Determination of Natural Blood Plasma Melatonin Concentration of Tsigai Ewes Characteristic for Gestation and Early Postpartum Period Between Autumnal Equinox and Winter Solstice
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Sampling and Determination of the Melatonin Concentration
2.3. Statistical Processing
- -
- Identification number: ear tag with life number;
- -
- Sampling date: the same day that the blood was collected;
- -
- Sampling time: the exact time when the blood was collected;
- -
- Days to winter solstice: the number of days between the sampling date and 21 December 2023 (ranging between −83 and −13 days);
- -
- Minutes around midnight: the number of minutes between midnight and the exact time of the blood collection (ranging between −345 and 323 min);
- -
- Lambing date: the day when the parturition was to occur;
- -
- Days to lambing: the number of days between the date of sampling and the date of lambing (ranging between −138 and −2);
- -
- Days from lambing: the number of days between the date of lambing and the date of sampling (ranging between 0 and 19).
3. Results
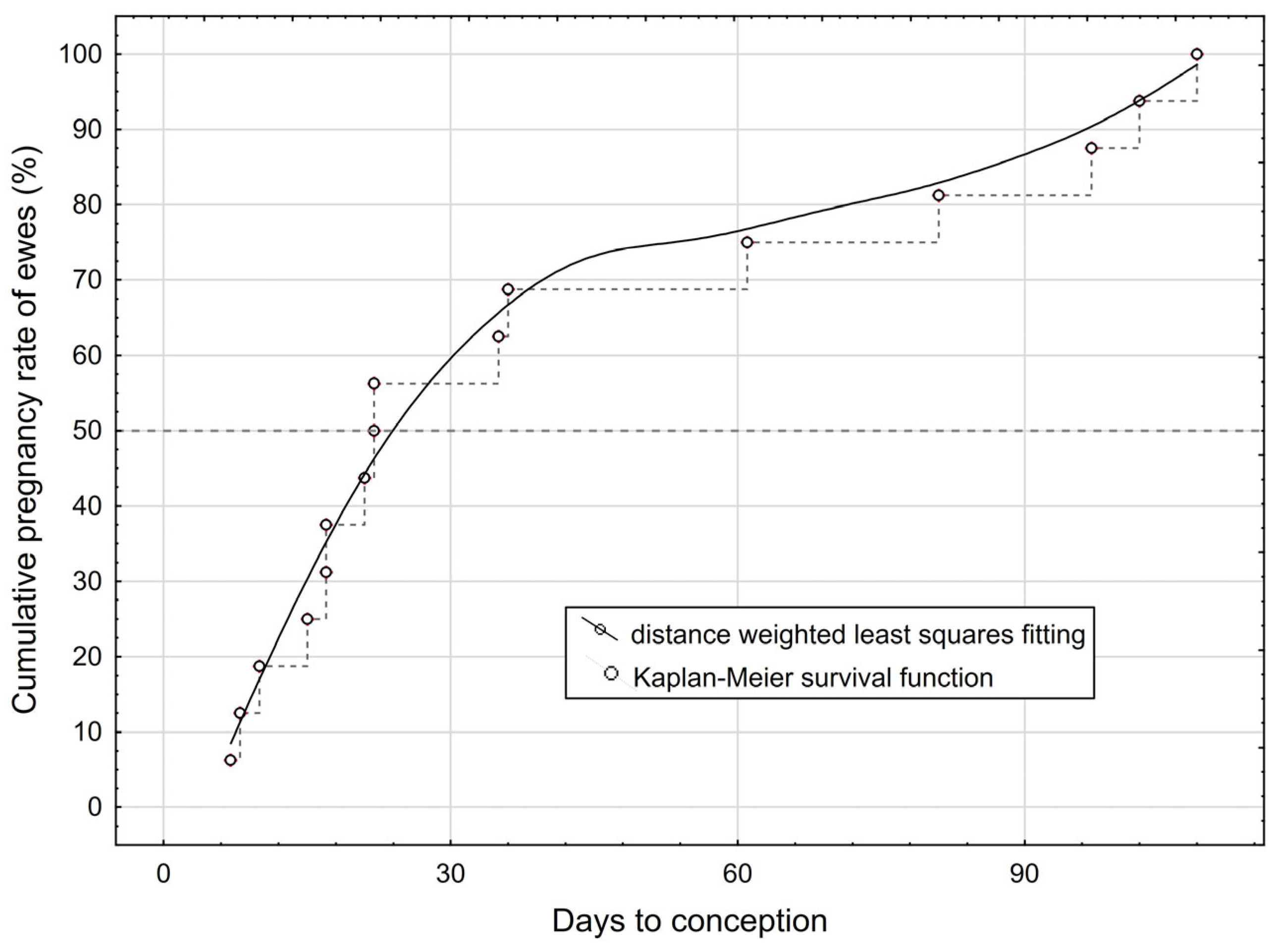
3.1. Conception Results
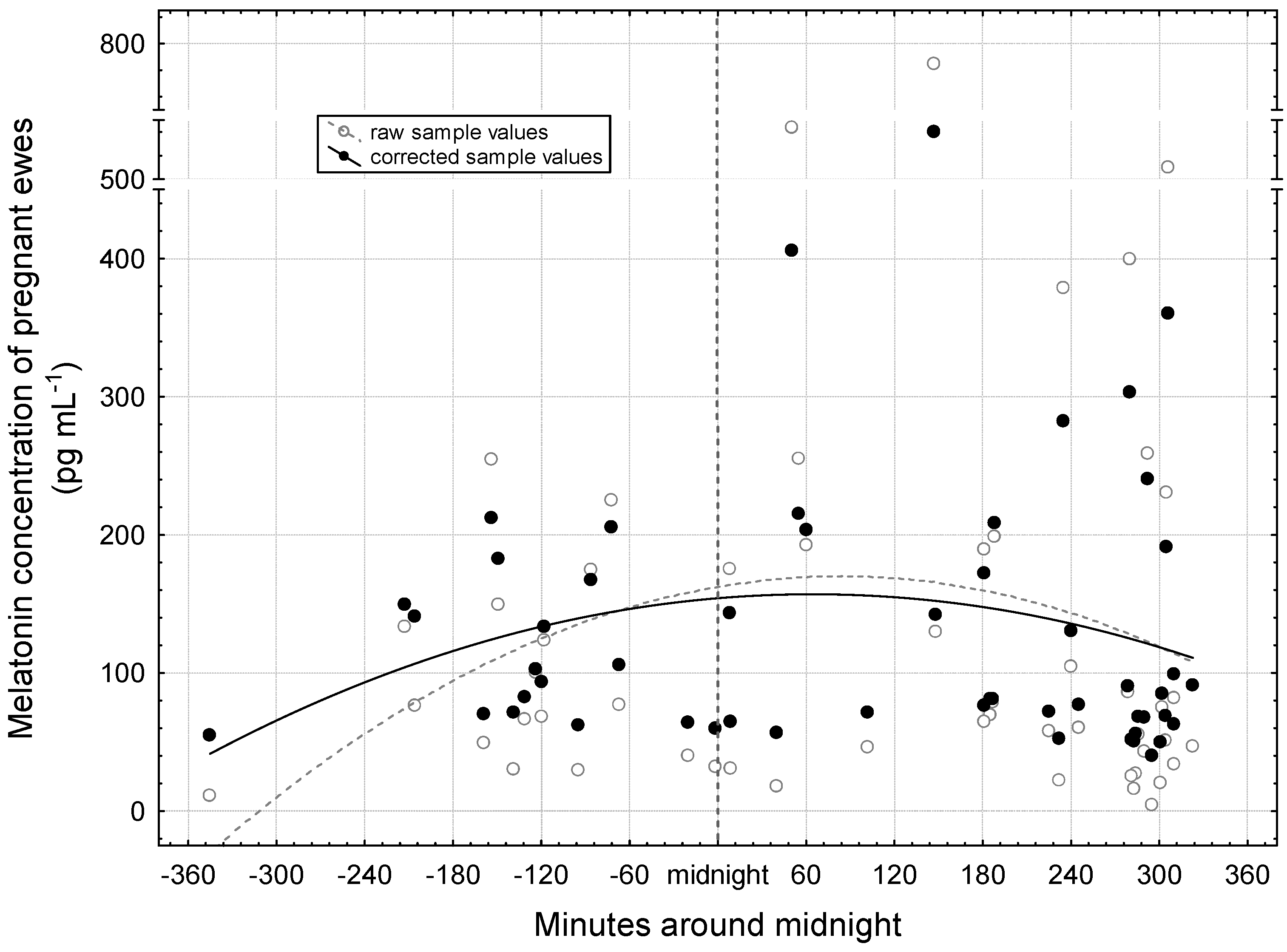
3.2. Period Around Midnight
3.3. Period Between Autumnal Equinox and Winter Solstice
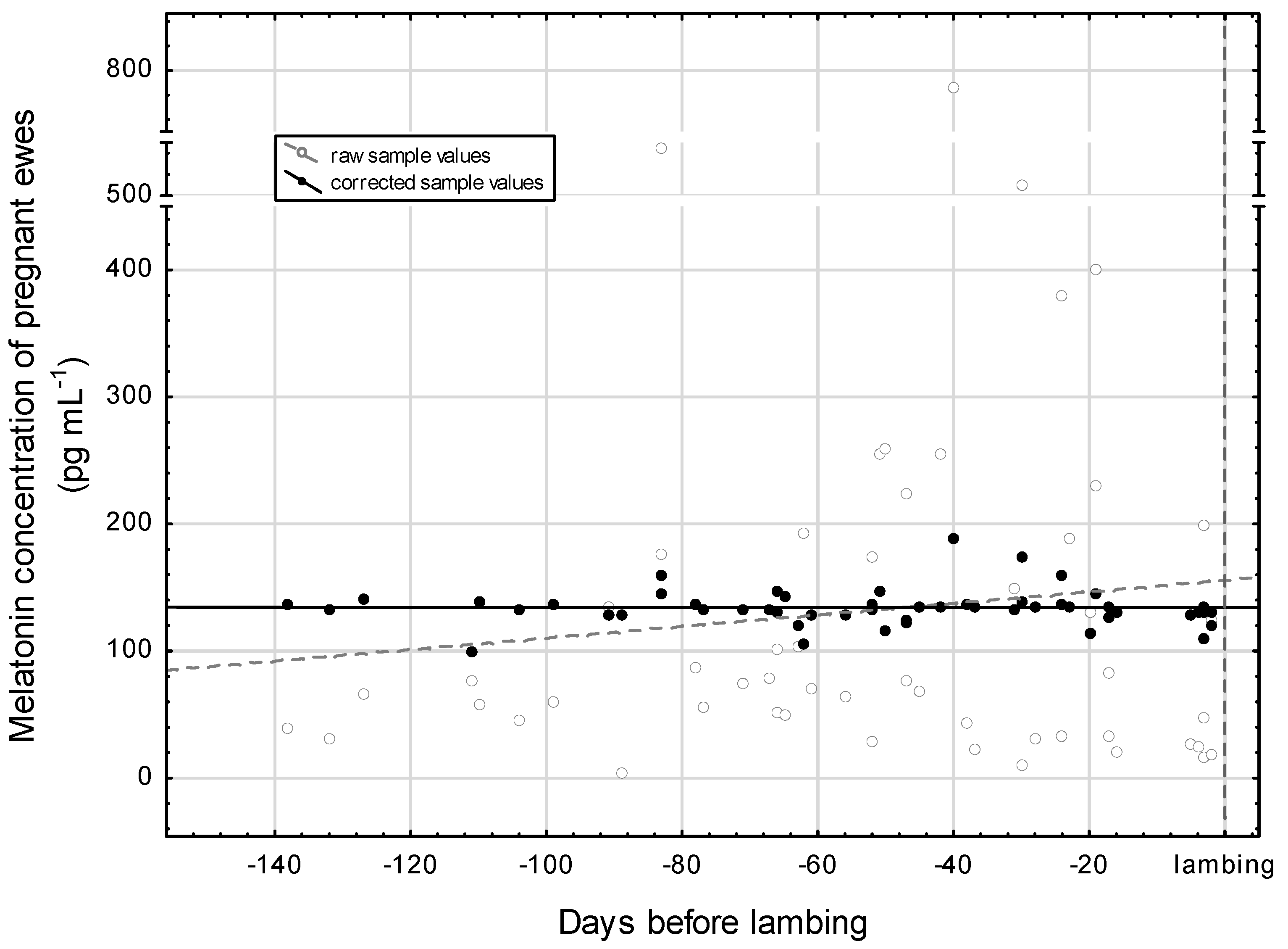
3.4. Period of Gestation
3.5. Period After Lambing
4. Discussion
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lerner, A.B.; Case, J.D.; Takahashi, Y.; Lee, T.H.; Mori, W. Isolation of melatonin, the pineal gland factor that lightens melanocytes1. J. Am. Chem. Soc. 1958, 80, 2587. [Google Scholar] [CrossRef]
- Dýrmundsson, Ó.R. Natural factors affecting puberty and reproductive performance in ewe lambs: A review. Livest. Prod. Sci. 1981, 8, 55–65. [Google Scholar] [CrossRef]
- Pandi-Perumal, S.; Trakht, I.; Srinivasan, V.; Spence, D.W.; Maestroni, G.J.M.; Zisapel, N.; Cardinali, D.P. Physiological effects of melatonin: Role of melatonin receptors and signal transduction pathways. Prog. Neurobiol. 2008, 85, 335–353. [Google Scholar] [CrossRef]
- Beersma, D.G.M.; Gordijn, M.C.M. Circadian control of the sleep–wake cycle. Physiol. Behav. 2007, 90, 190–195. [Google Scholar] [CrossRef]
