Simple Summary
This study investigated the therapeutic potential of Angelicae Pubescentis Radix (APR) in mice infected with Escherichia coli (E. coli) and found that APR treatment significantly reduced both bacterial load and intestinal injury. Serum analysis indicated that APR treatment also alleviated the inflammation and oxidative stress induced by E. coli infection. Intestinal microbiota sequencing further showed that APR treatment increased the abundance of intestinal probiotics such as Ligilactobacillus, Paludicola, and Blautia_A_1417806 while also enhancing the enrichment of functional pathways associated with antioxidant defense.
Abstract
This study aims to explore the therapeutic potential of Angelicae Pubescentis Radix (APR), a traditional Chinese medicine that is widely known for its anti-inflammatory, anti-oxidative, and anti-microbial properties, using a mouse model. In this study, 30 mice were selected and divided into three groups: control group (CD), infection group (ED), and treatment group (TD). Mice in the TD were gavaged with APR oil (0.15 mL/kg/day) for 20 days, while mice in the CD and ED received an equal volume of normal saline. On the 21st day, mice in the ED and TD were infected with multi-drug-resistant E. coli (1 × 107 CFU/mL) derived from diarrheal yak. Twenty-four hours later, all mice were euthanized, and blood, organs, and intestinal samples were collected for analysis. The results of intestinal sections and intestinal bacterial load revealed that APR treatment significantly reduced (p < 0.05) both bacterial load and intestinal injury. Serum analysis indicated that APR treatment also alleviated the inflammation and oxidative stress induced by E. coli infection. Intestinal microbiota sequencing further showed that APR treatment increased the abundance of intestinal probiotics such as Ligilactobacillus, Paludicola, and Blautia_A_1417806 while also enhancing the enrichment of functional pathways associated with antioxidant defense. In conclusion, APR treatment effectively alleviates diseases caused by E. coli infection, promotes the growth of beneficial intestinal bacteria, and improves the antioxidant capacity in animals. Additionally, these findings confirm APR’s role in addressing immediate effects rather than chronic adaptations. Future studies should investigate the prolonged effects of APR treatment beyond the acute phase.
1. Introduction
Bacterial diarrhea, primarily caused by enteropathogenic bacteria such as E. coli, Salmonella [], and Shigella [], is a major public health issue worldwide. These pathogens typically enter the human body via contaminated food or water [], disturbing the intestinal microecological balance [], triggering an inflammatory response [], and resulting in symptoms such as diarrhea, abdominal pain, fever, and others. Traditional treatments largely rely on antibiotics to rapidly control the infection by killing or inhibiting the growth of bacteria []. However, the overuse and misuse of antibiotics have led to the emergence of drug-resistant “superbugs” [] and can disrupt the natural intestinal microbiota, leading to secondary infections or chronic intestinal diseases [], such as Clostridium difficile-associated diarrhea (CDAD), which complicates treatment and increases risks []. In this regard, traditional Chinese medicine (TCM) has gained attention as a promising alternative due to its natural, safe, and relatively inexpensive characteristics [,,,]. Many studies have shown that compound Chinese herbal extracts can significantly improve immune functions [], maintain the balance of intestinal flora [], and enhance antioxidant defense, thereby reducing disease incidence [,]. However, the mechanisms of action of TCM and antibiotics differ, and with increasing bacterial resistance, blindly replacing antibiotics with Chinese medicine may not achieve the desired effects.
Angelicae Pubescentis Radix (APR), a member of the Umbelliferae family, is rich in volatile oils, coumarins, polysaccharides, and other bioactive compounds []. These compounds demonstrate significant immune-regulating, anti-inflammatory, and antioxidant effects []. Specifically, coumarin compounds, such as imperatorin, possess remarkable anti-inflammatory effects [] by inhibiting the nuclear factor kappa B (NF-κB) signaling pathway, thereby alleviating intestinal inflammatory mediators and promoting intestinal mucosal repair []. Additionally, polysaccharides in APR can activate immune cells and enhance the body’s ability to resist bacterial invasion [,]. Similarly, coumarins like columbianadin (CBN) and osthole also inhibit NF-κB activation, reducing the expression of pro-inflammatory cytokines like tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), and interleukin-1β (IL-1β) [,]. The suppression of Nucleotide-binding oligomerization domain-containing protein 1 (NOD1)/NF-κB signaling to block LPS-induced cytokine production in immune cells has been proved []. CBN inhibits Janus Kinase 2/Signal Transducer and Activator of Transcription 3 (JAK2/STAT3) phosphorylation, interrupting cytokine-derived inflammatory cascades []. Additionally, coumarins modulate Signal Transducer and Activator of Transcription (MAPK) pathways (e.g., p. 38), reducing oxidative stress and tissue damage [,]. Hence, coumarins like osthole and CBN enhance intestinal barrier function by reducing oxidative stress and inflammatory mediators’ release []. Additionally, Bergapten and umbelliferone exhibit analgesic and anti-inflammatory effects, potentially stabilizing gut epithelial cells during immune challenges [].
APR polysaccharides bind to Toll-like receptors (TLRs) and pattern recognition receptors (PRRs), activating macrophages and dendritic cells [,]. This triggers the production of cytokines, e.g., Granulocyte-Macrophage Colony-Stimulating Factor (GM-CSF), that promote hematopoiesis and immune cell differentiation, increased phagocytic activity, and antigen presentation, boosting adaptive immune response [,]. Angelica sinensis polysaccharides (ASP) activate the Wingless-related integration site/β-catenin pathway (Wnt/β-catenin), reducing oxidative injury in gut epithelial cells []. Similarly, ASP-Hb-AP (a polysaccharide variant) upregulates antioxidant enzyme expression, e.g., superoxide dismutase (SOD) and catalase (CAT), in intestinal cells, alleviating oxidative damage. Polysaccharides bind TLRs on epithelial cells, inducing anti-inflammatory cytokine release [] and also stimulating mucin (MUC2) [] production via gut microbiota, strengthening the mucosal layer [].
The gut, as the largest digestive organ in the body, plays a crucial role in overall health. The intestinal microbiota is closely associated with various physiological processes, including metabolism, immunity, and nutrient absorption []. The intestinal flora, comprising the microbiota, its metabolites, and the environment in which they exist, is fundamental to maintaining body health []. Once disrupted, it can lead to a range of diseases []. At present, over 1000 species of intestinal microbial members have been identified in the human gut []. Under normal conditions, the intestinal microbiota remains in a stable state, but an abnormal physiological condition can trigger a variety of health issues []. Additionally, this intestinal microbiota provides essential nutrients and supports the normal functioning of the intestinal barrier, playing a key role in defending against pathogens [,,]. The interaction between symbiotic bacteria, metabolites, intestinal epithelial cells, and the immune system helps to maintain the balance of the intestinal environment and serves as a natural barrier against pathogen invasion []. Therefore, in this study, E. coli was employed to induce bacterial diarrhea in model animals, and the therapeutic effects of APR were investigated from the perspectives of intestinal microecological changes, antioxidant stress, and inflammation, as well as the potential of replacing antibiotics with traditional Chinese medicine.
2. Materials and Methods
2.1. Oil Extraction
Angelicae Pubescentis Radix (1.0 kg) was purchased from a Chinese herb farm in Yichang, China. The main roots were carefully collected and shade-dried. Then, the velamina of the roots were removed, and the roots were soaked in hexyl hydride (5 L) for 10 h. The resulting extracts were concentrated at 45 °C and dried via vacuum to achieve oil with a concentration of (2 g/mL) as depicted in a previous study []. The key active ingredients in the APR oil extract include coumarins such as imperatorin, CBN, osthole, Bergapten, and umbelliferone, along with volatile oils and APR polysaccharides [,].
