Diagnostic Accuracy of Lung Ultrasound in Rabbit Subclinical Lung Lesions
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Subject Inclusion
2.2. LUS Protocol
2.3. Thoracic CT Protocol
2.4. Image Analysis
3. Statistical Analysis
4. Results
4.1. Study Population
4.2. Thoracic LUS
4.3. Thoracic CT
4.4. Performance of LUS
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CT | Computed tomography |
| FN | False negatives |
| FP | False positives |
| GGO | Ground-glass opacities |
| HU | Hounsfield unit |
| LUS | Lung ultrasound |
| NPV | Negative predictive value |
| PPV | Positive predictive value |
| TN | True negatives |
| TP | True positives |
References
- Hedley, J. Respiratory Disease. In BSAVA Manual of Rabbit Medicine; Meredith, L., Ed.; British Small Animal Veterinary Association: Gloucestershire, UK, 2014; pp. 160–167. ISBN 978-1-905319-49-7. [Google Scholar]
- Lennox, A.M.; Mancinelli, E. 15—Respiratory Disease. In Ferrets, Rabbits, and Rodents, 4th ed.; Quesenberry, K.E., Orcutt, C.J., Mans, C., Carpenter, J.W., Eds.; W.B. Saunders: Philadelphia, PA, USA, 2020; pp. 188–200. ISBN 978-0-323-48435-0. [Google Scholar]
- Johnson-Delaney, C.A.; Orosz, S.E. Rabbit Respiratory System: Clinical Anatomy, Physiology and Disease. Vet. Clin. N. Am. Exot. Anim. Pract. 2011, 14, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Jekl, V. Respiratory Disorders in Rabbits. Vet. Clin. N. Am. Exot. Anim. Pract. 2021, 24, 459–482. [Google Scholar] [CrossRef]
- Varga, M. Chapter 11—Cardiorespiratory Disease. In Textbook of Rabbit Medicine, 2nd ed.; Varga, M., Ed.; Butterworth-Heinemann: Oxford, UK, 2014; pp. 390–404. ISBN 978-0-7020-4979-8. [Google Scholar]
- Mancinelli, E. Respiratory Disease in Rabbits. Practice 2019, 41, 121–129. [Google Scholar] [CrossRef]
- Grint, N. Anaesthesia. In BSAVA Manual of Rabbit Surgery, Dentistry and Imaging; Harcourt-Brown, C., Ed.; British Small Animal Veterinary Association: Gloucestershire, UK, 2013; pp. 1–25. ISBN 978-1-905319-41-1. [Google Scholar]
- Lennox, A.M.; Mancinelli, E. Respiratory Disease. In Ferrets, Rabbits, and Rodents; Elsevier: Amsterdam, The Netherlands, 2020; pp. 188–200. ISBN 978-0-323-48435-0. [Google Scholar]
- Müllhaupt, D.; Wenger, S.; Kircher, P.; Pfammatter, N.; Hatt, J.-M.; Ohlerth, S. Computed Tomography of the Thorax in Rabbits: A Prospective Study in Ten Clinically Healthy New Zealand White Rabbits. Acta Vet. Scand. 2017, 59, 72. [Google Scholar] [CrossRef]
- Veraa, S.; Schoemaker, N. CT and MRI Scanning and Interpretation. In BSAVA Manual of Rabbit Surgery, Dentistry and Imaging; Harcourt-Brown, C., Ed.; British Small Animal Veterinary Association: Gloucestershire, UK, 2013; pp. 107–114. ISBN 978-1-905319-41-1. [Google Scholar]
- Sargo, R.; Tomé, I.; Silva, F.; Ginja, M. Evaluation of the Effects of Sedation and Anesthesia on Total Lung Volume and Attenuation in Rabbit Lung CT Exams. Animals 2024, 14, 3473. [Google Scholar] [CrossRef]
- Capello, V.; Lennox, A.M. Diagnostic Imaging of the Respiratory System in Exotic Companion Mammals. Vet. Clin. N. Am. Exot. Anim. Pract. 2011, 14, 369–389. [Google Scholar] [CrossRef]
- Hunt, T.D.; Wallack, S.T. Minimal Atelectasis and Poorly Aerated Lung on Thoracic CT Images of Normal Dogs Acquired under Sedation. Vet. Radiol. Ultrasound 2021, 62, 647–656. [Google Scholar] [CrossRef]
- Ali, R.M.M.; Ghonimy, M.B.I. Semi-Quantitative CT Imaging in Improving Visualization of Faint Ground Glass Opacities Seen in Early/Mild Coronavirus (COVID-19) Cases. Egypt. J. Radiol. Nucl. Med. 2020, 51, 244. [Google Scholar] [CrossRef]