- Basini, G.; Grasselli, F. Role of Melatonin in Ovarian Function. Animals 2024, 14, 644. [Google Scholar] [CrossRef] [PubMed]
- Cardinali, D.P.; Pévet, P. Basic aspects of melatonin action. Sleep Med. Rev. 1998, 2, 175–190. [Google Scholar] [CrossRef]
- Zhao, D.; Yu, Y.; Shen, Y.; Liu, Q.; Zhao, Z.; Sharma, R.; Reiter, R.J. Melatonin Synthesis and Function: Evolutionary History in Animals and Plants. Front. Endocrinol. 2019, 10, 249. [Google Scholar] [CrossRef]
- Bartlewski, P.M.; Baby, T.E.; Giffin, J.L. Reproductive cycles in sheep. Anim. Reprod. Sci. 2011, 124, 259–268. [Google Scholar] [CrossRef]
- Ahmad Pampori, Z.; Ahmad Sheikh, A.; Aarif, O.; Hasin, D.; Ahmad Bhat, I. Physiology of reproductive seasonality in sheep—An update. Biol. Rhythm Res. 2020, 51, 586–598. [Google Scholar] [CrossRef]
- Zarazaga, L.Á.; Guzmán, J.L.; Malpaux, B. Melatonin for the Control of Reproduction in Small Ruminants. In Melatonin in the Promotion of Health, 2nd ed.; Watson, R.R., Ed.; Taylor & Francis: Abingdon-on-Thames, UK, 2011; pp. 339–350. [Google Scholar]
- Vasantha, I. Physiology of Seasonal Breeding: A Review. J. Vet. Sci. Technol. 2016, 7, 331. [Google Scholar] [CrossRef]
- Rosa, H.J.D.; Bryant, M.J. Seasonality of reproduction in sheep. Small Rumin. Res. 2003, 48, 155–171. [Google Scholar] [CrossRef]
- Cruz, M.H.C.; Leal, C.L.V.; Cruz, J.F.; Tan, D.X.; Reiter, R.J. Essential actions of melatonin in protecting the ovary from oxidative damage. Theriogenology 2014, 82, 925–932. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J.; Sharma, R. Central and peripheral actions of melatonin on reproduction in seasonal and continuous breeding mammals. Gen. Comp. Endocrinol. 2021, 300, 113620. [Google Scholar] [CrossRef]
- González-Arto, M.; Aguilar, D.; Gaspar-Torrubia, E.; Gallego, M.; Carvajal-Serna, M.; Herrera-Marcos, L.V.; Serrano-Blesa, E.; Hamilton, T.R.d.S.; Pérez-Pé, R.; Muiño-Blanco, T.; et al. Melatonin MT1 and MT2 Receptors in the Ram Reproductive Tract. Int. J. Mol. Sci. 2017, 18, 662. [Google Scholar] [CrossRef]
- Sosa, C.; Laurenzana, E.; de Brun, V.; Meikle, A.; Abecia, J.A. The melatonin system is expressed in the ovine uterus: Effect of the day of the oestrous cycle and undernutrition. Reprod. Fert. Dev. 2023, 35, 563–574. [Google Scholar] [CrossRef]