2.2. Animal Experiment
Thirty ICR mice (15 male and 15 female), four weeks old and weighing 24.16 ± 0.75 g, were obtained from the Experimental Animal Center at Yangzhou University. Mice were acclimatized for 3 days under controlled conditions (26 °C, 50–60% humidity, 12 h light/dark cycle). Feed and water were provided ad libitum, with environmental parameters monitored daily. Four-week-old ICR mice were selected to standardize immune and metabolic responses, as juvenile rodents are widely used in enteric infection models to ensure uniformity in pathogen susceptibility and therapeutic outcomes [,]. This age minimizes developmental variability while allowing acute observation of E. coli-induced intestinal damage and APR intervention. The feed used in the given study was purchased from Yizheng Anliu Biotechnology Co. Ltd. (Yizheng, China; standards: GB14924.1-2010, GB14924.2-2010, GB14924.3-2010) and mainly consisted of corn, wheat, soybean meal, bran, fish meal, various amino acids, and multivitamins. The feed provided was composed of ≤10% moisture, ≥19% protein, ≥4% crude protein, ≤5% crude fiber, ≤8% crude ash, 1.0–1.8% calcium, 0.6–1.2% phosphorus, with calcium to phosphorus ratio (Ca: P); 1.2:1–1.7:1, ≥0.92% lysine, and methionine + cystine ≥ 0.53%. The remaining ~48% was carbohydrate (corn) content. The feed was stored in dry, ventilated conditions at a temperature of 20–25 °C, with a relative humidity of 50–60%, in a sealed (air-tight) pack to avoid environmental exposure. The shelf life of feed was 3 months (2 months in the rainy season).
After three days of acclimatization, the mice were randomly divided into three groups: the control group (CD), the infection group (ED), and the treatment group (TD). ICR mice in the TD were gavaged with APR oil (0.15 mL/kg/day) for 20 days, while those in the CD and ED received an equal volume of normal saline. On the 21st day, the ED and TD were inoculated with multi-drug-resistant E. coli (clinical isolate PP859186, 1 × 10⁷ CFU/mL) derived from diarrheal yak []. The strain’s pathogenicity is attributed to its clinical origin and resistance profile, though serotype-specific markers (e.g., K88/K99) were not characterized. Meanwhile, mice in the CD were administered an equivalent amount of Luria–Bertani nutrient medium (Solarbio Science & Technology Co., Ltd., Beijing, China). Twenty-four hours post-inoculation, all mice were euthanized to collect blood, organs, and intestinal samples. The key parameters, such as body weight, diarrhea rate and mortality, organ index, organ bacterial loads, serum oxidation resistance, inflammation levels, and microbiota composition, were analyzed (Figure 1).
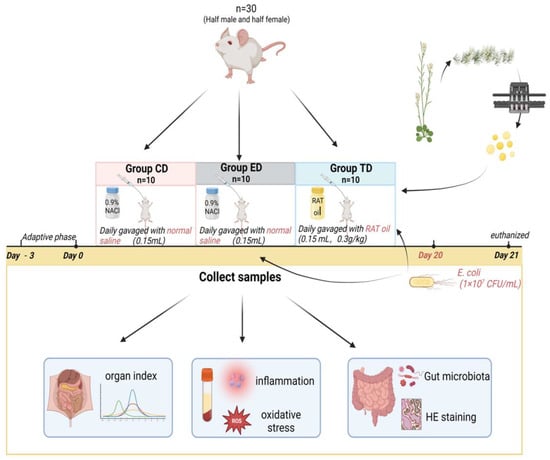
Figure 1.
Experimental design of the mice model induced by E. coli.
2.3. Pathological Analysis
Jejunum tissues (0.5–1 cm/animal) from mice in CD, TD, and ED were collected and fixed in a 4% formaldehyde solution for 48 h. The fixed samples were then sent to Wuhan Pinuofei Biological Technology (China) for hematoxylin and eosin (H&E) staining. Pathological analysis of villus integrity and damage was conducted using an Olympus CX33 microscope (Olympus, Hachioji, Tokyo, Japan), as described in a previous study [].
2.4. Examination of Antioxidant and Inflammatory Levels in Mice
Blood samples were collected and centrifuged at 3500× g for 20 min to separate the serum. The obtained serum samples were analyzed for antioxidant and inflammatory markers using commercial assay kits, including malondialdehyde (MDA), SOD, total antioxidant capacity (T-AOC), glutathione peroxidase (GSH-Px), IL-6, IL-10, TNF-α and IL-1β, sourced from Solarbio Science & Technology Co, Ltd. (Beijing, China) and Jiancheng Bioengineering Research Institute (Nanjing, China).
2.5. Microbiota Sequencing and Analysis
The total microbial DNA was extracted from rectal samples of mice in the CD (n = 5), TD (n = 5), and ED (n = 5) groups using the FastDNA Spin Kit for Feces (Beijing Think-Far Technology Co, Ltd., Beijing, China). The quality and quantity of DNA were checked via Ultramicrospectrophotometer YSNano-100 (Yeasen Biotechnology, Shanghai, China) and agarose gel electrophoresis (1.5%), following the protocol described in a previous study [,]. The V3-V4 region of the 16S rRNA gene was amplified using 338F-806R primer pairs (F: 5′-ACTCCTACGGGAGGCAGCAG-3′, R:5′-GGACTACHVGGGTWTCTAAT-3′) []. The amplified products were used to construct sequencing libraries via the Hieff NGS® OnePot II DNA Library Prep Kit for Illumina® (Yeasen, China). Microbiota sequencing was subsequently performed on the Illumina platform at Bioyi Biotechnology Co, Ltd. (Wuhan, China).
The raw sequencing data from the CD, TD, and ED underwent quality control and denoising via Divisive Amplicon Denoising Algorithm 2 (DADA2, Version: 1.26.0) []. The filtered data were used to produce amplicon sequence variants (ASVs) by blasting with the Greengenes database with ≥97% similarity and assigned taxonomy table via Quantitative Insights into Microbial Ecology (QIIME 2 Version: 2024.10) platform []. A Venn diagram was generated to visualize shared ASVs among the CD, TD, and ED []. The analysis of alpha and beta diversities of mice in different groups was conducted via the QIIME2 platform to explore the species diversity of samples and groups []. Species difference and biomarker analyses were performed via Linear discriminant effect Size (LefSe) analysis and analysis of variance (ANOVA) []. Finally, microbiota function prediction was performed using the Phylogenetic Investigation of Communities by Reconstruction of Unobserved States (PICRUSt2; Version: 2.6.) [], and the abundance of metabolic pathways among different groups was analyzed by employing the Kyoto Encyclopedia of Genes and Genomes (KEGG) and MetaCyc Metabolic Pathway (MetaCyc) databases [].
2.6. Statistical Analysis
Variances among different mice groups (CD, TD, and ED) were analyzed using ANOVA and Student’s t-test via IBM SPSS (25.0). One-way ANOVA with Tukey’s post hoc test was used for comparisons across all three groups (e.g., body weight changes, organ indices, bacterial loads across intestinal segments, antioxidant markers, inflammatory cytokines, alpha diversity indices, and taxonomic and functional pathway abundances), while Student’s t-test was applied for pairwise group comparisons (e.g., weight loss in ED vs. CD/TD, bacterial load reduction in TD vs. ED, recovery of antioxidant markers in TD vs. ED). Results are presented as means ± standard error of the mean (SEM), with statistical significance set at p < 0.05.
