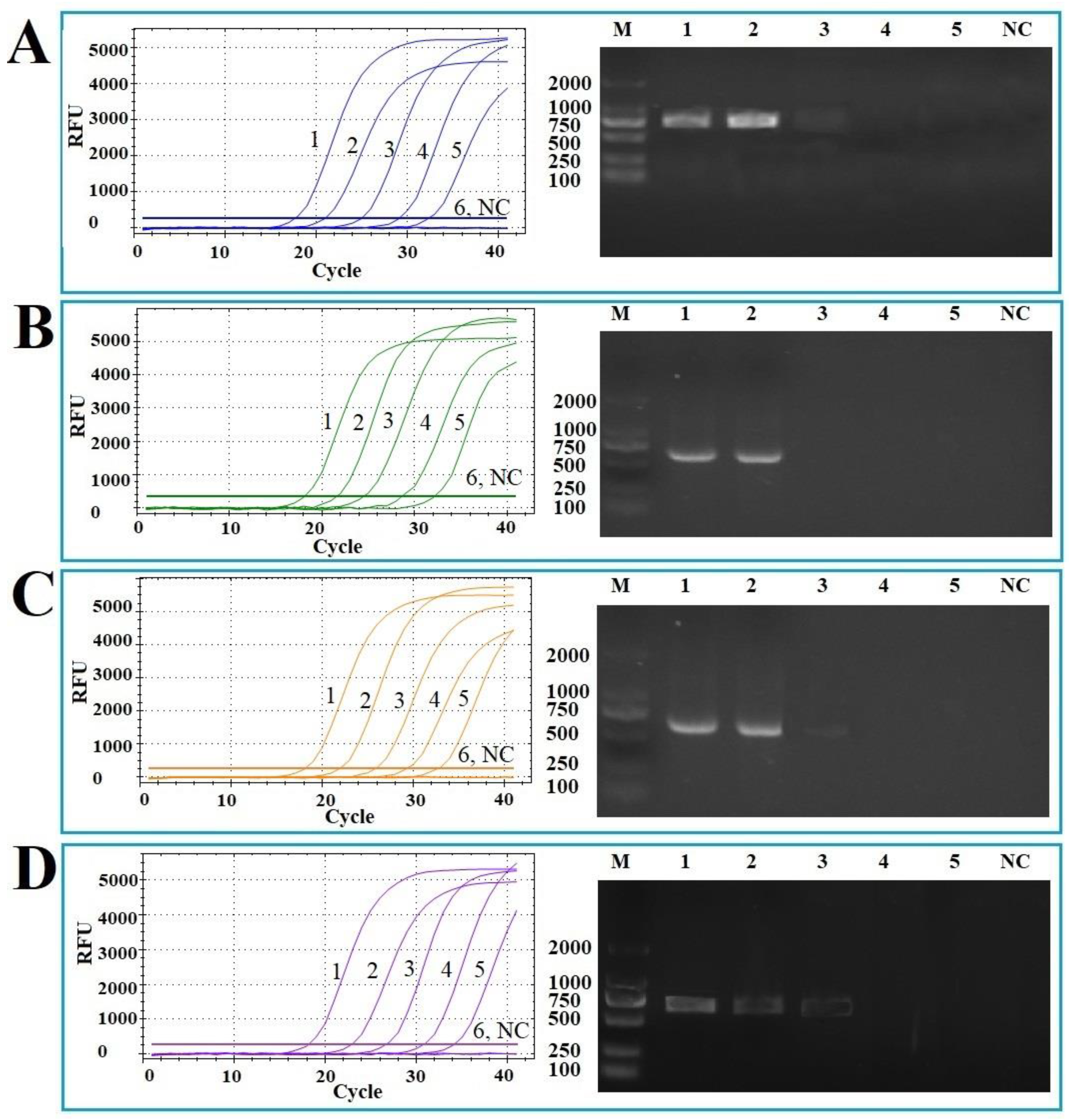
3.5. Sensitivity of the Multiplex qPCR
3.6. Repeatability of the Multiplex qPCR
3.7. Testing of Clinical and Environmental Samples Using the Multiplex qPCR
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Oktariani, A.F.; Ramona, Y.; Sudaryatma, P.E.; Dewi, I.A.M.M.; Shetty, K. Role of marine bacterial contaminants in histamine formation in seafood products: A review. Microorganisms 2022, 10, 1197. [Google Scholar] [CrossRef] [PubMed]
- Janda, J.M.; Duman, M. Expanding the spectrum of diseases and disease associations caused by Edwardsiella tarda and related species. Microorganisms 2024, 12, 1031. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Wang, Y.; Yang, W.; Cai, H.; Zhang, Y.; Huang, L. Strategies for prevention and control of Vibriosis in Asian fish culture. Vaccines 2022, 11, 98. [Google Scholar] [CrossRef]
- Lages, M.A.; Balado, M.; Lemos, M.L. The expression of virulence factors in Vibrio Anguillarum is dually regulated by iron levels and temperature. Front. Microbiol. 2019, 10, 2335. [Google Scholar] [CrossRef] [PubMed]
- Akinbowale, O.L.; Peng, H.; Grant, P.; Barton, M.D. Antibiotic and heavy metal resistance in motile aeromonads and pseudomonads from rainbow trout (Oncorhynchus mykiss) farms in Australia. Int. J. Antimicrob. Agents 2007, 30, 177–182. [Google Scholar] [CrossRef]
- Fauzi, I.A.; Haga, Y.; Kondo, H.; Hirono, I.; Satoh, S. Dietary citrulline improves survival of rainbow trout Oncorhynchus mykiss juveniles challenged with Vibrio anguillarum. Aquaculture 2020, 528, 735491. [Google Scholar] [CrossRef]
- Akter, T.; Lindegaard, M.; Pedersen, K.; Strube, M.L.; Ronco, T.; Dalsgaard, I. Sequence analysis of plasmids in Vibrio anguillarum from different fish and locations. J. Aquat. Anim. Health 2020, 32, 21–27. [Google Scholar] [CrossRef]
- Austin, B. Vibrios as causal agents of zoonoses. Vet. Microbiol. 2010, 140, 310–317. [Google Scholar] [CrossRef]
- Citil, B.E.; Derin, S.; Sankur, F.; Sahan, M.; Citil, M.U. Vibrio alginolyticus associated chronic myringitis acquired in mediterranean waters of Turkey. Infect. Disnor. 2015, 2015, 187212. [Google Scholar]
- Abbate, F.; Guerrera, M.C.; Montalbano, G.; Ciriaco, E.; Germana, A. Morphology of the tongue dorsal surface of gilthead seabream (Sparus aurata). Microsc. Res. Tech. 2012, 75, 1666–1671. [Google Scholar] [CrossRef]
- Liu, P.C.; Lin, J.Y.; Hsiao, P.T.; Lee, K.K. Isolation and characterization of pathogenic Vibrio alginolyticus from diseased cobia Rachycentron canadum. J. Basic. Microbiol. 2004, 44, 23–28. [Google Scholar] [PubMed]