- Viola, I.; Sosa, C.; Accornero, P.; Manenti, I.; Canto, F.; Miretti, S.; Abecia, J.A.; Toschi, P. Exogenous melatonin ameliorates embryo–maternal cross-talk in early pregnancy in sheep. Reproduction 2024, 168, e240172. [Google Scholar] [CrossRef]
- Palacin, I.; Forcada, F.; Abecia, J.A. Meta-analysis of the efficacy of melatonin implants for improving reproductive performance in sheep. Span. J. Agric. Res. 2011, 9, 730–743. [Google Scholar] [CrossRef]
- Kárpáti, E.; Fürlinger, D.; Móczáné Pleskó, A.; Gulyás, L.; Gáspárdy, A.; Becskei, Z. Various approaches to influence melatonin level in sheep reproduction. Vet. Glas. 2023, 77, 16–34. [Google Scholar] [CrossRef]
- Durotoye, L.A.; Rajkumar, R.; Argo, C.M.; Nowak, R.; Webley, G.E.; McNeil, M.E.; Graham, N.B.; Rodway, R.G. Effect of constant-release melatonin implants on the onset of oestrous activity and on reproductive performance in the ewe. Anim. Sci. 1991, 52, 489–497. [Google Scholar] [CrossRef]
- Abecia, J.A.; Forcada, F.; Casao, A.; Palacín, I. Effect of exogenous melatonin on the ovary, the embryo and the establishment of pregnancy in sheep. Animal 2008, 2, 399–404. [Google Scholar] [CrossRef]
- DeNicolo, G.; Morris, S.T.; Kenyon, P.R.; Morel, P.C.H.; Parkinson, T.J. Melatonin-improved reproductive performance in sheep bred out of season. Anim. Reprod. Sci. 2008, 109, 124–133. [Google Scholar] [CrossRef] [PubMed]
- Bouroutzika, E.; Ciliberti, M.G.; Caroprese, M.; Theodosiadou, E.; Papadopoulos, S.; Makri, S.; Skaperda, Z.-V.; Kotsadam, G.; Michailidis, M.-L.; Valiakos, G.; et al. Association of Melatonin Administration in Pregnant Ewes with Growth, Redox Status and Immunity of Their Offspring. Animals 2021, 11, 3161. [Google Scholar] [CrossRef]
- Kivelä, A. Serum melatonin during human pregnancy. Acta Endocrinol. 1991, 124, 233–237. [Google Scholar] [CrossRef]
- Ejaz, H.; Figaro, J.K.; Woolner, A.M.F.; Thottakam, B.M.V.; Galley, H.F. Maternal Serum Melatonin Increases During Pregnancy and Falls Immediately After Delivery Implicating the Placenta as a Major Source of Melatonin. Front. Endocrinol. 2021, 11, 623038. [Google Scholar] [CrossRef]