3. Results
3.1. Effect of APR Oil on Body Weight in E. coli-Induced Mice
Following E. coli induction, no significant differences (p ≥ 0.05) in body weight were observed among the groups (Figure 2a). However, post-infection, the ED displayed a net weight loss (~3%), while the TD and CD experienced weight gain. By the experiment’s conclusion, the CD exhibited the most substantial relative weight gain, averaging approximately 10%, while the TD showed a moderate increase of ~5%. In contrast, the ED demonstrated a significant (p < 0.05) average weight loss of ~3% (Figure 2b). These findings highlight distinct weight trends among the groups, reflecting the physiological impacts of infection and treatment. Additionally, the CD maintained a 100% survival rate throughout the experiment, while the TD had a slight reduction in survival, and the ED experienced a significant drop (p < 0.05) in survival rate by day 21 (Figure 2c).
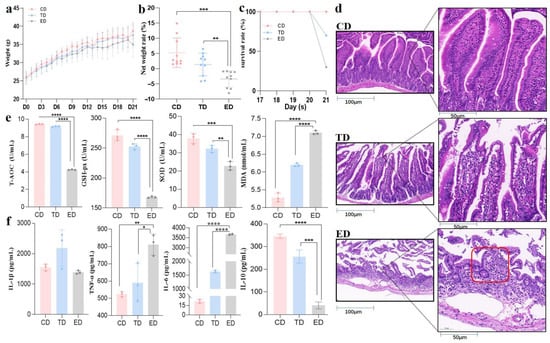
Figure 2.
APR oil regulated body weight, intestinal integrity, inflammation, and antioxidant capacity in E. coli-induced mice. (a) Body weight, (b) Net weight change on day 21, (c) Survival rate, (d) H&E staining (red circled area in group ED shows severely damaged and atrophied villi, (e) Antioxidant capacity, (f) Inflammation levels. Scale bar = 50 μm. Statistical significance is indicated as * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001. Data are presented as mean ± SEM.
The pathological examination (Figure 2d) of jejunum tissues revealed clear differences in the intestinal tissue integrity. The CD exhibited intact intestinal mucosa, a well-organized tissue structure, and complete, neatly arranged villi. In contrast, the ED showed severe intestinal damage, including inflammatory cell infiltration, disrupted tissue structure, and villi characterized by obvious shortening, aggregation, and destruction. After treatment with APR oil, the infected mice maintained relatively complete intestinal mucosa and villi, although there were some defects in the upper mucosa and other phenomena. The integrity was markedly higher compared to the ED, and the structure was close to normal.
Serum oxidative stress markers also highlighted the protective effects of APR oil. T-AOC, GSH-px, and SOD levels, which were significantly decreased (p < 0.05) in the ED, showed recovery in the TD, approaching levels observed in the CD. Conversely, MDA levels, which were elevated significantly (p < 0.05) in the ED, were slightly alleviated in the APR oil treatment group (Figure 2e). Inflammatory cytokine analysis further supported these findings. IL-6 and TNF-α levels were significantly elevated (p < 0.05) in the serum of the ED but returned to normal in the TD. Additionally, IL-10, an anti-inflammatory cytokine, was substantially higher in the TD compared to the ED, which exhibited minimal levels (Figure 2f). Therefore, these results suggest that APR oil effectively mitigates weight loss, restores intestinal integrity, and alleviates inflammation and oxidative stress in E. coli-induced mice.
3.2. Effect of APR Oil on Organ Index and Bacterial Load in E. coli-Induced Mice
After blood collection from the eyeballs, about 0.1 g of heart, liver, spleen, lung, kidney, duodenum, jejunum, ileum, cecum, and colon were weighed and placed in a sterilized 2 mL centrifuge tube, and 1 mL of normal saline and two grinding beads were added. After grinding, the supernatant was taken and diluted 10 times its original volume. The diluted 20 μL bacterial solution was inoculated into the MacConkey agar and cultured in the incubator at 37 °C for 18 h. The colony number was recorded, and the bacterial load in each organ and intestine was calculated.
The organ indices of the spleen, liver, and lungs in the ED were significantly different (p < 0.05) from those in the control group, indicating notable organ changes due to E. coli infection. In contrast, the organ indices in the APR oil treatment group were similar to those observed in the CD, suggesting that APR oil may help mitigate these changes. The bacterial load in the intestinal tract also displayed interesting trends. In the ED, the bacterial load in the duodenum, jejunum, ileum, cecum, and colon were significantly increased (p < 0.05) compared to the CD and TD. Specifically, the bacterial load in the ED was approximately 100 times higher than that in the TD, indicating a substantial increase in intestinal bacteria due to E. coli infection (Figure 3a,b).
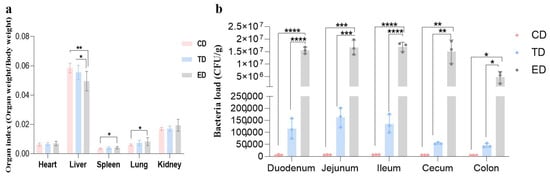
Figure 3.
Organ index and bacterial loads in different intestinal segments of mice in different groups. (a) Organ index, (b) Bacterial loads. Statistical significance is indicated as * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001. Data are presented as mean ± SEM.
3.3. Effect of APR Oil on Intestinal Flora Composition in Mice Induced by E. coli
Sequencing data revealed more than 72,000, 64,000, and 62,000 unfiltered original sequences from the CD, ED, and TD, respectively. After denoising and filtering, at least 51,000, 42,000, and 41,000 high-quality sequences were obtained from these groups (Table 1).

Table 1.
Analysis of sequencing data for different groups.
Alpha diversity analysis was employed to compare the microbial diversity within each group (Figure 4a). The Good’s coverage index ranged from 99.43 to 99.96% across the three groups, indicating that the sequencing results could reflect the entire bacterial structure. The α-diversity correlation index, a measure of species richness, was highest in the TD (1139.61 ± 406.73), followed by the ED (814.89 ± 367.2), and lowest in the CD (611.41 ± 679.61).
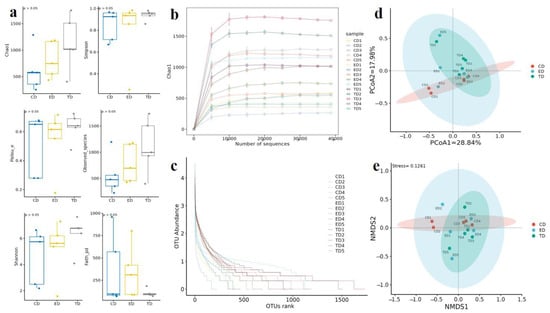
Figure 4.
Alpha and beta diversity analysis. (a) Diversity indexes, (b) Rarefaction curve, (c) Rank abundance curve, (d) Principal coordinate analysis (PCoA), (e) Non-metric multidimensional scaling (NMDS).
