Potential Therapeutic Effect of ZnO/CuO Nanocomposite as an Acaricidal, Immunostimulant, and Antioxidant in Rabbits
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of AZ
2.3. Experimental Schemes
2.3.1. Nanocomposite
2.3.2. Experimental Animals
2.4. Growth Performance Measurement
2.5. Body Weights and Spleen Somatic Index
2.6. Induction of Infection
Parasites
2.7. Experimental Protocol
2.7.1. In Vitro Assay
2.7.2. In Vivo Assay
2.8. Samples
2.8.1. Blood Sample
2.8.2. Tissue Samples
2.9. Antioxidants Profile
2.10. Serum Immunity Profile
2.11. AZ Residuals
2.12. Histopathological Examination
2.13. Statistical Analysis
3. Results and Discussion
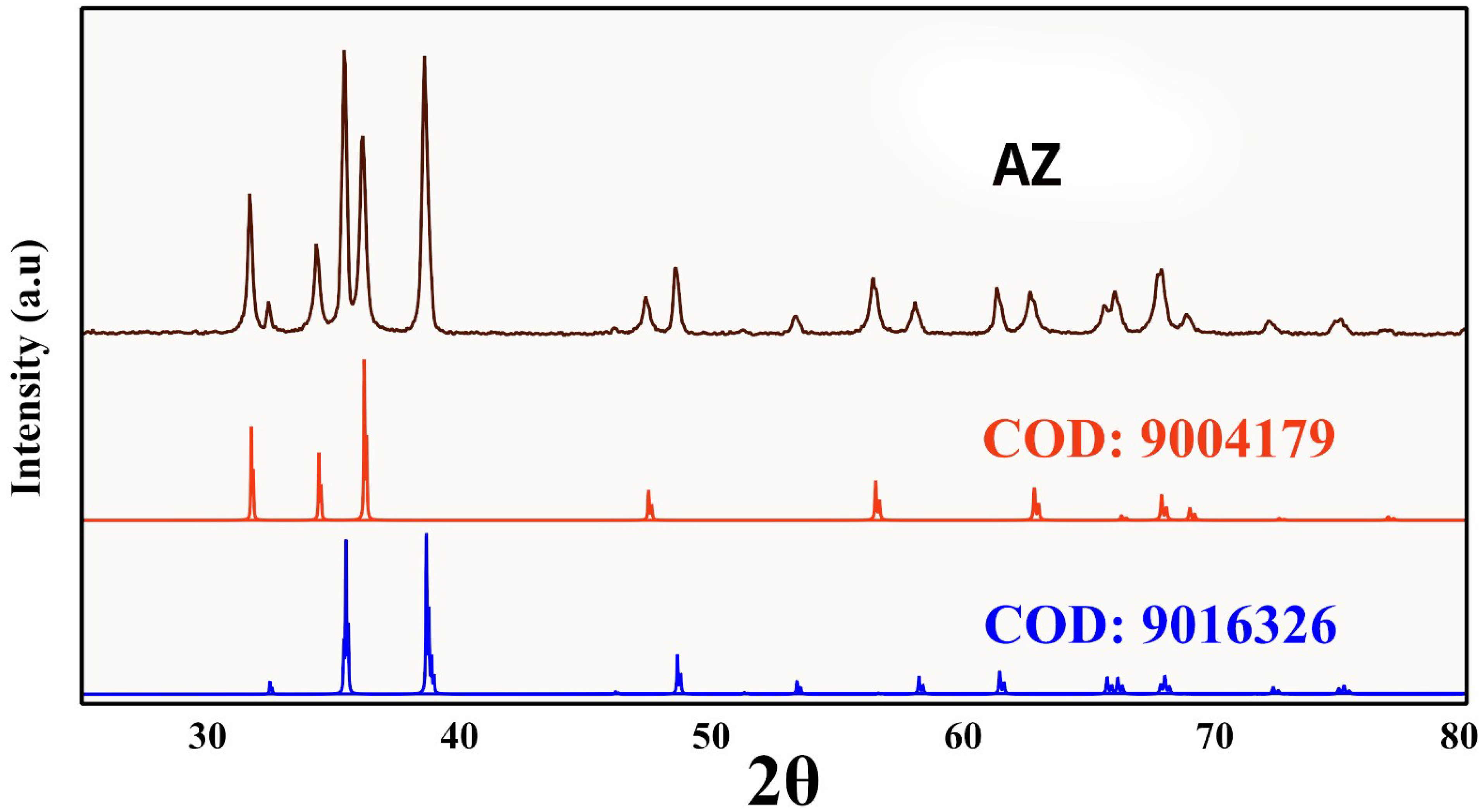
3.1. XRD Characterization
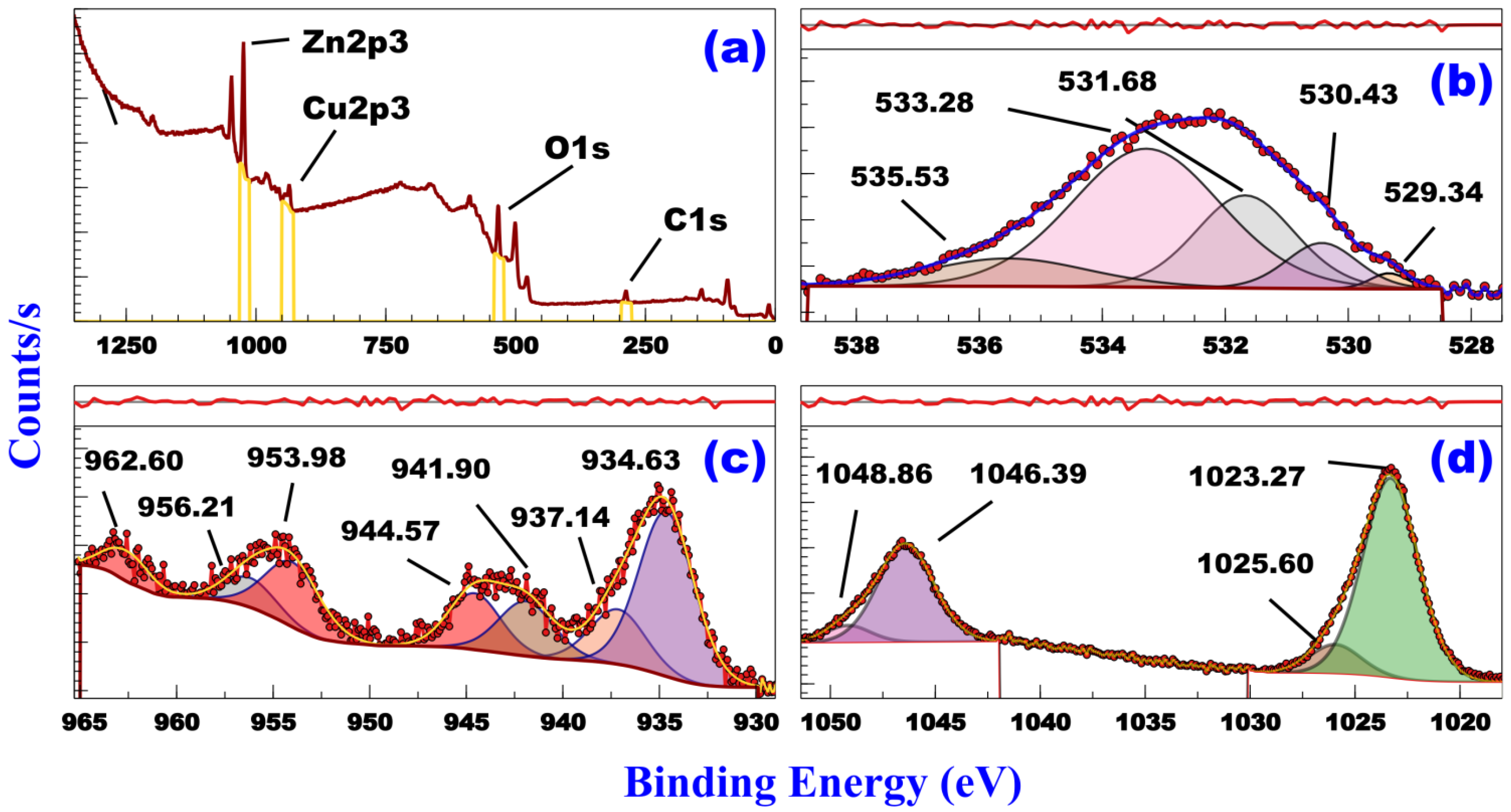
3.2. XPS
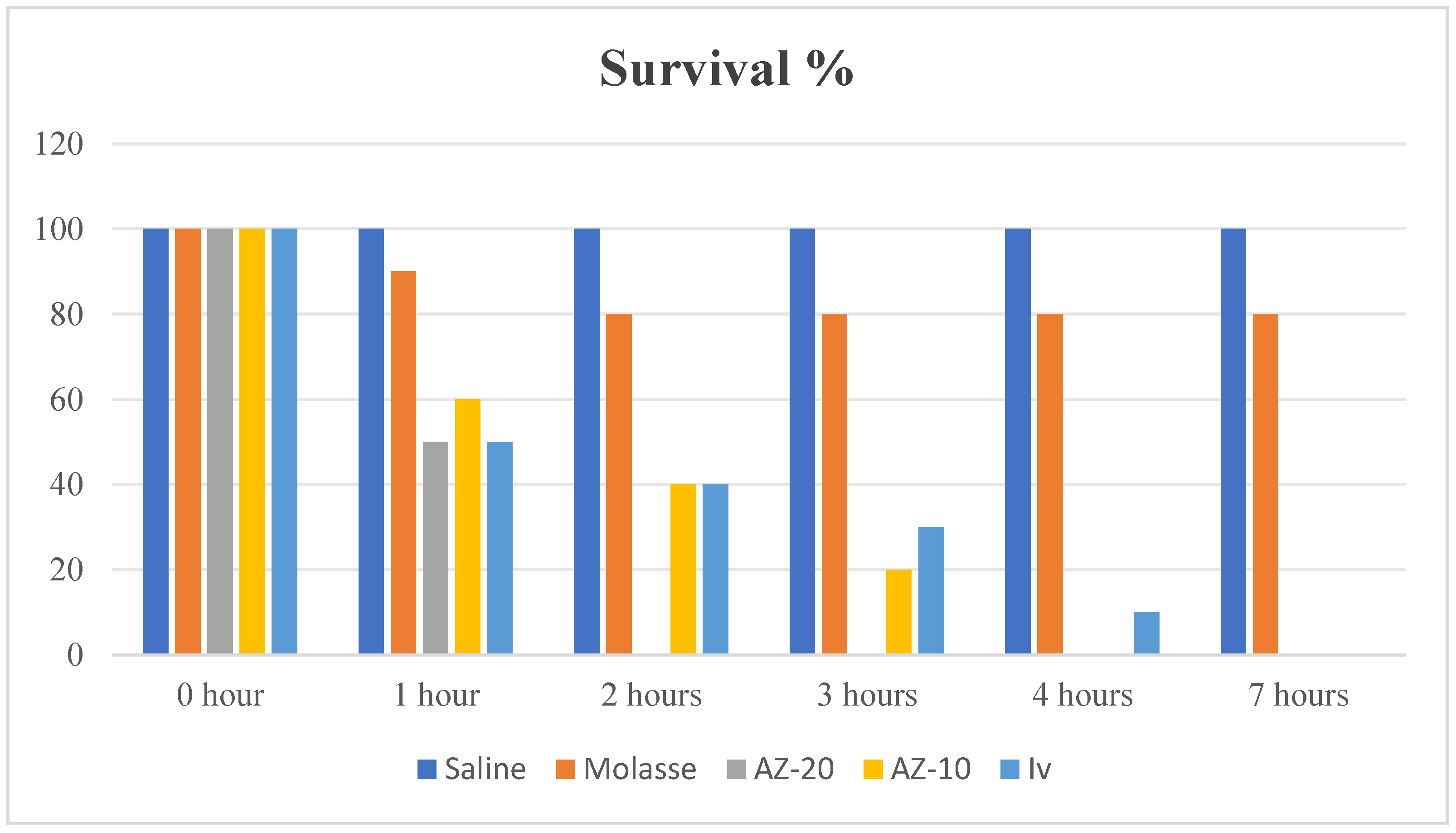
3.3. In Vitro Assessment
3.4. In Vivo Assessment
3.5. Effect of AZ on the Growth Parameters, Leucogram, Immunity Profile, and Some Biochemical Parameters
3.5.1. Effect of Nanocomposite of AZ on Growth Performance and Spleen Somatic Index of the Rabbits
3.5.2. The Effect of Nanocomposite of AZ on Blood WBCs, Neutrophils, Lymphocytes, Monocytes, and Eosinophils Counts of the Rabbits
3.5.3. Effect of Nano Composite of AZ on Immunity Profile (Interferon Gamma, IgG, IgM, Phagocytic Activity and Phagocytic Index) of the Rabbits
3.5.4. The Effect of AZ on Total Protein, Albumin, and Globulin of Rabbit
3.5.5. Effect of AZ on the Antioxidant Profile of the Rabbits
3.5.6. AZ Residues in the Liver, Back Muscle, and Brain of Rabbits
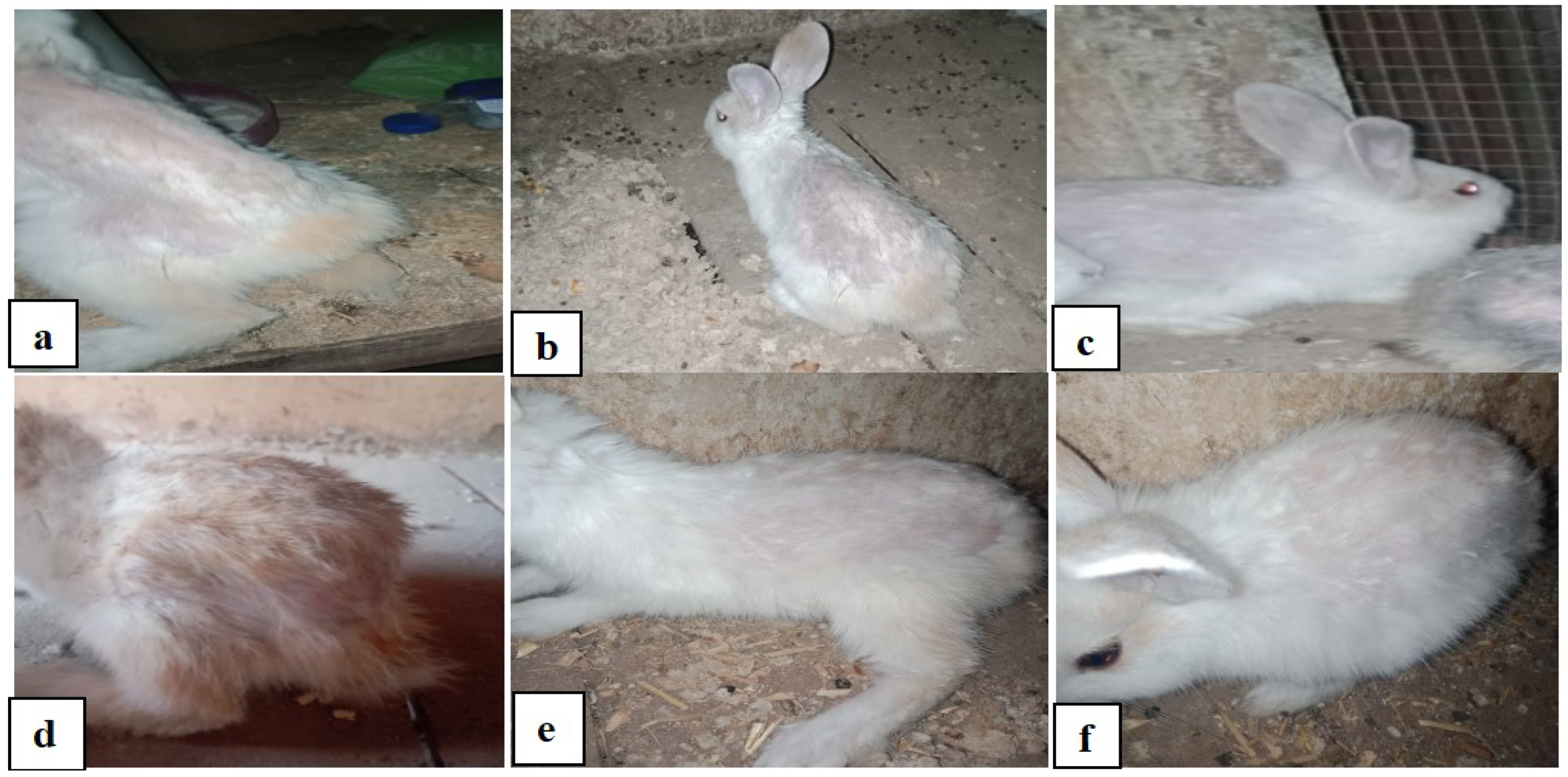
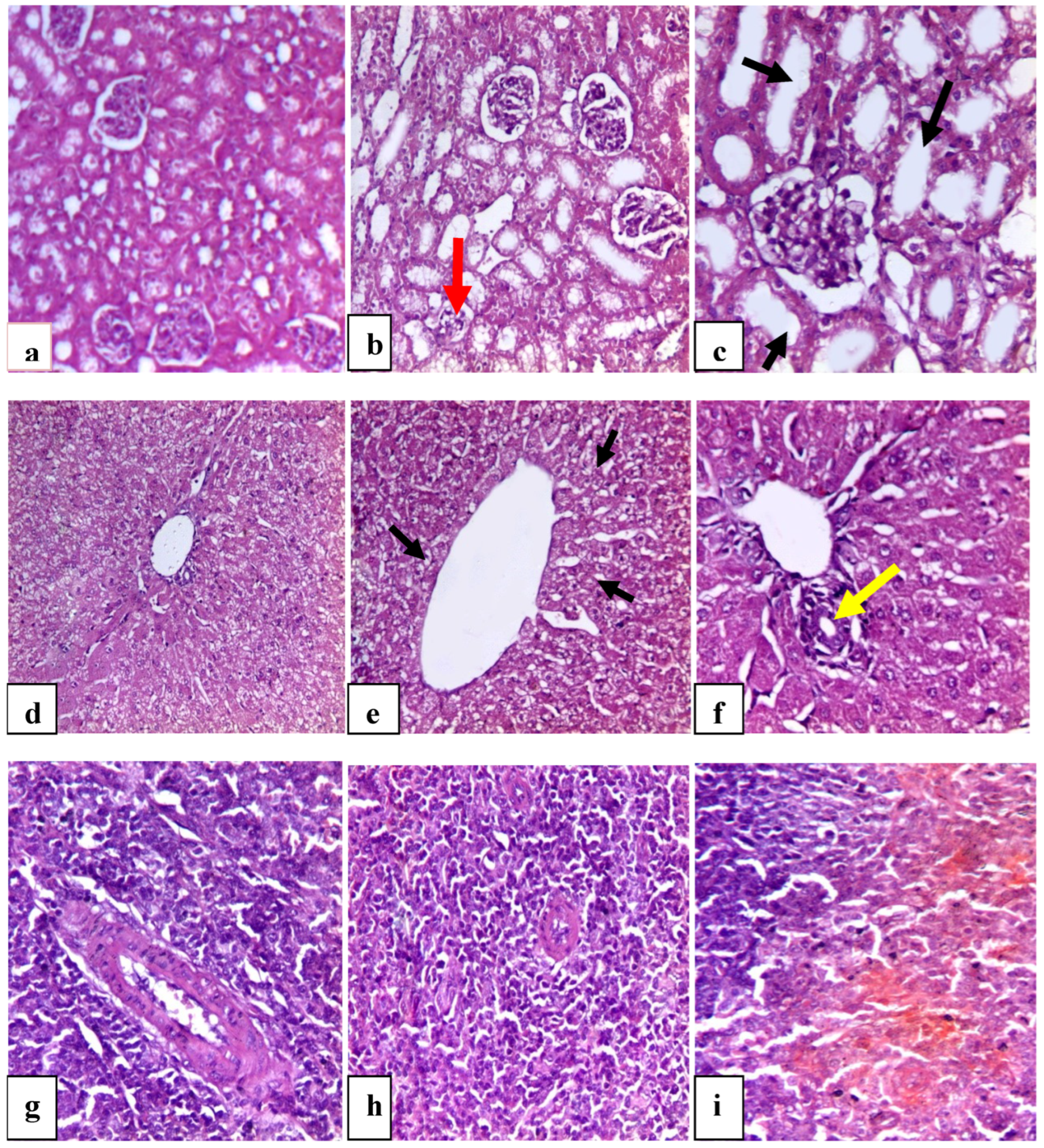
3.5.7. AZ Histopathological Pictures of Rabbits’ Kidney, Liver, and Spleen
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- El-Moghazy, M.M.; El-Fadaly, H.A.; Khalifa, E.I.; Mohamed, M.A. Effect of Dietary Zinc-Methionine on Growth, Carcass Traits, Antioxidants and Immunity of Growing Rabbits. J. Anim. Poult. Prod. 2019, 10, 59–66. [Google Scholar]
- Tsai, Y.H.; Mao, S.Y.; Li, M.Z.; Huang, J.T.; Lien, T.F. Effects of nanosize zincoxide on zinc retention, eggshell quality, immune response and serum parameters of aged laying hens. Anim. Feed Sci. Technol. 2016, 213, 99–107. [Google Scholar]
- Sloup, V.; Jankovská, I.; Nechybová, S.; Peřinková, P.; Langrová, I. Zinc in the animal organism: A review. SAB 2017, 48, 13–21. [Google Scholar]
- Abdel-Wareth, A.A.A.; Amer, S.A.; Mobashar, M.; El-Sayed, H.G.M. Use of zinc oxide nanoparticles in the growing rabbit diets to mitigate hot environmental conditions for sustainable production and improved meat quality. BMC Vet. Res. 2022, 18, 354. [Google Scholar]
- Hussein, M.A.; Ismail, Z.S.H.; Abdel-Wareth, A.A.A. Application of zinc oxide nanoparticles on productive performance in rabbit nutrition: A Review. SVU-IJAS 2020, 2, 278–290. [Google Scholar]
- Pei, X.; Jiang, H.; Xu, G.; Li, C.; Li, D.; Tang, S. Lethality of Zinc Oxide Nanoparticles Surpasses Conventional Zinc Oxide via Oxidative Stress, Mitochondrial Damage and Calcium Overload: A Comparative Hepatotoxicity Study. Int. J. Mol. Sci. 2022, 23, 6724. [Google Scholar] [CrossRef]
- Metwally, A.A.; Abdel-Hady, A.A.A.; Ebnalwaled, K.; Morad, S.A.F.; Soliman, A.A. Wound-Healing Activity of Green and Chemical Zinc Oxide Nanoparticles (ZnO-NPs) Gels in Equine Wounds: A clinical Study. SVU-IJVS 2020, 3, 66–79. [Google Scholar]
- Kaur, H.; Kaur, H.; Kaur, H.; Srivastava, S. The beneficial roles of trace and ultratrace elements in plants. Plant Growth Regul. 2023, 100, 219–236. [Google Scholar]