- Jayaprakash, N.S.; Pai, S.S.; Philip, R.; Singh, I.S. Isolation of a pathogenic strain of Vibrio alginolyticus from necrotic larvae of Macrobrachium rosenbergii (de man). J. Fish Dis. 2006, 29, 187–191. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.K. Pathogenesis studies on Vibrio alginolyticus in the grouper, Epinephelus malabaricus. Blochet. Schneider Microb. Pathog. 1995, 19, 39. [Google Scholar]
- Selvin, J.; Lipton, A.P. Vibrio alginolyticus associated with white spot disease of Penaeus monodon. Dis. Aquat. Org. 2003, 57, 147–150. [Google Scholar]
- Lee, K.K.; Yu, S.R.; Yang, T.I.; Liu, P.C.; Chen, F.R. Isolation and characterization of Vibrio alginolyticus isolated from diseased kuruma prawn, Penaeus japonicus. Lett. Appl. Microbiol. 1996, 22, 111–114. [Google Scholar] [CrossRef] [PubMed]
- Austin, B.; Zhang, X.H. Vibrio harveyi: A significant pathogen of marine vertebrates and invertebrates. Lett. Appl. Microbiol. 2006, 43, 119–124. [Google Scholar]
- Ransangan, J.; Mustafa, S. Identification of Vibrio harveyi isolated from diseased Asian seabass Lates calcarifer by use of 16S ribosomal DNA sequencing. J. Aquat. Anim. Health 2009, 21, 150–155. [Google Scholar]
- Ruwandeepika, H.A.D.; Jayaweera, T.S.P.; Bhowmick, P.P.; Karunasagar, I.; Bossier, P.; Defoirdt, T. Pathogenesis, virulence factors and virulence regulation of vibrios belonging to the Harveyi clade. Rev. Aquacult. 2012, 4, 59–74. [Google Scholar]
- Shen, G.M.; Shi, C.Y.; Fan, C.; Jia, D.; Wang, S.Q.; Xie, G.S.; Li, G.Y.; Mo, Z.L.; Huang, J. Isolation, identification and pathogenicity of Vibrio harveyi, the causal agent of skin ulcer disease in juvenile hybrid groupers Epinephelus fuscoguttatus×Epinephelus lanceolatus. J. Fish Dis. 2017, 40, 1351–1362. [Google Scholar]
- Defoirdt, T.; Boon, N.; Sorgeloos, P.; Verstraete, W.; Bossier, P. Alternatives to antibiotics to control bacterial infections: Luminescent vibriosis in aquaculture as an example. Trends Biotechnol. 2007, 25, 472–479. [Google Scholar] [CrossRef]
- Cerdà-Cuéllar, M.; Rossellò-Mora, R.A.; Lalucat, J.; Jofre, J.; Blanch, A. Vibrio scophthalmi sp. nov., a new species from turbot (Scophthalmus maximus). Int. J. Syst. Bacteriol. 1997, 47, 58–61. [Google Scholar]
- Qiao, G.; Lee, D.C.; Woo, S.H.; Li, H.; Xu, D.H.; Park, S.I. Microbiological characteristics of Vibrio scophthalmi isolates from diseased olive flounder Paralichthys olivaceus. Fish. Sci. 2012, 78, 853–863. [Google Scholar]