The Shannon index, which reflects both richness and evenness, was also highest in the TD (6.34 ± 2.24), followed by the ED (5.21 ± 3.65) and the CD (4.64 ± 2.18). The Pielou_e index, which measures evenness, ranged from 0.278 to 0.678 in the CD, 0.177–0.714 in the ED, and 0.474–0.724 in the TD. The Observed_species index, representing the number of distinct species observed, ranged from 288.1 to 1202.5 in the CD, 421.7–1174.2 in the ED, and 403.2–1734 in the TD (Table 2). Additionally, both the rarefaction curve (Figure 4b) and the rank-abundance curve (Figure 4c) approached a plateau with an increasing number of sequences, suggesting that the sequencing depth was sufficient to reflect the complete microbiota composition and maximum species diversity has been captured. However, some curves (e.g., CD1 and ED1) appear to continue increasing slightly, suggesting that additional sequencing might uncover more diversity in these samples. Overall, sequencing depth seems sufficient for robust analyses, but further sequencing could be considered for samples where saturation is not fully achieved.

Table 2.
Analysis of alpha diversity indices.
3.4. Effect of APR Oil on Intestinal Flora Composition in E. coli-Induced Mice
At the phylum level, 56 phyla were identified across the groups. Bacteroidota (CD: 28.47%, ED: 29.17%, TD: 47.29%) and Firmicutes_D (CD: 25.13%, ED: 32.93%, TD: 11.59%) were the most abundant. Campylobacterota was most abundant in the CD (32.97%), Proteobacteria dominated the ED (14.00%), and Firmicutes_A was prevalent in the TD (19.21%). Other less abundant phyla included Deferribacterota (CD: 5.72%, ED: 1.21%, TD: 0.54%), Actinobacteriota (CD: 0.48%, ED: 3.08%, TD: 1.29%), and Desulfobacterota_I (CD: 0.18%, ED: 0.46%, TD: 1.65%).
At the class level, Bacteroidia (CD: 28.47%, ED: 29.12%, TD: 47.27%) and Bacilli (CD: 25.13%, ED: 32.93%, TD: 11.59%) were the most prevalent. Campylobacteria, abundant in the CD (32.97%), was reduced in the ED (5.28%) and TD (7.27%) groups. Gammaproteobacteria was most abundant in the ED (12.85%), with lower levels in CD (0.52%) and TD (9.65%). Clostridia_258483 (CD: 4.17%, ED: 7.98%, TD: 19.20%) was present in all groups. In total, 9176 ASVs were generated, with 1880 unique to the CD, 2872 unique to the ED, and 4424 unique to the TD.
At the genus level, Ligilactobacillus was most abundant in the CD and TD (CD: 15.05%, TD: 10.86%) but scarce in the ED (2.18%). Helicobacter_C_479931 (29.34%) and Paramuribaculum (6.29%) were predominant in the CD, while Paramuribaculum (11.59%) and Helicobacter_D (8.74%) were more common in the TD. Malacoplasma_A_271108 (22.97%) was the most abundant genus in the ED. Additionally, 260 ASVs were shared across all three groups (Figure 5a). Figure 5b–f shows the top ten phyla, classes, orders, families, and genera among all three groups.
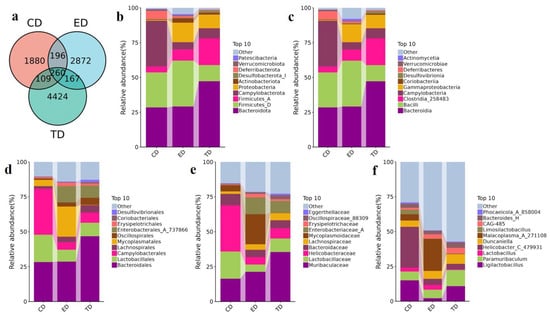
Figure 5.
Venn diagram of ASVs and APR-mediated changes in the intestinal microbiota of E. coli-induced mice in different taxa. (a) Venn diagram of ASVs, (b) Phylum, (c) Class, (d) Order, (e) Family, (f) Genus.
Then, the re-analysis demonstrated significant differences (p < 0.05) in both phylum and genus composition. Firmicutes A was notably higher in the TD (Figure 6a), while Ligilactobacillus was more abundant in the CD and TD. The TD also showed increased levels of Desulfovibrio R 446353, UBA644, Paludicola, Cupidesulfovibrio, and Blautia_A_141780, while CAG-95 and CAG-510 were more abundant in the ED (Figure 6b).
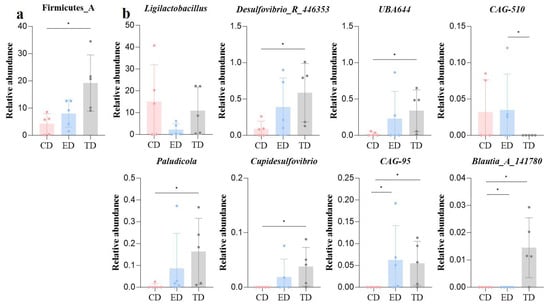
Figure 6.
Microbiota differences among mice in different groups revealed by ANOVA analysis (a) Phyla and (b) Genus. Statistical significance is indicated as * p < 0.05, Data are presented as mean ± SEM (n = 5).
3.5. Analysis of Differential Metabolic Pathways Using KEGG and MetaCyc Databases
Differential metabolic pathways were analyzed using the KEGG and MetaCyc databases. The KEGG results revealed several pathways with LDA scores greater than 2 in the CD, including aminoacyl-tRNA biosynthesis, D-glutamine and D-glutamate metabolism, D-alanine metabolism, photosynthesis, lysine biosynthesis, ribosome production, protein export, mismatch repair, and peptidoglycan biosynthesis. In contrast, the ED showed higher LDA scores in pathways such as the biosynthesis of ansamycins, African trypanosomiasis, and the degradation of valine, leucine, and isoleucine.
At LDA SCORE (log 10) in the CD, D-glutamine and D-glutamate metabolism, D-alanine metabolism, aminoacyl-tRNA biosynthesis, lysine biosynthesis in group ED; biosynthesis of ansamycins, valine, and leucine and isoleucine degradation, in group TD; pentose and glucuronate interconversions, ascorbate and aldarate metabolism, tropane, piperidine, and pyridine alkaloid biosynthesis, beta-lactam resistance, etc., related pathways were found to be significantly (p < 0.05) upregulated (Figure 7a). Similarly, MetaCyc pathway analysis indicated that in group CD, PWY-7228, PWY0-162, PWY-5100, PWY-7197, PWY-7229, in group ED; PWY-7013, glucargalactsuper-PWY, galactardeg-PWY, in group TD; PWY0-1261, PWY-6629, sulfate-CYS-PWY, FAO-PWY and PWY-7242 pathways were found to be significant (p < 0.05) expressed (Figure 7b). These findings highlight distinct metabolic shifts across the groups in response to E. coli infection and APR oil treatment (Figure 7a,b).
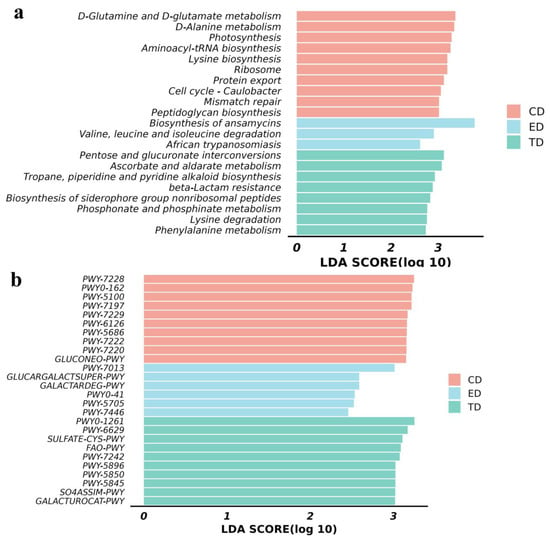
Figure 7.