- Lu, J.; Liu, X.; Li, X.; Yang, X. Copper regulates the host innate immune response against bacterial infection via activation of ALPK1 kinase. Proc. Natl. Acad. Sci. USA 2023, 121, e2311630121. [Google Scholar]
- Du, Y.; Tu, Y.; Zhou, Z.; Hong, R.; Yan, J.; Zhang, G.W. Effects of organic and inorganic copper on cecal microbiota and short-chain fatty acids in growing rabbits. Front. Vet. Sci. 2023, 10, 1179374. [Google Scholar]
- Kumar, N.; Singh, A.K.; Kumar, S.; Kumar, T.; Kochewad, S.A.; Tharat, S.T.; Patole, P.B.; Gite, A. Nano-copper enhances thermal efficiency and stimulates gene expression in response to multiple stresses in Pangasianodon hypophthalmus (Striped catfish). Aquacture 2023, 564, 739059. [Google Scholar] [CrossRef]
- Donahue, N.D.; Acar, H.; Wilhelm, S. Concepts of nanoparticle cellular uptake, intracellular trafficking, and kinetics in nanomedicine. Adv. Drug Deliv. Rev. 2019, 143, 68–96. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, W.X. Cell-type-dependent dissolution of CuO nanoparticles and efflux of Cu ions following cellular internalization. Environ. Sci. Technol. 2022, 56, 12404–12415. [Google Scholar] [PubMed]
- Scott, A.; Vadalasetty, K.P.; Sawosz, E.; Łukasiewicz, M.; Vadalasetty, R.K.P.; Jaworski, S.; Chwalibog, A. Effect of copper nanoparticles and copper sulphate on metabolic rate and development of broiler embryos. Assoc. Food Sci. Technol. 2016, 220, 151–158. [Google Scholar]
- Easa, F.M.; Refaie, A.M.; Morsy, W.A.; Hekil, A.M. Effect of supplemental nano vs. conventional copper sources on growth performance of NewZealand white rabbits. Egypt. J. Remote Sens. 2018, 28, 93–113. [Google Scholar]
- Sazak, C.; Attar, A.; Yilmaz, A.; Yapaoz, M.A. Biofabrication of Acer palmatum-Mediated Multifunctional CuO Nanoparticles for Dye Removal, Antibacterial–Antifungal Activity, and Molecular Docking. ACS Omega 2023, 8, 36835–36844. [Google Scholar] [CrossRef]
- Abd Elwanees, M.; Fathalla, S.I.; Shawky, S.M.; El-Seidy, A.M.A.; Abu-alya, I.S.; Masoud, S.R. Importance of Metal Nanoparticles in Veterinary Medicine. Mat. J. Vet. Med. 2023, 3, 505. [Google Scholar]
- Adeyemi, J.O.; Onwudiwe, D.C.; Oyedeji, A.O. Biogenic synthesis of CuO, ZnO, and CuO–ZnO nanoparticles using leaf extracts of Dovyalis caffra and their biological properties. Molecules 2022, 27, 3206. [Google Scholar] [CrossRef]
- Cao, Y.; Dhahad, H.A.; El-Shorbagy, M.A.; Alijani, H.Q.; Zakeri, M.; Heydari, A.; Bahonar, E.; Slouf, M.; Khatami, M.; Naderifar, M.; et al. Green synthesis of bimetallic ZnO–CuO nanoparticles and their cytotoxicity properties. Sci. Rep. 2023, 11, 23479. [Google Scholar]
- El-Seidy, A.M.A.; Elbaset, M.A.; Ibrahim, F.A.A.; Abdelmottaleb Moussa, S.A.; Bashandy, S.A. Nanocerium oxide and cerium/zinc nanocomposites characterization and therapeutic role in combating obesity via controlling oxidative stress and insulin resistance in rat model. J. Trace Elem. Med. Biol. 2023, 80, 127312. [Google Scholar]
- Kustos, T.; Balint, L.; Than, P.; Bardos, T. Comparative study of autograft or allograft in primary anterior cruciate ligament reconstruction. Int. Orthop. 2004, 28, 290–293. [Google Scholar] [CrossRef] [PubMed]
- Zou, C.; Xing, X.; Li, S.; Zheng, X.; Zhao, J.; Liu, H. Effects of a Combined Chinese Herbal Medicine on Growth Performance, Intestinal Barrier Function, Immune Response, and Cecal Microflora in Broilers Infected with Salmonella enteritidis. Enteritidis. Anim. 2024, 14, 2670. [Google Scholar] [CrossRef] [PubMed]