- Soffientino, B.; Gwaltney, T.; Nelson, D.R.; Specker, J.L.; Mauel, M.; Gómez-Chiarri, M. Infectious necrotizing enteritis and mortality caused by Vibrio carchariae in summer flounder Paralichthys dentatus during intensive culture. Dis. Aquat. Org. 1999, 38, 201–210. [Google Scholar]
- Hidalgo, B.R.; Cleenwerck, I.; Balboa, S.; Wachter, M.D.; Thompson, F.L.; Swings, J.; De Vos, P.; Romalde, J. Diversity of Vibrios associated with reared clams in Galicia (NW Spain). Syst. Appl. Microbiol. 2008, 31, 215–222. [Google Scholar]
- Valdenegro-Vega, V.; Naeem, S.; Carson, J.; Bowman, J.P.; Tejedor, R.J.L.; Nowak, B. Culturable microbiota of ranched southern bluefin tuna (Thunnus maccoyii Castelnau). J. Appl. Microbiol. 2013, 115, 923–932. [Google Scholar]
- Rui, H.; Liu, Q.; Ma, Y.; Wang, Q.; Zhang, Y. Roles of LuxR in regulating extracellular alkaline serine protease a, extracellular polysaccharide and mobility of Vibrio alginolyticus. FEMS Microbiol. Lett. 2008, 285, 155–162. [Google Scholar]
- Zhou, Z.; Pang, H.; Ding, Y.; Cai, J.; Huang, Y.; Jian, J.; Wu, Z. VscO, a putative T3SS chaperone escort of Vibrio alginolyticus, contributes to virulence in fish and is a target for vaccine development. Fish Shellfish Immunol. 2013, 35, 1523–1531. [Google Scholar] [PubMed]
- Vandenberghe, J.; Thompson, F.L.; Gomez-Gil, B.; Swings, J. Phenotypic diversity among Vibrio isolates from marine aquaculture systems. Aquaculture 2003, 219, 9–20. [Google Scholar]
- Ina-Salwany, M.Y.; Al-Saari, N.; Aslah, M.; Mursidi, F.A.; Mohd-Aris, A.; Amal, M.N.A.; Kasai, H.; Mino, S.; Sawabe, T.; Zamri-Saad, M. Vibriosis in Fish: A review on disease development and prevention. J. Aquat. Anim. Health 2019, 31, 3–22. [Google Scholar]
- Kim, H.J.; Ryu, J.O.; Lee, S.Y.; Kim, E.S.; Kim, H.Y. Multiplex PCR for detection of the Vibrio genus and five pathogenic Vibrio species with primer sets designed using comparative genomics. BMC Microbiol. 2015, 15, 239. [Google Scholar]
- O’Hara, C.M.; Sowers, E.G.; Bopp, C.A.; Duda, S.B.; Strockbine, N.A. Accuracy of six commercially available systems for identification of members of the family Vibrionaceae. J. Clin. Microbiol. 2003, 41, 5654–5659. [Google Scholar] [PubMed]
- Pang, L.; Zhang, X.H.; Zhong, Y.; Chen, J.; Li, Y.; Austin, B. Identification of Vibrio harveyi using PCR amplification of the toxR gene. Lett. Appl. Microbiol. 2006, 43, 249–255. [Google Scholar]
- Xiao, P.; Mo, Z.L.; Mao, Y.X.; Wang, C.L.; Zou, Y.X.; Li, J. Detection of Vibrio anguillarum by PCR amplification of the empA gene. J. Fish Dis. 2009, 32, 293–296. [Google Scholar]