Functional analysis of mice microbiota (a) KEGG pathway analysis and (b) MetaCyc pathway analysis.
4. Discussion
Our findings provide strong evidence that APR exerts protective effects in the early stages of bacterial infection by reducing oxidative stress and modulating inflammatory responses. The significant reduction in bacterial load and improvement in antioxidant markers highlight the acute therapeutic potential of APR rather than suggesting prolonged microbiota shifts. While previous studies have explored the chronic benefits of herbal treatments, our study underscores the importance of early intervention []. During the experimental period, the body weight of mice in the CD showed a fluctuating upward trend. In contrast, the weight of mice in the TD and ED decreased initially after being exposed to E. coli, though the TD exhibited a slight recovery. This suggests that APR treatment may enhance resistance to bacterial infection, reducing symptoms such as diarrhea and weight loss [].
Additionally, cytokine analysis revealed that IL-6 and TNF-α levels were elevated in the infection group but significantly reduced in the APR treatment group within 24 h, emphasizing the immediate anti-inflammatory effects of APR. This aligned with the study’s objective of assessing acute responses rather than inferring long-term immune modulation. IL-10 is a key anti-inflammatory cytokine that blocks immune cell activation and cytokine production in innate immune cell types []. Conversely, pro-inflammatory cytokines such as IL-1β and TNF-α are secreted in significant quantities during pathological conditions [,]. The experimental results showed that APR treatment significantly reduced the inflammatory response in mice post-E. coli challenge. The literature indicated that other Chinese herbs, like Licorice root, have also shown anti-inflammatory and soothing effects on the gastrointestinal tract in treating ulcers [] and promoting healing by increasing mucus production in the stomach [,].
In the antioxidant defense system, T-AOC serves as an important measure of the antioxidant capacity of bioactive substances []. The enzymes such as SOD and GSH-Px work together to convert reactive oxygen species (ROS) into harmless substances, thereby protecting cells from oxidative damage []. Additionally, MDA content is an important parameter reflecting the antioxidant capacity of the body, which can reflect the rate and intensity of lipid peroxidation in the body and also indirectly reflect the degree of tissue peroxidation damage []. The T-AOC in the ED was significantly lower than that in the control group and APR treatment group (TD). All antioxidant indexes of TD mice showed that APR treatment could significantly enhance the ability of mice to resist oxidative stress and clear free radicals; this is consistent with previous results using APR to treat rheumatoid arthritis []. Furthermore, in the given study, it was observed that the intestinal bacterial load in the APR treatment group decreased by about 100 times compared with that in the ED. This is consistent with the results of intestinal biopsies, and APR can alleviate intestinal damage caused by E. coli [].
From the alterations in intestinal microecology, Bacteroidota and Firmicutes_D are the common phyla with high abundance among the three groups. Nevertheless, the abundance of Firmicutes_A in the TD was significantly elevated. Firmicutes, mostly Gram-positive bacteria, play a crucial role in host nutrition and metabolism through the synthesis of short-chain fatty acids (SCFAs) []. At the genus level, it was found that eight genera had abundance differences. The abundance of Ligilactobacillus, UBA644, Paludicola, Cupidesulfovibrio, and Blautia_A_1417806 had all increased in the APR treatment group. Among these, Ligilactobacillus is a well-established probiotic that has been shown to have antibacterial and anti-inflammatory properties [,] and has been used to treat or relieve diarrhea, colitis, mastitis, and other conditions [,]. Paludicola has rarely been reported in recent years, but it has been confirmed that it can produce propionate []. As a short-chain fatty acid, propionate has anti-inflammatory and immune homeostasis regulation functions [] and can maintain the stability and health of intestinal microecology []. Similarly, Blautia_A_1417806 is thought to have potential probiotic properties [], having previously been found to help humans adapt to hypoxic environments [], and it has also been found to produce butyrate, which is beneficial for gut health [].
Similar results have been observed with berberin, which modulated gut microbiome by reducing the harmful bacteria and enhancing probiotic bacteria, thus increasing intestinal barrier function and metabolic health [,]. Berberine acts by directly reducing bacterial growth and modulating inflammatory pathways. Its effects on gut microbiota are well-documented, showing a significant increase in beneficial bacteria like Lactobacillus and Bifidobacterium [,]. These findings suggest that APR treatment enhances the abundance of beneficial gut microbiota, particularly SCFA-producing bacteria, which likely play a key role in alleviating inflammation and repairing intestinal damage caused by bacterial diarrhea, comparable to established results [,].
In terms of functional pathways, several significant differences were observed in the TD, including pathways related to pentose and glucuronate interconversions. Ascorbate and aldarate metabolism, tropane, piperidine, and pyridine alkaloid biosynthesis beta-lactam resistance, etc. Ascorbate metabolism, in particular, was linked to increased activity in pentose and glucuronate interconversions and D-galacturonate degradation, pathways that are known to enhance ascorbate synthesis []. Ascorbate plays a vital role in promoting iron absorption, boosting immunity, preventing scurvy, and acting as a potent antioxidant by binding ROS and converting to monodehydroascorbic acid to mitigate oxidative stress []. Additionally, pathways related to vitamin K synthesis, such as menaquinol biosynthesis, showed increased expression. The reduced form of vitamin K (vitamin K-hydroquinone, VKH2) has been identified as an antioxidant that can effectively inhibit ferroptosis []. These findings suggest that APR treatment enhances the expression of antioxidant functional pathways, thereby alleviating oxidative stress in mice. These findings suggest that APR treatment can alleviate oxidative stress in mice by increasing the expression of functional pathways in antioxidants [].
While APR exhibited acute therapeutic efficacy against E. coli-induced intestinal injury, its clinical translation needs to address its stability, bioavailability, and regulatory measures. Coumarins and polysaccharides as bioactive compounds from APR require technical formulation strategies, e.g., nanoencapsulation and enteric coatings, to increase their stability and absorption [,]. Regulatory pathways need rigorous standardization of extracts, lengthy safety assessments, and adherence to good management practices (GMPs) protocols, as exemplified by successful TCM-derived therapeutics like artemisinin [,]. Future research should prioritize pharmacokinetic studies and collaborative regulatory frameworks to bridge traditional use with modern therapeutic validation.
5. Conclusions
This study showed that APR effectively neutralizes the acute intestinal damage caused by E. coli infection in mice. APR treatment significantly reduced bacterial load and alleviated oxidative burden by T-AOC, SOD, GSH-Px, and downregulated pro-inflammatory cytokines, e.g., IL-6, TNF-α, while upregulated the anti-inflammatory IL-10. Histopathological results confirmed APR’s role in preserving intestinal mucosal integrity and villus structure. Furthermore, APR enriched the beneficial genera such as Ligilactobacillus, Paludicola, and Blautia_A_1417806, which are linked to SCFA production and antioxidant pathway activation. These findings explored the APR’s therapeutic potential for acute bacterial diarrhea, underscoring its dual role in combating oxidative stress and inflammation while modulating gut microbiota beneficially. Future investigations should explore long-term treatment effects to assess sustained benefits and broader clinical applicability.