- Bernigaud, C.; Fang, F.; Fischer, K.; Lespine, A.; Aho, L.S.; Dreau, D.; Kelly, A.; Sutra, J.F.; Moreau, F.; Lilin, T.; et al. Preclinical study of single-dose moxidectin, a new oral treatment for scabies: Efficacy, safety, and pharmacokinetics compared to two-dose ivermectin in a porcine model. PLoS Negl. Trop. Dis. 2016, 10, e0005030. [Google Scholar] [CrossRef]
- Bergvall, K. Advances in acquisition, identification, and treatment of equine ectoparasites. Clin. Tech. Equine Pract. 2005, 4, 296–301. [Google Scholar] [CrossRef]
- Sharaf, M.S.; Othman, A.A.; Abd El Ghafar, A.E.; Ali, D.M.; Eid, M.M. Evaluation of the scabicidal effect of a single dose of furalaner in a rabbit model of crusted scabies. Parasitol. Res. 2023, 122, 2477–2490. [Google Scholar] [CrossRef]
- Sreelakshmi, P.; Harish, K.K.; Kuruvilla, J.; Theck-el, P.G. Estimation of Phagocytic Activity by Normal Human Peripheral Blood Mononuclear Cells on Various Oral Isolates of Candida Species: An in-vitro Study. J. Med. Sci. 2024, 93, e953. [Google Scholar]
- Buzanovskii, V.A. Determination of proteins in blood. Part 1: Determination of total protein and albumin. Rev. J. Chem. 2017, 7, 79–124. [Google Scholar] [CrossRef]
- Wang, W.; Lin, M.; Song, W.; Huang, F.; Wang, B. Discussion on the Information Collection Methods of Public Health Emergency Files. Open J. Prev. Med. 2021, 11, 325–333. [Google Scholar] [CrossRef]
- Rashed, R.R.; Moustafa, E.M.; Rashed, E.R. Modulation of Sirtuin-1, Apoptosis and Redox Signaling Pathways by Astringenin: A Potential Parkinsonism Therapeutic Effect. EJRSA 2022, 35, 99–112. [Google Scholar] [CrossRef]
- Chen, L.; Chi, H.; Teng, J.; Meng, J.; Zhang, H.; Su, Y.; Liu, H.; Ye, J.; Shi, H.; Hu, Q.; et al. Neutralizing anti-IFN-γ IgG was increased in patients with systemic lupus erythematosus and associated with susceptibility to infection. Clin. Rheumatol. 2024, 43, 189–198. [Google Scholar] [CrossRef]
- Baraud, F.; Zaiter, A.; Porée, S.; Leleyter, L. New approach for determination of Cd, Cu, Cr, Ni, Pb, and Zn in sewage sludges, fired brick, and sediments using two analytical methods by microwave-induced plasma optical spectrometry and induced coupled plasma optical spectrometry. SN Appl. Sci. 2020, 2, 1536. [Google Scholar]
- El-Gendy, H.F.; Tahoun, E.A.; Elfert, A.Y.; Mady, R. Trial for decreasing ifosfamide-induced hematological toxicity, oxidative stress, inflammation, and hepatotoxicity by beetroot extract in male albino rats. Comp. Clin. Pathol. 2024, 31, 699–712. [Google Scholar]
- ElBaset, M.A.; Salem, R.S.; Ayman, F.; Ayman, N.; Shaban, N.; Afifi, S.M.; Esatbeyoglu, T.; Abdelaziz, M.; Elalfy, Z.S. Effect of Empagliflozin on Thioacetamide-Induced Liver Injury in Rats: Role of AMPK/SIRT-1/HIF-1α Pathway in Halting Liver Fibrosis. Antioxidants 2022, 11, 2152. [Google Scholar] [CrossRef] [PubMed]
- Coultas, S.J.; Counsell, J.D.P.; Gerrard, N. First row transition metals Fe, Co, Ni, Cu, and Zn analyzed by XPS using monochromatic Ag Lα x rays. Surf. Sci. Spectra 2021, 28, 024004. [Google Scholar]
- Salvadori, M.R.; Ando, R.A.; Nascimento, C.A.O.; Corrêa, B. Dead biomass of Amazon yeast: A new insight into bioremediation and recovery of silver by intracellular synthesis of nanoparticles. J. Environ. Sci. Health A Tox. 2017, 52, 1112–1120. [Google Scholar]
- Qiu, J.; Peng, Y.; Tang, M.; Lu, S.; Li, X.; Yan, J. Catalytic activity, selectivity, and stability of co-precipitation synthesized Mn-Ce mixed oxides for the oxidation of 1,2-dichlorobenzene. Environ. Sci. Pollut. Res. 2021, 28, 65416–65427. [Google Scholar]