- Zarazaga, L.A.; Malpaux, B.; Chemineau, P. The characteristics of the melatonin secretory rhythm are not modified by the stage of pregnancy in ewes. Reprod. Nutr. Dev. 1997, 37, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Gáspárdy, A. The Tsigai or in Hungarian Berke Sheep. In Living Heritage—Old Historical Hungarian Livestock, 1st ed.; Bodó, I., Ed.; Agroinform Kiadó: Budapest, Hungary, 2001; pp. 60–62. [Google Scholar]
- Cunningham, K.; Van Burgel, A.; Kelman, K.R.; Macleay, C.M.; Paganoni, B.L.; Thompson, A.N. Interactions between Ewes and Rams during Mating Can Be Used to Predict Lambing Dates Accurately, but Not Sire. Animals 2022, 12, 1707. [Google Scholar] [CrossRef]
- Pedigree Viewer. Version 6.5f. 2015. Available online: http://bkinghor@une.edu.au (accessed on 4 June 2024).
- TIBCO Software Inc. Data Science Workbench. Statistica Version 14. 2020. Available online: http://tibco.com (accessed on 2 April 2025).
- Szikszai, I. (Tsigai Seedstock Breeder, Csanádpalota, Csongrád-Csanád County, Hungary). Personal communication. 2024. [Google Scholar]
- Bittman, E.L.; Dempsey, R.J.; Karsch, F.J. Pineal Melatonin Secretion Drives the Reproductive Response to Daylength in the Ewe. Endocrinology 1983, 113, 2276–2283. [Google Scholar] [CrossRef]
- Carcangiu, V.; Mura, M.C.; Parmeggiani, A.; Piccione, G.; Bini, P.P.; Cosso, G.; Luridiana, S. Daily rhythm of blood melatonin concentrations in sheep of different ages. Biol. Rhythm Res. 2013, 44, 908–915. [Google Scholar] [CrossRef]
- Williams, L.M.; Helliwell, R.J.A. Melatonin and seasonality in the sheep. Anim. Reprod. Sci. 1993, 33, 159–182. [Google Scholar] [CrossRef]
- Zarazaga, L.A.; Malpaux, B.; Bodin, L.; Chemineau, P. The large variability in melatonin blood levels in ewes is under strong genetic influence. Am. J. Physiol. 1998, 274, E607–E610. [Google Scholar] [CrossRef]
- Coelho, L.A.; Rodrigues, P.A.; Nonaka, K.O.; Sasa, A.; Balieiro, J.C.C.; Vicente, W.R.R.; Cipolla-Neto, J. Annual pattern of plasma melatonin and progesterone concentrations in hair and wool ewe lambs kept under natural photoperiod at lower latitudes in the southern hemisphere. J. Pineal Res. 2006, 41, 101–107. [Google Scholar] [CrossRef]