- Kolta, M.F.F.; Abouheif, M.A.A.-E.A.-E.; Abd El-Mageed, M.R. Semiquantitative CT Imaging as a Tool in Improving Detection of Ground Glass Patches in Patients with COVID-19 Pneumonia and for Better Follow-Up. Egypt. J. Radiol. Nucl. Med. 2022, 53, 180. [Google Scholar] [CrossRef]
- Chen, A.; Karwoski, R.A.; Gierada, D.S.; Bartholmai, B.J.; Koo, C.W. Quantitative CT Analysis of Diffuse Lung Disease. RadioGraphics 2020, 40, 28–43. [Google Scholar] [CrossRef]
- Sun, D.; Li, X.; Guo, D.; Wu, L.; Chen, T.; Fang, Z.; Chen, L.; Zeng, W.; Yang, R. CT Quantitative Analysis and Its Relationship with Clinical Features for Assessing the Severity of Patients with COVID-19. Korean J. Radiol. 2020, 21, 859–868. [Google Scholar] [CrossRef] [PubMed]
- Blazic, I.; Cogliati, C.; Flor, N.; Frija, G.; Kawooya, M.; Umbrello, M.; Ali, S.; Baranne, M.-L.; Cho, Y.-J.; Pitcher, R.; et al. The Use of Lung Ultrasound in COVID-19. ERJ Open Res. 2023, 9, 00196-2022. [Google Scholar] [CrossRef] [PubMed]
- Cole, L.; Pivetta, M.; Humm, K. Diagnostic Accuracy of a Lung Ultrasound Protocol (Vet BLUE) for Detection of Pleural Fluid, Pneumothorax and Lung Pathology in Dogs and Cats. J. Small Anim. Pract. 2021, 62, 178–186. [Google Scholar] [CrossRef]
- Ma, H.; Huang, D.; Guo, L.; Chen, Q.; Zhong, W.; Geng, Q.; Zhang, M. Strong Correlation between Lung Ultrasound and Chest Computerized Tomography Imaging for the Detection of Acute Lung Injury/Acute Respiratory Distress Syndrome in Rats. J. Thorac. Dis. 2016, 8, 1443–1448. [Google Scholar] [CrossRef]
- Rademacher, N.; Pariaut, R.; Pate, J.; Saelinger, C.; Kearney, M.T.; Gaschen, L. Transthoracic Lung Ultrasound in Normal Dogs And Dogs with Cardiogenic Pulmonary Edema: A Pilot Study: Transthoracic Ultrasound of the Lungs in Dogs. Veter.-Radiol. Ultrasound 2014, 55, 447–452. [Google Scholar] [CrossRef]
- Armenise, A. Point-of-Care Lung Ultrasound in Small Animal Emergency and Critical Care Medicine: A Clinical Review. Animals 2025, 15, 106. [Google Scholar] [CrossRef]
- Lin, C.-H.; Lo, P.-Y.; Lam, M.-C.; Wu, H.-D. Usefulness of Chest Ultrasonography in Predicting Diagnosis in Non-Emergency Small Animal Patients with Lung Parenchymal and Pleural Disease. Front. Vet. Sci. 2020, 7, 616882. [Google Scholar] [CrossRef]
- Kraszewska, K.; Gajewski, M.; Boysen, S.; Buda, N. Retrospective Evaluation of Subpleural Consolidations Using Lung Ultrasound in 634 Dogs and 347 Cats. Animals 2025, 15, 549. [Google Scholar] [CrossRef]
- Grune, J.; Beyhoff, N.; Hegemann, N.; Lauryn, J.H.; Kuebler, W.M. From Bedside to Bench: Lung Ultrasound for the Assessment of Pulmonary Edema in Animal Models. Cell Tissue Res. 2020, 380, 379–392. [Google Scholar] [CrossRef]
- Zhu, Z.; Lian, X.; Zeng, Y.; Wu, W.; Xu, Z.; Chen, Y.; Li, J.; Su, X.; Zeng, L.; Lv, G. Point-of-Care Ultrasound—A New Option for Early Quantitative Assessment of Pulmonary Edema. Ultrasound Med. Biol. 2020, 46, 1–10. [Google Scholar] [CrossRef]
- Lichtenstein, D.A. Lung Ultrasound in the Critically Ill. J. Med. Ultrasound 2009, 17, 125–142. [Google Scholar] [CrossRef]
- Bujang, M.A.; Adnan, T.H. Requirements for Minimum Sample Size for Sensitivity and Specificity Analysis. J. Clin. Diagn. Res. 2016, 10, YE01–YE06. [Google Scholar] [CrossRef] [PubMed]
- Lisciandro, G.R.; Fosgate, G.T.; Fulton, R.M. Frequency and Number of Ultrasound Lung Rockets (b-Lines) Using a Regionally Based Lung Ultrasound Examination Named Vet Blue (Veterinary Bedside Lung Ultrasound Exam) in Dogs with Radiographically Normal Lung Findings. Vet. Radiol. Ultrasound 2014, 55, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Slicer/SlicerLungCTAnalyzer. 2024. Available online: https://github.com/Slicer/SlicerLungCTAnalyzer/ (accessed on 30 October 2024).