- Ali, H.M.; Ibrahim, S.M.; Abo Zeid, E.F.; Al-Hossainy, A.F.; El-Aal, M.A. A comparative study of Cu-anchored 0 D and 1 D ZnO nanostructures for the reduction of organic pollutants in water. RSC Adv. 2022, 12, 16496–16509. [Google Scholar] [CrossRef]
- Chen, L.; Arellano, U.; Wang, J.; Balcázar, L.; Sotelo, R.; Solis, S.; Azomosa, M.; González, J.; González Vargas, O.; Song, Y.; et al. Oxygen defect, electron transfer and photocatalytic activity of Ag/CeO2/SBA-15 hybrid catalysts. Catal. Today 2022, 394–396, 62–80. [Google Scholar]
- El-Okaily, M.S.; El-Seidy, A.M.A.; Ismail, E.H.; Allam, R.M.; Saeed, A.A.; Bhaumik, A.; Mostaf, A.A. Design, fabrication and structural analysis of catalytic tubular nanomotors based on Cu/Fe@SBA-15 for lung cancer treatment. J. Mark. Res. 2024, 39, 1741–1757. [Google Scholar]
- Chandrappa, K.; Venkatesha, T. Electrochemical bulk synthesis and characterisation of hexagonal-shaped CuO nanoparticles. J. Exp. Nanosci. 2013, 8, 516–532. [Google Scholar]
- Miura, R.; Kitada, A.; Fukami, K.; Murase, K. Thermodynamic Design of Electrolyte for CuO/Cu2O Bilayer by Anodic Electrodeposition. J. Electrochem. Soc. 2021, 168, 062506. [Google Scholar]
- El-Seidy, A.M.A.; Sallam, O.I.; Nabil, I.M.; Rammah, Y.S.; MEl-Okaily, S.; Alshater, H. Preparation, physical, Optical, ESR and γ-rayattenuation efficacy investigation of copper oxide/silver borosilicate glass. Sci. Rep. 2024, 14, 25354. [Google Scholar] [CrossRef] [PubMed]
- Chuaicham, C.; Sekar, K.; Balakumar, V.; Zhang, L.; Trakulmututa, J.; Smith, S.M.; Sasaki, K. Fabrication of Hydrotalcite-like Copper Hydroxyl Salts as a Photocatalyst and Adsorbent for Hexavalent Chromium Removal. Minerals 2022, 12, 182. [Google Scholar] [CrossRef]
- Woo, J.-M.; Seo, J.Y.; Kim, H.; Lee, D.-H.; Park, Y.C.; Yi, C.-K.; Park, Y.S.; Moon, J.-H. CuY zeolite catalysts prepared by ultrasonication-assisted ion-exchange for oxidative carbonylation of methanol to dimethyl carbonate. Ultrason. Sonochem. 2018, 44, 146–151. [Google Scholar] [CrossRef]
- Simonart, T.; Lam Hoai, X.L. Escalating Threat of Drug-Resistant Human Scabies: Current Insights and Future Directions. J. Clin. Med. 2024, 13, 5511. [Google Scholar] [CrossRef]
- Mendes, C.R.; Dilarri, G.; Forsan, C.F.; Sapata, V.M.R.; Lopes, P.R.M.; de Moraes, P.B.; Montagnolli, R.N.; Ferreira, H.; Bidoia, E.D. Antibacterial action and target mechanisms of zinc oxide nanoparticles against bacterial pathogens. Sci. Rep. 2022, 12, 2658. [Google Scholar]
- Joe, A.; Park, S.; Shim, K.; Kim, D.; Jhee, K.; Lee, H.; Heo, C.; Kim, H.; Jang, E. Antibacterial mechanism of ZnO nanoparticles under dark conditions. J. Ind. Eng. Chem. 2017, 45, 430–439. [Google Scholar]
- Zudyte, B.; Luksiene, Z. Visible light-activated ZnO nanoparticles for microbial control of wheat crop. J Photochem. Photobiol. 2021, 219, 112206. [Google Scholar]
- Sabourian, P.; Yazdani, G.; Ashraf, S.S.; Frounchi, M.; Mashayekhan, S.; Kiani, S.; Kakkar, A. Effect of Physico-Chemical Properties of Nanoparticles on Their Intracellular Uptake. Int. J. Mol. Sci. 2020, 21, 8019. [Google Scholar] [CrossRef]
- Vo, T.T.T.; Peng, T.Y.; Nguyen, T.H.; Bui, T.N.H.; Wang, C.S.; Lee, W.J.; Chen, Y.L.; Wu, Y.C.; Lee, I.T. The crosstalk between copper-induced oxidative stress and cuproptosis: A novel potential anticancer paradigm. Cell Commun. Signal 2024, 22, 353. [Google Scholar]
- Mavil-Guerrero, E.; Vazquez-Duhalt, R.; Juarez-Moreno, K. Exploring the cytotoxicity mechanisms of copper ions and copper oxide nanoparticles in cells from the excretory system. Chemosphere 2023, 347, 140713. [Google Scholar] [CrossRef] [PubMed]