- Rollag, M.D.; Niswender, G.D. Radioimmunoassay of Serum Concentrations of Melatonin in Sheep Exposed to Different Lighting Regimens. Endocrinology 1976, 98, 482–489. [Google Scholar] [CrossRef] [PubMed]
- Harmat, L.; Nagy, J.; Somoskői, B.; Alpár, A.; Fekete, S.G.; Gáspárdy, A. Determination of Rhythmicity and Gestational Stage-Related Distribution of Blood Plasma Melatonin Concentrations in Donkey Mares. Vet. Sci. 2024, 11, 310. [Google Scholar] [CrossRef] [PubMed]
- Szczesna, M.; Kirsz, K.; Misztal, T.; Molik, E.; Zieba, D.A. The effects of leptin on plasma concentrations of prolactin, growth hormone, and melatonin vary depending on the stage of pregnancy in sheep. J. Anim. Sci. 2018, 96, 3348–3357. [Google Scholar] [CrossRef] [PubMed]
- McMillen, I.C.; Walker, D.W. Effects of different lighting regimes on daily hormonal and behavioral rhythms in the pregnant ewe and sheep fetus. J. Physiol. 1991, 442, 465–476. [Google Scholar] [CrossRef]
- Zemdegs, I.Z.; McMillen, I.C.; Walker, D.W.; Thorburn, G.D.; Nowak, R. Diurnal rhythms in plasma melatonin concentrations in the fetal sheep and pregnant ewe during late gestation. Endocrinology 1988, 123, 284–289. [Google Scholar] [CrossRef]
- Suarez-Trujillo, A.; Hoang, N.; Robinson, L.; McCabe, C.J.; Conklin, D.; Minor, R.C.; Townsend, J.; Plaut, K.; George, U.Z.; Boerman, J.; et al. Effect of circadian system disruption on the concentration and daily oscillations of cortisol, progesterone, melatonin, serotonin, growth hormone, and core body temperature in periparturient dairy cattle. J. Dairy Sci. 2022, 105, 2651–2668. [Google Scholar] [CrossRef]
- Kennaway, D.J.; Matthews, C.D.; Seamark, R.F.; Phillipou, G.; Schilthuis, M. On the presence of melatonin in the pineal glands and plasma of foetal sheep. J. Steroid Biochem. 1977, 8, 559–563. [Google Scholar] [CrossRef]
- Eifert, A.W.; Wilson, M.E.; Vonnahme, K.A.; Camacho, L.E.; Borowicz, P.P.; Redmer, D.A.; Romero, S.; Dorsam, S.; Haring, J.; Lemley, C.O. Effect of melatonin or maternal nutrient restriction on vascularity and cell proliferation in the ovine placenta. Anim. Reprod. Sci. 2015, 153, 13–21. [Google Scholar] [CrossRef]