- Osman, E.; Kim, N.; Lee, Y.; Yoo, J.; Kim, S.H.; Kim, D.H. Molecular approaches for detection and quantification of Vibrio scophthalmi based on recA. J. Fish Dis. 2022, 45, 373–378. [Google Scholar]
- Cao, Y.T.; Wu, Z.H.; Jian, J.C.; Lu, Y.S. Evaluation of a loop-mediated isothermal amplification method for the rapid detection of Vibrio harveyi in cultured marine shellfish. Lett. Appl. Microbiol. 2010, 51, 24–29. [Google Scholar] [PubMed]
- Fukui, Y.; Sawabe, T. Rapid detection of Vibrio harveyi in seawater by real-time PCR. Microbes Environ. 2008, 23, 172–176. [Google Scholar]
- Zhao, J.J.; Chen, C.; Luo, P.; Ren, C.H.; Jiang, X.; Zhao, Z.; Hu, C.Q. SYBR Green I-based real-time PCR targeting the rpoX gene for sensitive and rapid detection of Vibrio alginolyticus. Mol. Cell Probes 2011, 25, 137–141. [Google Scholar]
- Crisafi, F.; Denaro, R.; Genovese, M.; Yakimov, M.; Genovese, L. Application of relative real-time PCR to detect differential expression of virulence genes in Vibrio anguillarum under standard and stressed growth conditions. J. Fish Dis. 2014, 37, 629–640. [Google Scholar]
- Li, D.R.; Zhao, J.Y.; Lan, W.Q.; Zhao, Y.; Sun, X.H. Effect of food matrix on rapid detection of Vibrio parahaemolyticus in aquatic products based on toxR gene. World J. Microbiol. Biotechnol. 2023, 39, 188. [Google Scholar]
- Law, J.W.F.; Ab Mutalib, N.S.; Chan, K.G.; Lee, L.H. Rapid methods for the detection of foodborne bacterial pathogens: Principles, applications, advantages and limitations. Front. Microbiol. 2015, 5, 770. [Google Scholar]
- Hu, Y.H.; Deng, T.; Sun, B.G.; Sun, L. Molecular analysis of the copper-responsive CopRSCD of a pathogenic Pseudomonas fluorescens strain. Fish Shellfish Immunol. 2012, 32, 1155–1161. [Google Scholar]
- Yu, L.P.; Hu, Y.H.; Sun, B.G.; Sun, L. C312M: An attenuated Vibrio anguillarum strain that induces immunoprotection as an oral and immersion vaccine. Dis. Aquat. Organ. 2012, 102, 33–42. [Google Scholar] [PubMed]
- Dong, Y.; Zhou, D.; Zhang, B.; Xu, X.; Zhang, J. Development of a real-time recombinase-aided amplification assay for rapid and sensitive detection of Edwardsiella piscicida. Front. Cell. Infect. Microbiol. 2024, 14, 1355056. [Google Scholar]
- Zhou, D.D.; Zhang, B.Z.; Dong, Y.C.; Li, X.P.; Zhang, J. Coinfection of cage-cultured spotted sea bass (Lateolabrax maculatus) with Vibrio harveyi and Photobacterium damselae subsp. piscicida associated with skin ulcer. Microorganisms 2024, 12, 503. [Google Scholar]
- Zhang, J.; Zhang, M.; Sun, L. Junctional adhesion molecule A of red drum (Sciaenops ocellatus): A possible immunomodulator and a target for bacterial immune evasion. Vet. Immunol. Immunopathol. 2014, 161, 99–107. [Google Scholar] [PubMed]