- Moustafa, K.-E.M.E.; El-Hosseiny, H.M.; Shaheen, G.F.; El-Kotamy, E.M.; Ghoniem, A.E.; Younan, G.E.; El-Nahrawy, M.M.; Farag, M.E.; Mohamed, M.S. Impact of different forms of selenium supplementation on growth and physiological performance of New Zealand white rabbits. Trop. Anim. Health Prod. 2024, 56, 131. [Google Scholar] [CrossRef] [PubMed]
- Effah-Yeboah, E.; Asare, E.A.; Abraham, J.D.; Reynolds, P.K.A.; Dwomoh, J.; Adongo, E.; Appiah, S.; Balali, G.I. Effects of Copper and Zinc Supplementation on Haematological, Renal and Liver Function in Healthy Wistar Rats. As. J. Im. 2021, 5, 27–36. [Google Scholar]
- Mahmoud, A.S.; Sayed, A.E.D.H.; Mahmoud, U.T.; Mohammed, A.A.A.; Darwish, M.H.A. Impact of zinc oxide nanoparticles on the behavior and stress indicators of African catfish (Clarias gariepinus) exposed to heat stress. BMC Vet. Res. 2024, 20, 474. [Google Scholar] [CrossRef] [PubMed]
- Refaie, A.; Ghazal, M.; Barakat, S.; Morsy, W.; Meshreky, S.; Younan, G.; Eisa, W. Nano copper as a new growth promoter in the diet of growing New Zealand white rabbits. Egypt. J. Remote Sens. 2015, 25, 39–57. [Google Scholar] [CrossRef]
- Ganatra, H.A.; Varisco, B.M.; Harmon, K.; Lahni, P.; Opoka, A.; Wong, H.R. Zinc supplementation leads to immune modulation and improved survival in a juvenile model of murine sepsis. Innate Immun. 2017, 23, 67–76. [Google Scholar] [CrossRef]
- Cui, D.; Tang, Y.; Jiang, Q.; Jiang, D.; Zhang, Y.; Lv, Y.; Xu, D.; Wu, J.; Xie, J.; Wen, C.; et al. Follicular helper T cells in the immunopathogenesis of SARS-CoV-2 infection. Front. Immunol. 2021, 12, 731100. [Google Scholar] [CrossRef]
- Chand, N.S.; Naz Khan, A.; Khan, S.; Khan, R.U. Performance traits and immune response of broiler chicks treated with zinc and ascorbic acid supplementation during cyclic heat stress. Int. J. Biometeorol. 2014, 58, 2153–2157. [Google Scholar] [CrossRef]
- Bartlett, J.R.; Smith, M.O. Effects of different levels of zinc on the performance and immunecompetence of broilers under heat stress. Poult. Sci. 2023, 82, 1580–1588. [Google Scholar] [CrossRef]
- Sunder, G.S.; Panda, A.K.; Gopinath, N.C.S.; Rao, S.V.R.; Raju, M.V.L.N.; Reddy, M.R.; Kumar, C.V. Effects of higher levels of zinc supplementation on performance, mineral availability, and immune competence in broiler chickens. J. Appl. Poult. Res. 2008, 17, 79–86. [Google Scholar] [CrossRef]
- Spears, J.W. Zinc Methionine for Ruminants: Relative Bioavailability of Zinc in Lambs and Effects of Growth and Performance of Growing Heifers. J. Anim. Sci. 1989, 67, 835–843. [Google Scholar] [PubMed]
- Badawi, M.; Ali, M.; Behairy, A. Effects of zinc sources supplementation on performance of broiler chickens. J. Am. Sci. 2017, 13, 35–43. [Google Scholar]
- Wang, C.; Xie, P.; Liu, L.L.; Lu, J.J.; Zou, X.T. Effects of dietary capsulated zinc oxide on growth performance, blood metabolism and mineral concentrations in weaning piglets. Asian J. Anim. Vet. Adv. 2013, 8, 502–510. [Google Scholar]
- Cho, J.H.; Upadhaya, S.D.; Kim, I.H. Effects of dietary supplementation of modified zinc oxide on growth performance, nutrient digestibility, blood profiles, fecal microbial shedding and fecal score in weanling pigs. Anim. Sci. J. 2015, 86, 617–623. [Google Scholar]
- Oconitrillo, M.; Wickramasinghe, J.; Omale, S.; Beitz, D.; Appuhamy, R. Effects of Elevating Zinc Supplementation on the Health and Production Parameters of High-Producing Dairy Cows. Animals 2024, 14, 395. [Google Scholar] [CrossRef]