Author Contributions
Conceptualization: K.D., X.L. and K.L.; methodology: K.D., C.X. and Q.H.; software: K.L.; validation: K.D., C.X. and Q.H.; formal analysis: K.D., C.X. and Q.H.; investigation: K.L.; resources: K.D., C.X. and Q.H.; data curation: K.D. and K.L.; writing—original draft preparation: K.L., M.S., M.N. and X.L.; writing—review and editing: K.L., M.S., M.N. and X.L.; visualization: K.L.; supervision: K.L.; project administration: K.L.; funding acquisition: X.L. and K.L. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the Yichang Science and Technology Plan Projects (A23-3-063) and the Yichang Natural Science Research Project (A24-3-032).
Institutional Review Board Statement
The study was conducted by the Institutional Review Board of the ethics committee of Nanjing Agricultural University (NJAU.No20240226021).
Informed Consent Statement
Not applicable.
Data Availability Statement
All raw sequence data from animals were deposited in the NCBI Sequence Read Archive database under accession number PRJNA1196629 (https://dataview.ncbi.nlm.nih.gov/object/PRJNA1196629; accessed on 10 December 2024).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Wang, L.-P.; Zhou, S.-X.; Wang, X.; Lu, Q.-B.; Shi, L.-S.; Ren, X.; Zhang, H.-Y.; Wang, Y.-F.; Lin, S.-H.; Zhang, C.-H.; et al. Etiological, Epidemiological, and Clinical Features of Acute Diarrhea in China. Nat. Commun. 2021, 12, 2464. [Google Scholar] [CrossRef] [PubMed]
- Lόpez-Vélez, R.; Lebens, M.; Bundy, L.; Barriga, J.; Steffen, R. Bacterial Travellers’ Diarrhoea: A Narrative Review of Literature Published over the Past 10 Years. Travel. Med. Infect. Dis. 2022, 47, 102293. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Lu, S.; Wang, J.; Xu, C.; Qu, W.; Nawaz, S.; Ataya, F.S.; Wu, Y.; Li, K. Lactobacillus salivarius and Berberine Alleviated Yak Calves’ Diarrhea via Accommodating Oxidation Resistance, Inflammatory Factors, and Intestinal Microbiota. Animals 2024, 14, 2419. [Google Scholar] [CrossRef]
- Liu, M.; Ma, J.; Xu, J.; Huangfu, W.; Zhang, Y.; Ali, Q.; Liu, B.; Li, D.; Cui, Y.; Wang, Z.; et al. Fecal Microbiota Transplantation Alleviates Intestinal Inflammatory Diarrhea Caused by Oxidative Stress and Pyroptosis via Reducing Gut Microbiota-Derived Lipopolysaccharides. Int. J. Biol. Macromol. 2024, 261, 129696. [Google Scholar] [CrossRef]
- Goodoory, V.C.; Ford, A.C. Antibiotics and Probiotics for Irritable Bowel Syndrome. Drugs 2023, 83, 687–699. [Google Scholar] [CrossRef]
- Aslam, B.; Khurshid, M.; Arshad, M.I.; Muzammil, S.; Rasool, M.; Yasmeen, N.; Shah, T.; Chaudhry, T.H.; Rasool, M.H.; Shahid, A.; et al. Antibiotic Resistance: One Health One World Outlook. Front. Cell Infect. Microbiol. 2021, 11, 771510. [Google Scholar] [CrossRef]
- Mekonnen, S.A.; Merenstein, D.; Fraser, C.M.; Marco, M.L. Molecular Mechanisms of Probiotic Prevention of Antibiotic-Associated Diarrhea. Curr. Opin. Biotechnol. 2020, 61, 226–234. [Google Scholar] [CrossRef]
- Ramirez, J.; Guarner, F.; Bustos Fernandez, L.; Maruy, A.; Sdepanian, V.L.; Cohen, H. Antibiotics as Major Disruptors of Gut Microbiota. Front. Cell Infect. Microbiol. 2020, 10, 572912. [Google Scholar] [CrossRef]
- Sun, Y.; Mao, W.; Cao, J.; Hao, P.G.; Su, J.; Yin, K.; Gu, K.; Zhao, H. Chinese Medicine Monomers Inhibit Biofilm Formation in Multidrug-Resistant Pasteurella multocida Isolated from Cattle Respiratory Infections. Pak. Vet. J. 2024, 44, 1095–1104. [Google Scholar] [CrossRef]
- Zhu, Y.; Lu, S.; Cidan, Y.; Ali, M.; Zhang, X.; Pubu, P.; Kiani, F.A.; Saleem, M.U.; Basang, W.; Li, K. Protective Effects of Traditional Chinese Herbal Medicine Formulas (TCHMFs) ViaInfluencing Anti-Oxidative Capacity, Inflammatory Mediators, and Gut Microbiota inWeaned Yaks. Pak. Vet. J. 2025; in press. [Google Scholar] [CrossRef]
- Meng, A.; Zhang, X.; Pubu, P.; Ali, M.; Wang, J.; Xu, C.; Almutairi, M.H.; Li, K. Protective Effect of Lentinan against LPS-Induced Injury in Mice Via Influencing Antioxidant Enzyme Activity, Inflammatory Pathways and Gut Microbiota. Pak. Vet. J. 2024, 44, 647–656. [Google Scholar] [CrossRef]
- Hu, J.; Sun, J.; Zhong, Q.; Chen, S.; Yin, W.; Wei, X.; Li, L.; Li, K.; Ali, M.; Sun, W.; et al. Edgeworthia gardneri (Wall.) Meisn Mitigates CCL4-Induced Liver Injury in Mice by Modulating Gut Microbiota, Boosting Antioxidant Defense, and Reducing Inflammation. Ecotoxicol. Environ. Saf. 2025, 293, 118042. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, Q.; Chen, Y.; Liang, C.-L.; Liu, H.; Qiu, F.; Dai, Z. Antitumor Effects of Immunity-Enhancing Traditional Chinese Medicine. Biomed. Pharmacother. 2020, 121, 109570. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Fu, R.; Xu, D.; Chen, Y.; Yue, S.; Zhang, S.; Tang, Y. Traditional Chinese Medicine: A Promising Strategy to Regulate the Imbalance of Bacterial Flora, Impaired Intestinal Barrier and Immune Function Attributed to Ulcerative Colitis through Intestinal Microecology. J. Ethnopharmacol. 2024, 318, 116879. [Google Scholar] [CrossRef]
- Xu, C.; Ali, M.; Sun, J.; Li, X.; Fouad, D.; Iqbal, M.; Kulyar, M.F.-E.-A.; Wu, Y.; Li, K. Protective Effects of Abrus Cantoniensis Hance against Liver Injury through Modulation of Intestinal Microbiota and Liver Metabolites. Ecotoxicol. Environ. Saf. 2024, 279, 116495. [Google Scholar] [CrossRef]
- Li, L.; Xiao, S.; Dai, X.; Tang, Z.; Wang, Y.; Ali, M.; Ataya, F.S.; Sahar, I.; Iqbal, M.; Wu, Y.; et al. Multi-Omics Analysis and the Remedial Effects of Swertiamarin on Hepatic Injuries Caused by CCl4. Ecotoxicol. Environ. Saf. 2024, 282, 116734. [Google Scholar] [CrossRef]