- Bumm, R.; Zaffino, P.; Lasso, A.; Estépar, R.S.J.; Pieper, S.; Wasserthal, J.; Spadea, M.F.; Latshang, T.; Kawel-Böhm, N.; Wäckerlin, A.; et al. Artificial Intelligence (AI)-Assisted Chest Computer Tomography (CT) Insights: A Study on Intensive Care Unit (ICU) Admittance Trends in 78 Coronavirus Disease 2019 (COVID-19) Patients. J. Thorac. Dis. 2024, 16, 1009. [Google Scholar] [CrossRef]
- Reimegård, E.; Lee, H.T.N.; Westgren, F. Prevalence of Lung Atelectasis in Sedated Dogs Examined with Computed Tomography. Acta Vet. Scand. 2022, 64, 25. [Google Scholar] [CrossRef]
- Staffieri, F.; Franchini, D.; Carella, G.L.; Montanaro, M.G.; Valentini, V.; Driessen, B.; Grasso, S.; Crovace, A. Computed Tomographic Analysis of the Effects of Two Inspired Oxygen Concentrations on Pulmonary Aeration in Anesthetized and Mechanically Ventilated Dogs. Am. J. Vet. Res. 2007, 68, 925–931. [Google Scholar] [CrossRef]
- Vishwas, K.; Sandhyarani, K.; Madhuri, D.; Jeevanalatha, M.; Dhanalakshmi, K. Incidence and Mortality Due to Respiratory Diseases in Rabbits. J. Entomol. Zool. Studies. 2000, 8, 706–711. [Google Scholar]