- Kumar, N.; Thorat, S.T.; Gite, A.; Patole, P.B. Nano-copper Enhances Gene Regulation of Non-specific Immunity and Antioxidative Status of Fish Reared Under Multiple Stresses. Biol. Trace Elem. Res. 2023, 201, 4926–4950. [Google Scholar]
- Mohammed, A.B.; Hamad, O.K.; Khttab, T.A. Effect of Zinc Oxide Nanoparticles in Drinking Water on Growth Rate, Biochemical Parameters, and Intestinal Histology of Broilers. Adv. Agric. 2023, 2023, 8523516. [Google Scholar]
- Anil, T.S.V.; Seshaiah, C.V.; Ashalatha, P.; Sudhakar, K. Effect of Dietary Nano Zinc Oxide Supplementation on Haematological Parameters, Serum Biochemical Parameters and Hepato-Renal Bio-Markers in Crossbred Calves. Int. J. Curr. Microbiol. Appl. Sci. 2020, 9, 2034–2044. [Google Scholar]
- AL-Ruwad, S.H.; Attia, A.I.; Abdel Monem, U.M.; Abdel-Maksoud, A.; Thagfan, F.A.; Alqahtani, H.A.; Alkahtani, A.M.; Salah, A.S.; Reda, F.M. Dietary supplementation with copper nanoparticles enhances broiler performance by improving growth, immunity, digestive enzymes, and gut microbiota. Poult. Sci. 2023, 103, 104026. [Google Scholar]
- Wang, J.; Ping, S.; Chen, X.; Zhong, Y.; Lin, M.; He, M.; Liu, Y.; Zhou, Y.; Pang, X.; Han, L.; et al. A one-two punch targeting reactive oxygen species and fibril for rescuing Alzheimer’s disease. Nat. Commun. 2024, 15, 705. [Google Scholar]
- Afifi, M.; Almaghrabi, O.A.; Kadasa, N.M. Ameliorative Effect of Zinc Oxide Nanoparticles on Antioxidants and Sperm Characteristics in Streptozotocin-Induced Diabetic Rat Testes. Biomed. Res. Int. 2015, 2015, 153573. [Google Scholar] [CrossRef] [PubMed]
- Stremmel, W.; Weiskirchen, R. Wilson disease: More complex than just simply a copper overload condition?—A narrative review. AME Med. J. 2022, 7, 26. [Google Scholar] [CrossRef]
- Linder, M.C. Copper homeostasis in mammals, with emphasis on secretion and excretion. A review. Int. J. Mol. Sci. 2020, 21, 4932. [Google Scholar] [CrossRef]
- Mavromati, J.; Shaqiri, L. Environmental contamination by Zinc and the risk of its introduction into the food chain. Vet. Stanica 2023, 55, 409–419. [Google Scholar] [CrossRef]
- Beckett, J.M.; Ball, M.J. Effect of hereditary haemochromatosis genotypes and iron overload on other trace elements. Eur. J. Nutr. 2013, 52, 255–261. [Google Scholar] [CrossRef]
- Abd Elmonem, H.A.; Mahmoud, A.H.; Abbas, M.M. Ameliorative Effect of Zinc Oxide Nanoparticles and vitamin E on some Biochemical and Histological changes in Irradiated Albino Rats. Egypt. J. Rad. Sci. Appl. 2021, 34, 1–10. [Google Scholar] [CrossRef]
- Mohammed, R.S.; Aadim, K.A.; Ahmed, K.A. Histological, haematological, and thyroid hormones toxicity of female rats orally exposed to CuO/ZnO core/shell nanoparticles synthesized by Ar plasma jets. Arch. Toxicol. 2023, 97, 1017–1031. [Google Scholar] [CrossRef]
- Kielbik, P.; Kaszewski, J.; Dominiak, B.; Damentko, M.; Serafińska, I.; Rosowska, J.; Gralak, M.A.; Krajewski, M.; Witkowski, B.S.; Gajewski, Z.; et al. Preliminary studies on biodegradable zinc oxide nanoparticles doped with Fe as a potential form of iron delivery to the living organism. Nanoscale Res. Lett. 2019, 14, 373. [Google Scholar] [CrossRef]

| Chemical Composition | Ration |
|---|---|
| Dry matter | 90.7% |
| Crude protein | 18% |
| Digestible energy | 2600 Kcal/kg |
| Crude fiber | 16% |
| Calcium | 1.2% |
| Phosphorus | 0.8% |
| Lysine | 0.75% |
| Methionine and cysteine | 1.2% |
| Zinc | 50 mg/kg |
| Copper | 4 mg/kg |
| Zincite (ZnO) | Tenorite (CuO) | |
|---|---|---|
| COD: 9004179 | COD: 9016326 | |
| a * | 3.26 (3.25) | 4.70 (4.69) |
| b * | 3.43 (3.43) | |
| c * | 5.22 (5.21) | 5.15(5.14) |