- Guerra, V.; Beule, L.; Lehtsaar, E.; Liao, H.L.; Karlovsky, P. Improved protocol for DNA extraction from subsoils using phosphate lysis buffer. Microorganisms 2020, 8, 532. [Google Scholar] [CrossRef] [PubMed]
- Kah Sem, N.A.D.; Abd Gani, S.; Chong, C.M.; Natrah, I.; Shamsi, S. Management and mitigation of Vibriosis in aquaculture: Nanoparticles as promising alternatives. Int. J. Mol. Sci. 2023, 24, 12542. [Google Scholar] [CrossRef]
- de Souza Valente, C.; Wan, A.H.L. Vibrio and major commercially important vibriosis diseases in decapod crustaceans. J. Invertebr. Pathol. 2021, 181, 107527. [Google Scholar]
- Frans, I.; Michiels, C.W.; Bossier, P.; Willems, K.A.; Lievens, B.; Rediers, H. Vibrio anguillarum as a fish pathogen: Virulence factors, diagnosis and prevention. J. Fish Dis. 2011, 34, 643–661. [Google Scholar]
- Wang, J.X.; Tang, W.L.; Chen, S.Q.; Zhang, J.; Ji, J.; Dong, J.Q.; Liu, G.; Gao, S. Rapid and sensitive detection of Vibrio alginolyticus pathogenic strains by real-time recombinase polymerase amplification. Acta Biochim. Biophys. Sin. 2021, 53, 950–954. [Google Scholar]
- Siddique, M.P.; Jang, W.J.; Lee, J.M.; Hasan, M.T.; Kim, C.H.; Kong, I.S. Detection of Vibrio anguillarum and Vibrio alginolyticus by singleplex and duplex loop-mediated isothermal amplification (LAMP) assays targeted to groEL and fklB genes. Int. Microbiol. 2019, 22, 501–509. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.H.; He, X.X.; Austin, B. Vibrio harveyi: A serious pathogen of fish and invertebrates in mariculture. Mar. Life Sci. Technol. 2020, 2, 231–245. [Google Scholar]
- Saha, R.; Bestervelt, L.L.; Donofrio, R.S. Development and validation of a real-time TaqMan assay for the detection and enumeration of Pseudomonas fluorescens ATCC 13525 used as a challenge organism in testing of food equipments. J. Food Sci. 2012, 77, M150–M155. [Google Scholar] [CrossRef] [PubMed]
- Denkin, S.M.; Nelson, D. Regulation of Vibrio anguillarum empA metalloprotease expression and its role in virulence. Appl. Environ. Microbiol. 2004, 70, 4193–4204. [Google Scholar] [PubMed]
- Gao, H.; Li, F.; Zhang, X.; Wang, B.; Xiang, J. Rapid, sensitive detection of Vibrio anguillarum using loop-mediated isothermal amplification. Chin. J. Oceanol. Limnol. 2010, 28, 62–66. [Google Scholar]
- Dong, Y.; Zhao, P.; Chen, L.; Wu, H.; Si, X.; Shen, X.; Shen, H.; Qiao, Y.; Zhu, S.Y.; Chen, Q.; et al. Fast, simple and highly specific molecular detection of Vibrio alginolyticus pathogenic strains using a visualized isothermal amplification method. BMC Vet. Res. 2020, 16, 76. [Google Scholar]