- Baker, D.G. Natural Pathogens of Laboratory Mice, Rats, and Rabbits and Their Effects on Research. Clin. Microbiol. Rev. 1998, 11, 231–266. [Google Scholar] [CrossRef]
- Hansell, D.M.; Bankier, A.A.; MacMahon, H.; McLoud, T.C.; Müller, N.L.; Remy, J. Fleischner Society: Glossary of Terms for Thoracic Imaging. Radiology 2008, 246, 697–722. [Google Scholar] [CrossRef]
- Hewitt, M.G.; Miller, W.T.; Reilly, T.J.; Simpson, S. The Relative Frequencies of Causes of Widespread Ground-Glass Opacity: A Retrospective Cohort. Eur. J. Radiol. 2014, 83, 1970–1976. [Google Scholar] [CrossRef]
- Nemec, S.F.; Bankier, A.A.; Eisenberg, R.L. Lower Lobe—Predominant Diseases of the Lung. Am. J. Roentgenol. 2013, 200, 712–728. [Google Scholar] [CrossRef]
- Constantinescu, R.; Istrate, A.; Sumping, J.C.; Dye, C.; Schiborra, F.; Mortier, J.R. Computed Tomographic Findings in Dogs with Suspected Aspiration Pneumonia: 38 Cases (2014–2019). J. Small Anim. Pract. 2023, 64, 280–287. [Google Scholar] [CrossRef] [PubMed]
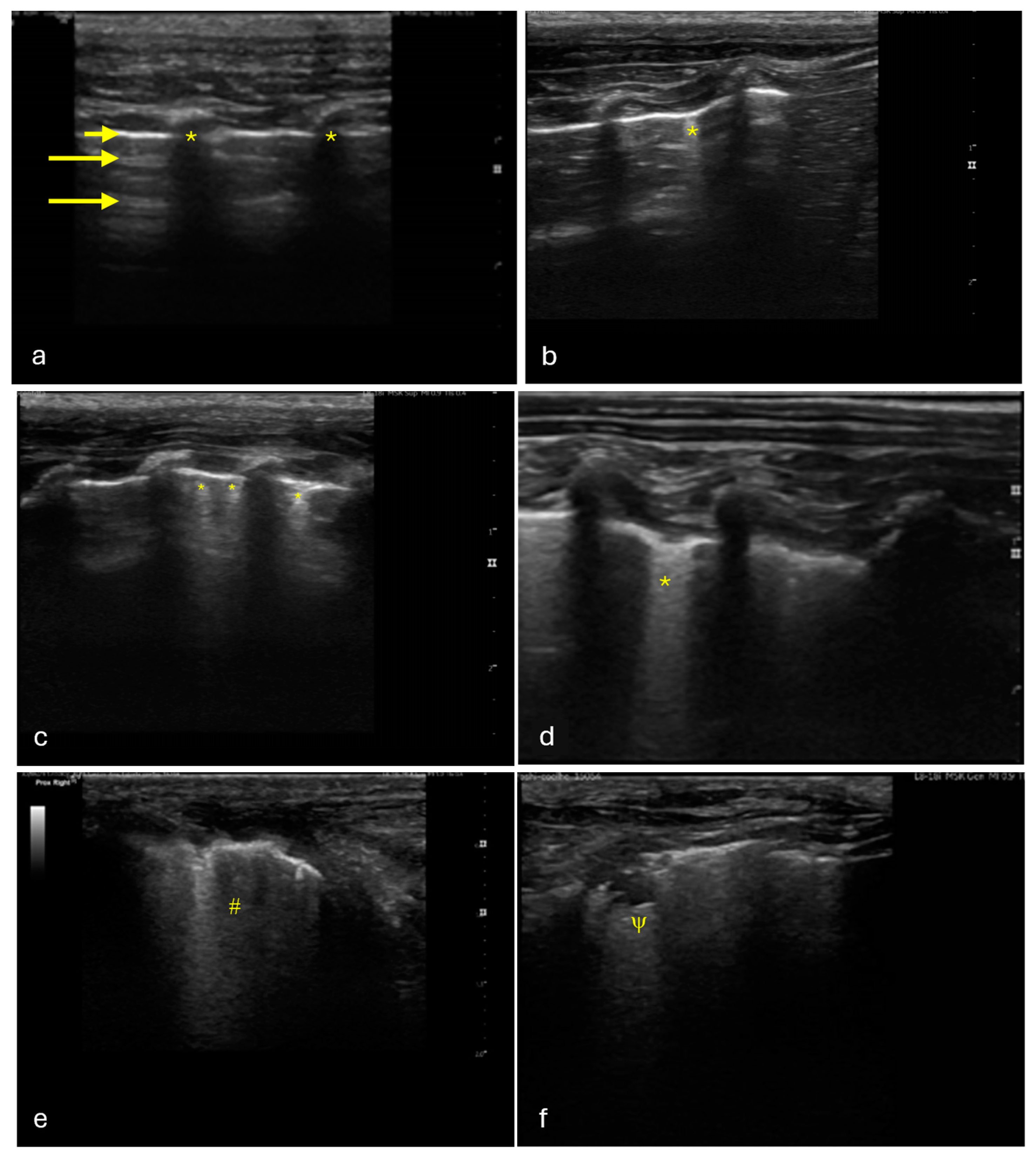
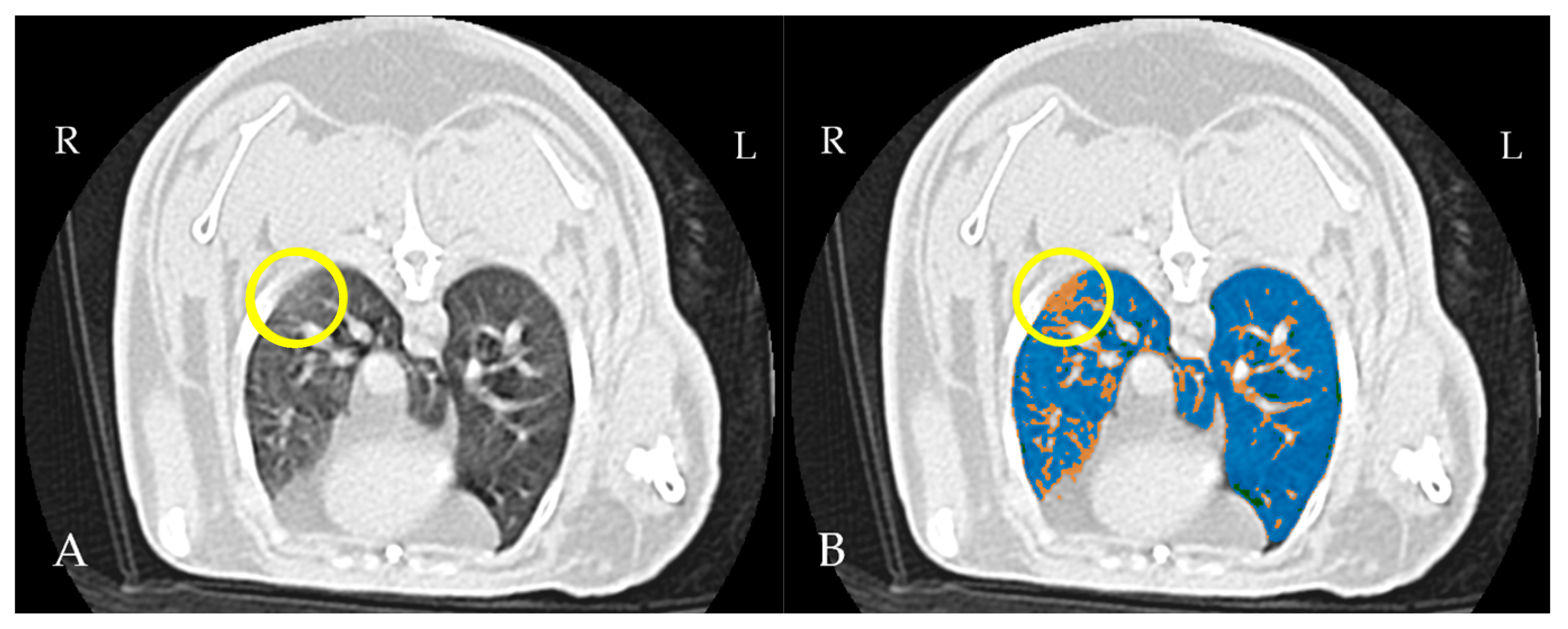
| LUS Scoring | Number of Regions | Percentage % |
|---|---|---|
| 0 | 181 | 75 |
| 1 | 14 | 6 |
| 2 | 7 | 3 |
| 3 | 29 | 12 |