| 2 θ * | 36.15° | 35.45° |
| FWHM | 0.40 | 0.35 |
| Crystallite size (nm) | 21.87 | 24.89 |
| Microstrain (ϵ × 10−3) | 5.33 | 4.78 |
| Specific surface area (S m2g−1) | 48.44 | 37.19 |
| Lorentz factor (Lf) | 2.73 | 2.83 |
| Lorentz polarization factor | 18.05 | 18.85 |
| Groups | Dead Rabbit/Total Rabbits | Mortality (%) |
|---|---|---|
| G1 | 0/10 | 0% |
| G2 | 2/10 | 20% |
| G3 | 0/10 | 0% |
| G4 | 1/10 | 10% |
| Weeks | Parameters | Treatments | |||
|---|---|---|---|---|---|
| G1 | G2 | G3 | G4 | ||
| Initial BW (gm) | 800 ± 11.0 | 805 ± 11.04 | 793 ± 9.14 | 810 ± 13.15 | |
| W1 | BW (gm) | 1100 ± 2.22 b | 1009 ± 23.71 b | 1291 ± 24.68 a | 962 ± 19.57 b |
| WG (gm) | 300 ± 15.65 ab | 204 ± 18.11 b | 497 ± 30.11 a | 151 ± 20.99 b | |
| FI (gm) | 440 ± 0.002 b | 474 ± 0.001 a | 425 ± 0.76 c | 438 ± 0.66 b | |
| FC | 1.46 ± 0.54 b | 2.42 ± 0.26 a | 0.86 ± 0.04 c | 3.14 ± 0.37 a | |
| W2 | BW | 1250 ± 10.54 b | 1091 ± 30.21 c | 1468 ± 9.60 a | 1276 ± 29.42 b |
| WG | 150 ± 10.55 b | 82.16 ± 9.79 c | 176 ± 29.12 b | 314 ± 19.95 a | |
| FI | 520 ± 0.76 a | 506 ± 0.76 b | 506 ± 0.76 b | 532 ± 0.76 a | |
| FC | 3.46 ± 0.67 b | 6.70 ± 0.97 a | 3.49 ± 0.81 b | 1.73 ± 0.11 b | |
| W3 | BW | 1390 ± 6.25 b | 1216 ± 5.62 c | 1693 ± 6.15 a | 1414 ± 6.02 b |
| WG | 140 ± 8.53 b | 124 ± 10.24 b | 224 ± 10.83 a | 137 ± 25.76 b | |
| FI | 530 ± 0.76 c | 670 ± 0.76 b | 582 ± 0.76 c | 689 ± 0.76 a | |
| FC | 3.78 ± 0.57 b | 5.56 ± 0.46 ab | 2.61.12 b | 6.59 ± 1.74 a | |
| W4 | BW | 1590 ± 3.52 b | 1398 ± 9.45 c | 1809 ± 9.69 a | 1636 ± 21.37 b |
| WG | 200 ± 6.5 ab | 181 ± 30.99 ab | 116 ± 10.44 b | 221 ± 20.42 a | |
| FI | 685 ± 0.76 c | 752 ± 0.76 b | 704 ± 0.76 c | 769 ± 0.76 a | |
| FC | 3.43 ± 1.25 | 6.19 ± 2.54 | 6.31 ± 0.60 | 3.63 ± 0.35 | |
| W5 | BW | 1850 ± 3.25 b | 1580 ± 17.52 c | 2017 ± 4.23 a | 1856 ± 23.79 b |
| WG | 260 ± 3.05 | 181 ± 10.15 | 208 ± 8.08 | 220 ± 23.53 | |
| FI | 690 ± 0.76 b | 737 ± 0.76 b | 612 ± 0.76 c | 767 ± 0.76 a | |
| FC | 2.65 ± 0.23 b | 4.11 ± 0.22 a | 2.96 ± 0.12 b | 3.70 ± 0.41 ab | |
| W6 | BW | 1985 ± 1.52 b | 1799 ± 9.28 c | 2243 ± 3.47 a | 2001 ± 5.04 b |
| WG | 135 ± 3.22 b | 219 ± 12.56 a | 225 ± 5.35 a | 145 ± 25.30 b | |
| FI | 700 ± 0.76 b | 747 ± 0.76 a | 690 ± 0.76 b | 749 ± 0.76 a | |
| FC | 5.18 ± 0.20 a | 3.45 ± 0.17 b | 3.07 ± 0.07 b | 5.83 ± 0.81 a | |
| Spleen weight (g) SSI | 1.95 ± 0.13 | 2.00 ± 0.06 | 2.00 ± 0.08 | 1.80 ± 0.02 | |
| 0.101 ± 0.04 | 0.11 ± 0.04 | 0.09 ± 0.06 | 0.09 ± 0.06 | ||
| Parameters | Time | Treatments | |||
|---|---|---|---|---|---|
| G1 | G2 | G3 | G4 | ||
| White blood cells (103/mm3) | Mid of Exp. | 6.50 ± 0.96 b | 6.42 ± 0.96 b | 10.32 ± 1.05 a | 5.90 ± 0.41 b |
| End of Exp. | 6.08 ± 0.26 b | 6.00 ± 0.26 b | 7.52 ± 0.15 a | 5.12 ± 0.24 c | |
| Neutrophil (%) | Mid of Exp. | 29.20 ± 1.25 b | 31.20 ± 1.25 b | 45.00 ± 2.02 a | 41.20 ± 1.06 a |
| End of Exp. | 32.83 ± 0.98 b | 33.83 ± 0.98 b | 43.16 ± 2.67 a | 28.83 ± 2.02 b | |
| Lymphocyte (%) | Mid of Exp. | 51.60 ± 2.58 b | 49.60 ± 2.58 b | 55.60 ± 1.12 a | 50.20 ± 1.70 b |
| End of Exp. | 52.03 ± 0.60 b | 50.03 ± 0.60 b | 52.86 ± 1.83 a | 46.86 ± 2.05 b | |
| Monocytes% | Mid of Exp. | 3.50 ± 0.71 a | 4.50 ± 0.71 a | 5.00 ± 0.44 a | 5.00 ± 0.51 a |
| End of Exp. | 2.50 ± 0.22 a | 3.50 ± 0.22 a | 3.33 ± 0.33 a | 3.50 ± 0.42 a | |
| Eosinophils% | Mid of Exp. | 1.22 ± 0.30 a | 1.83 ± 0.30 a | 2.00 ± 0.36 a | 1.50 ± 0.34 a |
| End of Exp. | 1.30 ± 0.22 a | 1.50 ± 0.22 a | 1.16 ± 0.16 a | 1.16 ± 0.16 a | |
| Items | Treatments | |||
|---|---|---|---|---|
| G1 | G2 | G3 | G4 | |