- McCarthy, R.; Jungheim, E.S.; Fay, J.C.; Bates, K.; Herzog, E.D.; England, S.K. Riding the Rhythm of Melatonin Through Pregnancy to Deliver on Time. Front. Endocrinol. 2019, 10, 616. [Google Scholar] [CrossRef]
- Huchton, J.D. Melatonin Levels in Ewes and Lambs Under Suckled and Non-Suckled Regimens During the First Eight Weeks Postpartum. Ph.D. Thesis, Texas A&M University, College Station, TX, USA, 1984. [Google Scholar]
- Seron-Ferre, M.; Torres-Farfan, C.; Valenzuela, F.J.; Castillo-Galan, S.; Rojas, A.; Mendez, N.; Reynolds, H.; Valenzuela, G.J.; Llanos, A.J. Deciphering the Function of the Blunt Circadian Rhythm of Melatonin in the Newborn Lamb: Impact on Adrenal and Heart. Endocrinology 2017, 158, 2895–2905. [Google Scholar] [CrossRef] [PubMed]
- Nowak, R.; Young, I.R.; McMillen, I.C. Emergence of the diurnal rhythm in plasma melatonin concentrations in newborn lambs delivered to intact or pinealectomized ewes. J. Endocrinol. 1990, 125, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Wyse, C.A.; Zhang, X.; McLaughlin, M.; Biello, S.M.; Hough, D.; Bellingham, M.; Curtis, A.M.; Robinson, J.E.; Evans, N.P. Circadian rhythms of melatonin and behaviour in juvenile sheep in field conditions: Effects of photoperiod, environment and weaning. Physiol. Behav. 2018, 194, 362–370. [Google Scholar] [CrossRef] [PubMed]
- Hooley, R.D.; Campbell, J.J.; Findlay, J.K. The importance of prolactin for lactation in the ewe. J. Endocrinol. 1978, 79, 301–310. [Google Scholar] [CrossRef]
- Molik, E.; Misztal, T.; Romanowicz, K.; Zieba, D. Short-day and melatonin effects on milking parameters, prolactin profiles and growth-hormone secretion in lactating sheep. Small Rumin. Res. 2013, 109, 182–187. [Google Scholar] [CrossRef]
- Misztal, T.; Górski, K.; Tomaszewska-Zaremba, D.; Molik, E.; Romanowicz, K. Identification of salsolinol in the mediobasal hypothalamus of lactating ewes and its relation to suckling-induced prolactin and GH release. J. Endocrinol. 2008, 198, 83–89. [Google Scholar] [CrossRef]
- Schmitt, B.; Povinelli, L.; Crodian, J.; Casey, T.; Plaut, K. Circadian rhythms of ewes suckling singletons versus twins during the second week of lactation. Bios 2014, 85, 207–217. Available online: http://www.jstor.org/stable/24367826 (accessed on 28 January 2025).