- Lu, Y.; Wu, H.; Yu, X.; Zhang, X.; Luo, H.; Tang, L.; Wang, Z. Traditional Chinese Medicine of Angelicae Pubescentis Radix: A Review of Phytochemistry, Pharmacology and Pharmacokinetics. Front. Pharmacol. 2020, 11, 335. [Google Scholar] [CrossRef]
- Chen, S.; Wang, Y.; Zhang, L.; Han, Y.; Liang, C.; Wang, S.; Qi, L.; Pang, X.; Li, J.; Chang, Y. Therapeutic Effects of Columbianadin from Angelicae Pubescentis Radix on the Progression of Collagen-Induced Rheumatoid Arthritis by Regulating Inflammation and Oxidative Stress. J. Ethnopharmacol. 2023, 316, 116727. [Google Scholar] [CrossRef]
- Jiang, X.; Lu, Z.; Zhang, Q.; Yu, J.; Han, D.; Liu, J.; Li, P.; Li, F. Osthole: A Potential AMPK Agonist That Inhibits NLRP3 Inflammasome Activation by Regulating Mitochondrial Homeostasis for Combating Rheumatoid Arthritis. Phytomedicine 2023, 110, 154640. [Google Scholar] [CrossRef]
- Ma, Z.; Peng, L.; Sheng, Y.; Chu, W.; Fu, Y. Anti-Inflammatory Effect of Columbianadin against D-Galactose-Induced Liver Injury In Vivo via the JAK2/STAT3 and JAK2/P38/NF-κB Pathways. Pharmaceuticals 2024, 17, 378. [Google Scholar] [CrossRef]
- Wang, D.; Liu, Y.; Zhao, W. The Adjuvant Effects on Vaccine and the Immunomodulatory Mechanisms of Polysaccharides From Traditional Chinese Medicine. Front. Mol. Biosci. 2021, 8, 655570. [Google Scholar] [CrossRef]
- Ren, C.; Luo, Y.; Li, X.; Ma, L.; Wang, C.; Zhi, X.; Zhao, X.; Li, Y. Pharmacological Action of Angelica sinensis Polysaccharides: A Review. Front. Pharmacol. 2025, 15, 1510976. [Google Scholar] [CrossRef] [PubMed]
- Dilixiati, Y.; Aipire, A.; Song, M.; Nijat, D.; Wubuli, A.; Cao, Q.; Li, J. The Potential Role of Plant Polysaccharides in Treatment of Ulcerative Colitis. Pharmaceutics 2024, 16, 1073. [Google Scholar] [CrossRef] [PubMed]
- Kuziel, G.A.; Rakoff-Nahoum, S. The Gut Microbiome. Curr. Biol. 2022, 32, R257–R264. [Google Scholar] [CrossRef]
- Brody, H. The Gut Microbiome. Nature 2020, 577, S5. [Google Scholar] [CrossRef]
- Morais, L.H.; Schreiber, H.L., IV; Mazmanian, S.K. The Gut Microbiota-Brain Axis in Behaviour and Brain Disorders. Nat. Rev. Microbiol. 2021, 19, 241–255. [Google Scholar] [CrossRef]
- Lynch, S.V.; Pedersen, O. The Human Intestinal Microbiome in Health and Disease. N. Engl. J. Med. 2016, 375, 2369–2379. [Google Scholar] [CrossRef]
- Healy, D.B.; Ryan, C.A.; Ross, R.P.; Stanton, C.; Dempsey, E.M. Clinical Implications of Preterm Infant Gut Microbiome Development. Nat. Microbiol. 2022, 7, 22–33. [Google Scholar] [CrossRef]
- Xie, Q.; Zhang, Y.; Zhang, Z.; Gong, S.; Mo, Q.; Li, J. Characteristics and Dynamic Changes of Gut Microbiota in Cats with Colitis. Pak. Vet. J. 2024, 44, 414–422. [Google Scholar] [CrossRef]
- Cidan, Y.; Lu, S.; Wang, H.; Wang, J.; Ali, M.; Fouad, D.; Ataya, F.S.; Zhu, Y.; Basang, W.; Li, K. Comparative Analysis of Microbiota in Jiani Yaks with Different Rib Structures. Life 2024, 14, 1458. [Google Scholar] [CrossRef]
- Tanes, C.; Bittinger, K.; Gao, Y.; Friedman, E.S.; Nessel, L.; Paladhi, U.R.; Chau, L.; Panfen, E.; Fischbach, M.A.; Braun, J.; et al. Role of Dietary Fiber in the Recovery of the Human Gut Microbiome and Its Metabolome. Cell Host Microbe 2021, 29, 394–407.e5. [Google Scholar] [CrossRef]
- Verdu, E.F.; Galipeau, H.J.; Jabri, B. Novel Players in Coeliac Disease Pathogenesis: Role of the Gut Microbiota. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Aly, S.H.; Eldahshan, O.A.; Al-Rashood, S.T.; Binjubair, F.A.; El Hassab, M.A.; Eldehna, W.M.; Dall’Acqua, S.; Zengin, G. Chemical Constituents, Antioxidant, and Enzyme Inhibitory Activities Supported by In-Silico Study of n-Hexane Extract and Essential Oil of Guava Leaves. Molecules 2022, 27, 8979. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Cidan, Y.; Ali, M.; Lu, S.; Javed, U.; Cisang, Z.; Gusang, D.; Danzeng, Q.; Li, K.; Basang, W. Evaluating the Effect of Dietary Protein–Energy Ratios on Yak Intestinal Microbiota Using High-Throughput 16S rRNA Gene Sequencing. Vet. Sci. 2025, 12, 208. [Google Scholar] [CrossRef]
- Stanzick, K.J.; Simon, J.; Zimmermann, M.E.; Schachtner, M.; Peterhoff, D.; Niller, H.-H.; Überla, K.; Wagner, R.; Heid, I.M.; Stark, K.J. DNA Extraction from Clotted Blood in Genotyping Quality. Biotechniques 2023, 74, 23–29. [Google Scholar] [CrossRef]
- Zhou, X.; Liu, X.; Liu, M.; Liu, W.; Xu, J.; Li, Y. Comparative Evaluation of 16S rRNA Primer Pairs in Identifying Nitrifying Guilds in Soils under Long-Term Organic Fertilization and Water Management. Front. Microbiol. 2024, 15, 1424795. [Google Scholar] [CrossRef]
- Plummer, E.; Bulach, D.; Carter, G.; Albert, M.J. Gut Microbiome of Native Arab Kuwaitis. Gut Pathog. 2020, 12, 10. [Google Scholar] [CrossRef]
- Ali, Z.; Shahzadi, I.; Majeed, A.; Malik, H.M.T.; Waseem, S.; Ahmed, I.; Anis, R.A.; Saeed, S.; Anees, M. Comparative Analysis of the Serum Microbiome of HIV Infected Individuals. Genomics 2021, 113, 4015–4021. [Google Scholar] [CrossRef]
- Ye, H.; Wen, Y.; Chen, Z.; Zhang, T.; Li, S.; Guan, M.; Zhang, Y.; Su, S. Relationship of Soil Microbiota to Seed Kernel Metabolism in Camellia oleifera Under Mulched. Front. Plant Sci. 2022, 13, 920604. [Google Scholar] [CrossRef]
- Jin, Y.; Lu, L.; Tu, W.; Luo, T.; Fu, Z. Impacts of Polystyrene Microplastic on the Gut Barrier, Microbiota and Metabolism of Mice. Sci. Total Environ. 2019, 649, 308–317. [Google Scholar] [CrossRef]