- Fu, K.; Li, J.; Wang, Y.; Liu, J.; Yan, H.; Shi, L.; Zhou, L. An innovative method for rapid identification and detection of Vibrio alginolyticus in different infection models. Front. Microbiol. 2016, 7, 651. [Google Scholar]
- Cai, S.; Cheng, H.; Pang, H.; Lu, Y.; Jian, J. Role of the toxR gene from fish pathogen Vibiro alginolyticus in the physiology and virulence. Indian J. Microbiol. 2017, 57, 477–484. [Google Scholar] [CrossRef]
- He, P.; Chen, Z.; Luo, J.; Wang, H.; Yan, Y.; Chen, L.; Gao, W. Multiplex real-time PCR assay for detection of pathogenic Vibrio parahaemolyticus strains. Mol. Cell Probes 2014, 28, 246–250. [Google Scholar] [CrossRef]
- Sun, K.; Hu, Y.H.; Bai, F.F.; Sun, L. Identification of vhhP2, a novel genetic marker of Vibrio harveyi, and its application in the quick detection of V. harveyi from animal specimens and environmental samples. J. Appl. Microbiol. 2009, 107, 1251–1257. [Google Scholar] [CrossRef]
- Cano-Gomez, A.; Hoj, L.; Owens, L.; Andreakis, N. Multilocus sequence analysis provides basis for fast and reliable identification of Vibrio harveyi-related species and reveals previous misidentification of important marine pathogens. Syst. Appl. Microbiol. 2011, 34, 561–565. [Google Scholar] [PubMed]
- García-Aljaro, C.; Melado-Rovira, S.; Milton, D.L.; Blanch, A.R. Quorum-sensing regulates biofilm formation in Vibrio scophthalmi. BMC Microbiol. 2012, 12, 287. [Google Scholar]
- Seok, B.; Kim, M.S.; Kim, B.S. Genome-wide analysis of quorum sensing regulon in marine fish pathogen Vibrio scophthalmi. Sci. Rep. 2024, 14, 27740. [Google Scholar] [CrossRef]
- Zhou, S.; Gao, Z.X.; Zhang, M.; Liu, D.Y.; Zhao, X.P.; Liu, Y. Development of a quadruplex loop-mediated isothermal amplification assay for field detection of four Vibrio species associated with fish disease. Springerplus 2016, 5, 1104. [Google Scholar] [CrossRef] [PubMed]
- Hickey, M.E.; Richards, G.P.; Lee, J.L. Development of a two-step, non-probed multiplex real-time PCR for surveilling Vibrio anguillarum in seawater. J. Fish Dis. 2015, 38, 551–559. [Google Scholar]
- Zou, J.; Yu, J.; Mu, Y.; Xie, X.; Wang, R.; Wu, H.; Liu, X.; Xu, F.; Wang, J.; Wang, Y. Development of a TaqMan-based multiplex real-time PCR for simultaneous detection of four feline diarrhea-associated viruses. Front. Vet. Sci. 2022, 9, 1005759. [Google Scholar]