- Guesdon, V.; Malpaux, B.; Delagrange, P.; Spedding, M.; Cornilleau, F.; Chesneau, D.; Haller, J.; Chaillou, E. Rapid effects of melatonin on hormonal and behavioral stressful responses in ewes. Psychoneuroendocrinology 2013, 38, 1426–1434. [Google Scholar] [CrossRef]
- Walker, C.D.; Lightman, S.L.; Steele, M.K.; Dallman, M.F. Suckling is a persistent stimulus to the adrenocortical system of the rat. Endocrinology 1992, 130, 115–125. [Google Scholar] [CrossRef]
- Lightman, S.L.; Birnie, M.T.; Conway-Campbell, B.L. Dynamics of ACTH and Cortisol Secretion and Implications for Disease. Endocr. Rev. 2020, 41, bnaa002. [Google Scholar] [CrossRef]
- Spiga, F.; Zavala, E.; Walker, J.J.; Zhao, Z.; Terry, J.R.; Lightman, S.L. Dynamic responses of the adrenal steroidogenic regulatory network. Proc. Natl. Acad. Sci. USA 2017, 114, E6466–E6474. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Yoon, M. Melatonin effects on animal behavior: Circadian rhythm, stress response, and modulation of behavioral patterns. J. Anim. Sci. Technol. 2025, 67, 1–16. [Google Scholar] [CrossRef]
- Konakchieva, R.; Mitev, Y.; Almeida, O.F.X.; Patchev, V.K. Chronic melatonin treatment and the hypothalamo-pituitary-adrenal axis in the rat: Attenuation of the secretory response to stress and effects on hypothalamic neuropeptide content and release. Biol. Cell 1997, 89, 587–596. [Google Scholar] [CrossRef] [PubMed]
- Pandi-Perumal, S.R.; Trakht, I.; Brown, G.M.; Cardinali, D.P. Melatonin, circadian dysregulation, and sleep in mental disorders. Prim. Psychiatry 2008, 15, 77–82. [Google Scholar]
- Stirrat, L.I.; Walker, J.J.; Stryjakowska, K.; Jones, N.; Homer, N.Z.M.; Andrew, R.; Norman, J.E.; Lightman, S.L.; Reynolds, R.M. Pulsatility of glucocorticoid hormones in pregnancy: Changes with gestation and obesity. Clin. Endocrinol. 2018, 88, 592–600. [Google Scholar] [CrossRef]
- Perkinson, M.R.; Kim, J.S.; Iremonger, K.J.; Brown, C.H. Visualising oxytocin neurone activity in vivo: The key to unlocking central regulation of parturition and lactation. J. Neuroendocrinol. 2021, 33, e13012. [Google Scholar] [CrossRef] [PubMed]
- Bienboire-Frosini, C.; Marcet-Rius, M.; Orihuela, A.; Domínguez-Oliva, A.; Mora-Medina, P.; Olmos-Hernández, A.; Casas-Alvarado, A.; Mota-Rojas, D. Mother–young bonding: Neurobiological aspects and maternal biochemical signaling in altricial domesticated mammals. Animals 2023, 13, 532. [Google Scholar] [CrossRef]
- Pissonnier, D.; Thiery, J.C.; Fabre-Nys, C.; Poindron, P.; Keverne, E.B. The Importance of olfactory bulb noradrenalin for maternal recognition in sheep. Physiol. Behav. 1985, 35, 361–363. [Google Scholar] [CrossRef]
- Keller, M.; Meurisse, M.; Poindron, P.; Nowak, R.; Ferreira, G.; Shayit, M.; Lévy, F. Maternal experience influences the establishment of visual/auditory, but not olfactory recognition of the newborn lamb by ewes at parturition. Dev. Psychobiol. 2003, 43, 167–176. [Google Scholar] [CrossRef]
- Lemley, C.O.; Vonnahme, K.A. PHYSIOLOGY AND ENDOCRINOLOGY SYMPOSIUM: Alterations in uteroplacental hemodynamics during melatonin supplementation in sheep and cattle. J. Anim. Sci. 2017, 95, 2211–2221. [Google Scholar] [CrossRef]