| Interferon gamma (pg/mL) | 645 ± 2.06 c | 518.71 ± 2.06 c | 1186.2 ± 2.91 a | 736.34 ± 1.20 b |
| IgM (pg/mL) | 322 ± 14.25 c | 284 ± 14.25 c | 588 ± 21.86 a | 338 ± 4.40 b |
| IgG (pg/mL) | 175 ± 3.05 c | 169 ± 3.05 c | 557 ± 9.59 a | 258 ± 1.85 b |
| Phagocytic activity, % | 52 ± 2.006 b | 44.37 ± 2.006 b | 63.12 ± 1.511 a | 61.72 ± 1.85 a |
| Phagocytic index | 1.75 ± 0.064 b | 1.57 ± 0.064 b | 2.04 ± 0.143 a | 2.20 ± 0.041 a |
| Parameters | Time | Treatments | |||
|---|---|---|---|---|---|
| G1 | G2 | G3 | G4 | ||
| Total protein (g/dL) | Mid of Exp. | 5.00 ± 0.17 ab | 4.55 ± 0.19 b | 5.78 ± 0.06 a | 5.46 ± 0.04 a |
| End of Exp. | 5.50 ± 0.07 a | 5.50 ± 0.09 a | 5.67 ± 0.13 a | 5.21 ± 0.05 b | |
| Albumin (g/dL) | Mid of Exp. | 3.20 ± 0.13 a | 3.034 ± 0.182 a | 3.33 ± 0.129 a | 3.40 ± 0.046 a |
| End of Exp. | 3.25 ± 0.02 a | 3.27 ± 0.08 a | 3.05 ± 0.035 b | 3.09 ± 0.031 b | |
| Globulin (g/dL) | Mid of Exp. | 1.80 ± 0.037 ab | 1.52 ± 0.037 c | 2.45 ± 0.08 a | 2.05 ± 0.04 b |
| End of Exp. | 2.25 ± 0.012 b | 2.22 ± 0.012 b | 2.60 ± 0.07 a | 2.11 ± 0.05 b | |
| A/G Ratio | Mid of Exp. | 1.77 ± 0.022 b | 1.99 ± 0.153 a | 1.35 ± 0.11081 b | 1.65 ± 0.058 b |
| End of Exp. | 1.44 ± 0.039 a | 1.47 ± 0.039 a | 1.17 ± 0.031 b | 1.46 ± 0.051 a | |
| Items | Treatments | |||
|---|---|---|---|---|
| G1 | G2 | G3 | G4 | |
| SOD, U/mL | 100.00 ± 1.45b | 70.00 ± 1.52c | 139.00 ± 0.57a | 118.00 ± 1.52b |
| MDA, nmol/mL | 6.59 ± 0.28 b | 9.59 ± 0.25 a | 3.83 ± 0.17638 b | 5.1667 ± 0.60 b |
| Item | Group | Tissue | ||
|---|---|---|---|---|
| Liver | Back muscle | Brain | ||
| Zinc | G1 | 97.88 ± 1.29 b | 58.98 ± 1.25 b | 40.88 ± 1.28 b |
| G2 | 98 ± 1.52 b | 60 ± 1.25 a | 40 ± 0.88 b | |
| G3 | 155.25 ± 1.44 a | 62.00 ± 0.57 a | 50.27 ± 0.14 a | |
| G4 | 100.00 ± 1.52 b | 59.50 ± 1.25 a | 42.66 ± 0.88 b | |
| Copper | G1 | 10.88 ± 0.29 b | 1.55 ± 0.29 b | 9.00 ± 0.19 b |
| G2 | 11.00 ± 0.29 b | 1.60 ± 0.06 b | 9.05 ± 0.09 b | |
| G3 | 30.50 ± 0.28 a | 2.55 ± 0.14 a | 9.38 ± 0.09 a | |
| G4 | 12.43 ± 0.29 b | 1.50 ± 0.06 b | 9.23 ± 0.07 b | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Masoud, S.R.; Fathalla, S.I.; Shawky, S.M.; El-Gendy, H.; Alakhras, M.A.Z.; Alhotan, R.A.; Ayyoub, A.; Selim, S.; Al-Otaibi, K.D.; El-Seidy, A.M.A. Potential Therapeutic Effect of ZnO/CuO Nanocomposite as an Acaricidal, Immunostimulant, and Antioxidant in Rabbits. Vet. Sci. 2025, 12, 333. https://doi.org/10.3390/vetsci12040333
Masoud SR, Fathalla SI, Shawky SM, El-Gendy H, Alakhras MAZ, Alhotan RA, Ayyoub A, Selim S, Al-Otaibi KD, El-Seidy AMA. Potential Therapeutic Effect of ZnO/CuO Nanocomposite as an Acaricidal, Immunostimulant, and Antioxidant in Rabbits. Veterinary Sciences. 2025; 12(4):333. https://doi.org/10.3390/vetsci12040333
Chicago/Turabian StyleMasoud, Shimaa R., Said I. Fathalla, Sherif M. Shawky, Hanem El-Gendy, Mahboba A. Z. Alakhras, Rashed A. Alhotan, Anam Ayyoub, Shaimaa Selim, Khaled Defallah Al-Otaibi, and Ahmed M. A. El-Seidy. 2025. "Potential Therapeutic Effect of ZnO/CuO Nanocomposite as an Acaricidal, Immunostimulant, and Antioxidant in Rabbits" Veterinary Sciences 12, no. 4: 333. https://doi.org/10.3390/vetsci12040333
APA StyleMasoud, S. R., Fathalla, S. I., Shawky, S. M., El-Gendy, H., Alakhras, M. A. Z., Alhotan, R. A., Ayyoub, A., Selim, S., Al-Otaibi, K. D., & El-Seidy, A. M. A. (2025). Potential Therapeutic Effect of ZnO/CuO Nanocomposite as an Acaricidal, Immunostimulant, and Antioxidant in Rabbits. Veterinary Sciences, 12(4), 333. https://doi.org/10.3390/vetsci12040333