- Bustin, S.A.; Benes, V.; Garson, J.A.; Hellemans, J.; Huggett, J.; Kubista, M.; Mueller, R.; Nolan, T.; Pfaffl, M.W.; Shipley, G.L.; et al. The MIQE guidelines: Minimum information for publication of quantitative real-time PCR experiments. Clin. Chem. 2009, 55, 611–622. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.Y.; Zhang, W.Y.; Ye, R.; Pan, Z.Z.; Li, G.R.; Su, S. One-step multiplex TaqMan probe-based method for real-time PCR detection of four canine diarrhea viruses. Mol. Cell. Probes 2020, 53, 101618. [Google Scholar]
- Fürer, F.; Fraefel, C.; Lechmann, J. Multiplex real-time PCR for the detection and differentiation of equid gammaherpesvirus 2 and 5. J. Virol. Methods 2022, 310, 114615. [Google Scholar]
- Xu, Z.X.; Li, B.S.; Jiang, Y.S.; Huang, J.; Su, L.B.; Wu, W.B.; Pang, Q.L.; Li, Z.L.; Zhang, J.Q.; Li, X.H.; et al. Development of a quadruple qRT-PCR assay for simultaneous identification of hypervirulent and carbapenem-resistant Klebsiella pneumoniae. Microbiol. Spectr. 2024, 12, e0071923. [Google Scholar] [CrossRef]

| Target | Gene | Primer/Probe | Sequence (5′-3′) |
|---|---|---|---|
| Va | empA | empA-F | TTATATTGATAGTTATGTGCACTATTAA |
| empA-R | ACAAAGAAGTCGACTAAATAAACCAT | ||
| empA-qF | AAGTCCGTTACCAGCAGATG | ||
| empA-qR | CGTAAACTTGGCCGATACCT | ||
| empA-P | [6-FAM]TCTCAGTTTGCGTTGCTACGACTGAC[BHQ1] | ||
| Val | toxR | toxR-F | GTGGAACGCTTGAGCCCATT |
| toxR-R | GCGTAGTGGGCCGACAGTAT | ||
| gyrB-qF | GTGTACCGGTGATGACACCTG | ||
| gyrB-qR | AATCACTTCAACTGGCAACGAG | ||
| gyrB-P | [HEX]CACGTAGCGCTCAATGCATTGCTC[BHQ1] | ||
| Vh | vhhP2 | vhhP2-F | ATGAAGAGAAGGAATCCTCAAGG |
| vhhP2-R | TTATTCCAATCTAGTTGGTTTTGATG | ||
| vhhR2-qF | CATCCTAGCTGTTGTTGCAGCT | ||
| vhhR2-qR | GTTCCACCATTGACTAACCATTGG | ||
| vhhR2-P | [ROX]TGGGTAAATCGCACGCCTGTTTCGA[BHQ2] | ||
| Vsc | luxR | luxR-F | ATGGACTCTATAGCAAAAAGACCC |
| luxR-R | TTACGCTTCTTCTTTGTAAATACACAG | ||
| luxR-qF | GTCGTGGTCATGCCGATATT | ||
| luxR-qR | ATTAGAGAACTGACGTACCACATAGTTC | ||
| luxR-P | [Cy5] CAACTACTTCCCAACACGTGAAGAC[BHQ2] |
| Component | Volume (μL) |
|---|---|
| empA-qF(20 μM) | 0.3 |
| empA-qR(20 μM) | 0.3 |
| empA-qP(20 μM) | 0.5 |
| toxR-qF(20 μM) | 0.2 |
| toxR-qR(20 μM) | 0.2 |
| toxR-qP(20 μM) | 0.6 |
| vhhR2-qF(20 μM) | 0.4 |
| vhhR2-qR(20 μM) | 0.4 |
| vhhR2-qP(20 μM) | 0.5 |
| luxR-qF(20 μM) | 0.2 |
| luxR-qR(20 μM) | 0.2 |
| luxR-qP(20 μM) | 0.3 |
| DNA-Va | 1 |
| DNA-Val | 1 |
| DNA-Vh | 1 |
| DNA-Vsc | 1 |
| Pro Taq HS Premix Probe real-time PCR Kit III | 10 |
| ddH2O | Up to 20 |
| Intra-Assay | Inter-Assay | ||||
|---|---|---|---|---|---|
| Targets | Templates (Copies/µL) | Cq Value (Mean ± SD) | CV/% | Cq Value (Mean ± SD) | CV/% |
| empA | 106 | 22.02 ± 0.18 | 0.82 | 23.33 ± 0.21 | 0.90 |