| 4 | 4 | 2 |
| 5 | 5 | 2 |
| CT Scoring | Number of Regions | Percentage % |
|---|---|---|
| 0 | 135 | 56.25 |
| 1 | 102 | 42.5 |
| 2 | 3 | 1.25 |
| LUS | |||||||
|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | ||
| CT | Positive | 63 | 5 | 6 | 9 | 3 | 5 |
| Negative | 118 | 9 | 1 | 20 | 1 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sargo, R.; Tomé, I.; Silva, F.; Ginja, M. Diagnostic Accuracy of Lung Ultrasound in Rabbit Subclinical Lung Lesions. Vet. Sci. 2025, 12, 340. https://doi.org/10.3390/vetsci12040340
Sargo R, Tomé I, Silva F, Ginja M. Diagnostic Accuracy of Lung Ultrasound in Rabbit Subclinical Lung Lesions. Veterinary Sciences. 2025; 12(4):340. https://doi.org/10.3390/vetsci12040340
Chicago/Turabian StyleSargo, Roberto, Inês Tomé, Filipe Silva, and Mário Ginja. 2025. "Diagnostic Accuracy of Lung Ultrasound in Rabbit Subclinical Lung Lesions" Veterinary Sciences 12, no. 4: 340. https://doi.org/10.3390/vetsci12040340
APA StyleSargo, R., Tomé, I., Silva, F., & Ginja, M. (2025). Diagnostic Accuracy of Lung Ultrasound in Rabbit Subclinical Lung Lesions. Veterinary Sciences, 12(4), 340. https://doi.org/10.3390/vetsci12040340