- Torres-Farfan, C.; Valenzuela, F.J.; Mondaca, M.; Valenzuela, G.J.; Krause, B.; Herrera, E.A.; Riquelme, R.; Llanos, A.J.; Seron-Ferre, M. Evidence of a role for melatonin in fetal sheep physiology: Direct actions of melatonin on fetal cerebral artery, brown adipose tissue and adrenal gland. J. Physiol. 2008, 586, 4017–4027. [Google Scholar] [CrossRef] [PubMed]
- Beñaldo, F.A.; Llanos, A.J.; Araya-Quijada, C.; Rojas, A.; Gonzalez-Candia, A.; Herrera, E.A.; Ebensperger, G.; Cabello, G.; Valenzuela, G.J.; Serón-Ferré, M. Effects of Melatonin on the Defense to Acute Hypoxia in Newborn Lambs. Front. Endocrinol. 2019, 10, 433. [Google Scholar] [CrossRef] [PubMed]


| Specific Day Time p = 0.085 | No. of Observations Considered † | Melatonin Concentration Mean, pg mL−1 | SEM * |
|---|---|---|---|
| Mid period (6–7 November 2023): | |||
| Afternoon 6:00 p.m. | 11 | 68.8 | 15.71 |
| Melatonin top after midnight 1:00 a.m. | 21 | 159.3 | 26.20 |
| Morning 6:00 a.m. | 30 | 122.4 | 20.60 |
| Specific Days p < 0.001 | No. of Observations Considered † | Melatonin Concentration Mean, pg mL−1 | SEM * |
|---|---|---|---|
| Autumnal equinox (23–24 September 2023) | 29 | 127.5 a | 9.13 |
| Mid time (6–7 November 2023) | 25 | 158.1 ab | 10.24 |
| Winter solstice (21–22 December 2023) | 22 | 188.3 b | 12.44 |
| Days to Lambing p = 0.783 | No. of Observations Considered † | Melatonin Concentration Mean, pg mL−1 | SEM * |
|---|---|---|---|
| 98 days before lambing | 13 | 162.6 | 3.66 |
| 49 days before lambing | 22 | 163.7 | 3.73 |
| 7 days before lambing | 18 | 160.5 | 2.67 |
| Specific Days p < 0.001 | No. of Observations Considered † | Melatonin Concentration Mean, pg mL−1 | SEM * |
|---|---|---|---|
| Adjusted for 2 days before lambing in pregnant ewes | 22 | 160.2 a | 2.85 |
| Adjusted for 1 day after lambing in ewes lambed | 12 | 36.18 b | 0.634 |
| Adjusted for 1 day after birth in lambs | 12 | 35.92 b | 0.578 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gáspárdy, A.; Gulyás, L.; Polland, I.; Alpár, A.; Fekete, S.G.; Harmat, L. Determination of Natural Blood Plasma Melatonin Concentration of Tsigai Ewes Characteristic for Gestation and Early Postpartum Period Between Autumnal Equinox and Winter Solstice. Vet. Sci. 2025, 12, 336. https://doi.org/10.3390/vetsci12040336
Gáspárdy A, Gulyás L, Polland I, Alpár A, Fekete SG, Harmat L. Determination of Natural Blood Plasma Melatonin Concentration of Tsigai Ewes Characteristic for Gestation and Early Postpartum Period Between Autumnal Equinox and Winter Solstice. Veterinary Sciences. 2025; 12(4):336. https://doi.org/10.3390/vetsci12040336
Chicago/Turabian StyleGáspárdy, András, László Gulyás, Ida Polland, Alán Alpár, Sándor György Fekete, and Levente Harmat. 2025. "Determination of Natural Blood Plasma Melatonin Concentration of Tsigai Ewes Characteristic for Gestation and Early Postpartum Period Between Autumnal Equinox and Winter Solstice" Veterinary Sciences 12, no. 4: 336. https://doi.org/10.3390/vetsci12040336
APA StyleGáspárdy, A., Gulyás, L., Polland, I., Alpár, A., Fekete, S. G., & Harmat, L. (2025). Determination of Natural Blood Plasma Melatonin Concentration of Tsigai Ewes Characteristic for Gestation and Early Postpartum Period Between Autumnal Equinox and Winter Solstice. Veterinary Sciences, 12(4), 336. https://doi.org/10.3390/vetsci12040336