| 105 | 24.83 ± 0.26 | 1.05 | 26.49 ± 0.24 | 0.91 | |
| 104 | 27.05 ± 0.13 | 0.48 | 29.11 ± 0.14 | 0.48 | |
| toxR | 106 | 25.12 ± 0.35 | 1.39 | 24.99 ± 0.31 | 1.24 |
| 105 | 28.22 ± 0.62 | 1.20 | 27.73 ± 0.35 | 1.26 | |
| 104 | 31.00 ± 0.77 | 1.48 | 30.46 ± 0.27 | 0.88 | |
| vhhR2 | 106 | 23.47 ± 0.07 | 0.30 | 21.97 ± 0.09 | 0.41 |
| 105 | 26.44 ± 0.11 | 0.42 | 25.35 ± 0.05 | 0.20 | |
| 104 | 29.76 ± 0.06 | 0.20 | 28.82 ± 0.14 | 0.49 | |
| luxR | 106 | 18.04 ± 0.14 | 0.78 | 18.85 ± 0.10 | 0.53 |
| 105 | 21.41 ± 0.17 | 0.79 | 21.71 ± 0.19 | 0.86 | |
| 104 | 24.54 ± 0.21 | 0.86 | 24.59 ± 0.17 | 0.69 | |
| Different Sample | Sample Size | Test Result (Positive/Negative) | Accuracy Rate/% |
|---|---|---|---|
| Clinical Sample | |||
| NC a | 9 | 0/9 | 100 |
| Va | 6 | 6/0 | 100 |
| Val | 6 | 6/0 | 100 |
| Vh | 6 | 6/0 | 100 |
| Vsc | 6 | 6/0 | 100 |
| Va+Val | 3 | 3/0 | 100 |
| Va+Vh | 3 | 3/0 | 100 |
| Va+Vsc | 3 | 3/0 | 100 |
| Val+Vh | 3 | 3/0 | 100 |
| Val+Vsc | 3 | 3/0 | 100 |
| Vh+Vsc | 3 | 3/0 | 100 |
| Va+Val+Vh | 3 | 3/0 | 100 |
| Va+Val+Vsc | 3 | 3/0 | 100 |
| Val+Vh+Vsc | 3 | 3/0 | 100 |
| Va+Val+Vh+Vsc | 3 | 3/0 | 100 |
| Water Sample | |||
| NC a | 6 | 0/6 | 100 |
| Va | 3 | 3/0 | 100 |
| Val | 3 | 3/0 | 100 |
| Vh | 3 | 3/0 | 100 |
| Vsc | 3 | 3/0 | 100 |
| Va+Val+Vh+Vsc | 3 | 3/0 | 100 |
| Sediment Sample | |||
| NC a | 6 | 0/6 | 100 |
| Va | 3 | 3/0 | 100 |
| Val | 3 | 3/0 | 100 |
| Vh | 3 | 3/0 | 100 |
| Vsc | 3 | 3/0 | 100 |
| Va+Val+Vh+Vsc | 3 | 3/0 | 100 |
| Positive Control | |||
| pVa | 3 | 3/0 | 100 |
| pVal | 3 | 3/0 | 100 |
| pVh | 3 | 3/0 | 100 |
| pVsc | 3 | 3/0 | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, B.; Qiu, Y.; Shi, C.; Zhang, J. Development of Multiple Real-Time Fluorescent Quantitative PCR for Vibrio Pathogen Detection in Aquaculture. Vet. Sci. 2025, 12, 327. https://doi.org/10.3390/vetsci12040327
Zhang B, Qiu Y, Shi C, Zhang J. Development of Multiple Real-Time Fluorescent Quantitative PCR for Vibrio Pathogen Detection in Aquaculture. Veterinary Sciences. 2025; 12(4):327. https://doi.org/10.3390/vetsci12040327
Chicago/Turabian StyleZhang, Binzhe, Yulie Qiu, Chenxi Shi, and Jian Zhang. 2025. "Development of Multiple Real-Time Fluorescent Quantitative PCR for Vibrio Pathogen Detection in Aquaculture" Veterinary Sciences 12, no. 4: 327. https://doi.org/10.3390/vetsci12040327
APA StyleZhang, B., Qiu, Y., Shi, C., & Zhang, J. (2025). Development of Multiple Real-Time Fluorescent Quantitative PCR for Vibrio Pathogen Detection in Aquaculture. Veterinary Sciences, 12(4), 327. https://doi.org/10.3390/vetsci12040327