- Yu, X.; Jiang, W.; Kosik, R.O.; Song, Y.; Luo, Q.; Qiao, T.; Tong, J.; Liu, S.; Deng, C.; Qin, S.; et al. Gut Microbiota Changes and Its Potential Relations with Thyroid Carcinoma. J. Adv. Res. 2022, 35, 61–70. [Google Scholar] [CrossRef]
- Douglas, G.M.; Maffei, V.J.; Zaneveld, J.R.; Yurgel, S.N.; Brown, J.R.; Taylor, C.M.; Huttenhower, C.; Langille, M.G.I. PICRUSt2 for Prediction of Metagenome Functions. Nat. Biotechnol. 2020, 38, 685–688. [Google Scholar] [CrossRef] [PubMed]
- Luan, M.; Niu, M.; Yang, P.; Han, D.; Zhang, Y.; Li, W.; He, Q.; Zhao, Y.; Mao, B.; Chen, J.; et al. Metagenomic Sequencing Reveals Altered Gut Microbial Compositions and Gene Functions in Patients with Non-Segmental Vitiligo. BMC Microbiol. 2023, 23, 265. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Li, H. Obesity: Epidemiology, Pathophysiology, and Therapeutics. Front. Endocrinol. 2021, 12, 706978. [Google Scholar] [CrossRef] [PubMed]
- York, A.G.; Skadow, M.H.; Oh, J.; Qu, R.; Zhou, Q.D.; Hsieh, W.-Y.; Mowel, W.K.; Brewer, J.R.; Kaffe, E.; Williams, K.J.; et al. IL-10 Constrains Sphingolipid Metabolism to Limit Inflammation. Nature 2024, 627, 628–635. [Google Scholar] [CrossRef]
- Wang, Y.; Che, M.; Xin, J.; Zheng, Z.; Li, J.; Zhang, S. The Role of IL-1β and TNF-α in Intervertebral Disc Degeneration. Biomed. Pharmacother. 2020, 131, 110660. [Google Scholar] [CrossRef]
- Han, Y.; Zhang, Y.-Y.; Pan, Y.-Q.; Zheng, X.-J.; Liao, K.; Mo, H.-Y.; Sheng, H.; Wu, Q.-N.; Liu, Z.-X.; Zeng, Z.-L.; et al. IL-1β-Associated NNT Acetylation Orchestrates Iron-Sulfur Cluster Maintenance and Cancer Immunotherapy Resistance. Mol. Cell 2023, 83, 1887–1902.e8. [Google Scholar] [CrossRef]
- Yin, S.; You, T.; Tang, J.; Wang, L.; Jia, G.; Liu, G.; Tian, G.; Chen, X.; Cai, J.; Kang, B.; et al. Dietary Licorice Flavonoids Powder Improves Serum Antioxidant Capacity and Immune Organ Inflammatory Responses in Weaned Piglets. Front. Vet. Sci. 2022, 9, 942253. [Google Scholar] [CrossRef]
- Zhang, Q.; Huang, H.; Qiu, M.; Wu, Z.; Xin, Z.; Cai, X.; Shang, Q.; Lin, J.; Zhang, D.; Han, L. Traditional Uses, Pharmacological Effects, and Molecular Mechanisms of Licorice in Potential Therapy of COVID-19. Front. Pharmacol. 2021, 12, 719758. [Google Scholar] [CrossRef]
- Hu, W.; He, Z.; Du, L.; Zhang, L.; Li, J.; Ma, Y.; Bi, S. Biomarkers of Oxidative Stress in Broiler Chickens Attacked by Lipopolysaccharide: A Systematic Review and Meta-Analysis. Ecotoxicol. Environ. Saf. 2023, 266, 115606. [Google Scholar] [CrossRef]
- Zhou, Y.; Jia, Z.; Wang, J.; Huang, S.; Yang, S.; Xiao, S.; Xia, D.; Zhou, Y. Curcumin Reverses Erastin-Induced Chondrocyte Ferroptosis by Upregulating Nrf2. Heliyon 2023, 9, e20163. [Google Scholar] [CrossRef]
- Attia, M.M.; Soliman, S.M.; Mahmoud, M.A.; Salem, M.A. Oxidative Stress Markers, Immune-Regulating Cytokines, and the Pathological Evaluation of Sheep Co-Infected with Oestrus ovis and Coenuruscerebralis. Microb. Pathog. 2022, 169, 105613. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Ni, Y.; Wang, Z.; Tu, W.; Ni, L.; Zhuge, F.; Zheng, A.; Hu, L.; Zhao, Y.; Zheng, L.; et al. Spermidine Improves Gut Barrier Integrity and Gut Microbiota Function in Diet-Induced Obese Mice. Gut Microbes 2020, 12, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Chuandong, Z.; Hu, J.; Li, J.; Wu, Y.; Wu, C.; Lai, G.; Shen, H.; Wu, F.; Tao, C.; Liu, S.; et al. Distribution and Roles of Ligilactobacillus murinus in Hosts. Microbiol. Res. 2024, 282, 127648. [Google Scholar] [CrossRef] [PubMed]
- He, T.; Hu, X.; Mi, J.; Hu, H.; Wang, H.; Qi, X.; Gao, L.; Zhang, Y.; Liu, C.; Wang, S.; et al. Ligilactobacillus salivarius XP132 with Antibacterial and Immunomodulatory Activities Inhibits Horizontal and Vertical Transmission of Salmonella pullorum in Chickens. Poult. Sci. 2024, 103, 104086. [Google Scholar] [CrossRef]
- Lukasik, J.; Dierikx, T.; Besseling-van der Vaart, I.; de Meij, T.; Szajewska, H. Multispecies Probiotic for the Prevention of Antibiotic-Associated Diarrhea in Children: A Randomized Clinical Trial. JAMA Pediatr. 2022, 176, 860–866. [Google Scholar] [CrossRef]
- Yan, S.; Yu, L.; Tian, F.; Zhao, J.; Chen, W.; Chen, H.; Zhai, Q. Ligilactobacillus salivarius CCFM 1266 Modulates Gut Microbiota and GPR109a-Mediated Immune Suppression to Attenuate Immune Checkpoint Blockade-Induced Colitis. Food Funct. 2023, 14, 10549–10563. [Google Scholar] [CrossRef]
- Akasaka, H.; Ueki, A.; Hanada, S.; Kamagata, Y.; Ueki, K. Propionicimonas paludicola Gen. Nov., Sp. Nov., a Novel Facultatively Anaerobic, Gram-Positive, Propionate-Producing Bacterium Isolated from Plant Residue in Irrigated Rice-Field Soil. Int. J. Syst. Evol. Microbiol. 2003, 53, 1991–1998. [Google Scholar] [CrossRef]
- Duan, H.; Wang, L.; Huangfu, M.; Li, H. The Impact of Microbiota-Derived Short-Chain Fatty Acids on Macrophage Activities in Disease: Mechanisms and Therapeutic Potentials. Biomed. Pharmacother. 2023, 165, 115276. [Google Scholar] [CrossRef]
- Yang, S.; Shang, J.; Liu, L.; Tang, Z.; Meng, X. Strains Producing Different Short-Chain Fatty Acids Alleviate DSS-Induced Ulcerative Colitis by Regulating Intestinal Microecology. Food Funct. 2022, 13, 12156–12169. [Google Scholar] [CrossRef]
- Liu, X.; Mao, B.; Gu, J.; Wu, J.; Cui, S.; Wang, G.; Zhao, J.; Zhang, H.; Chen, W. Blautia-a New Functional Genus with Potential Probiotic Properties? Gut Microbes 2021, 13, 1–21. [Google Scholar] [CrossRef]
- Su, Q.; Zhuang, D.-H.; Li, Y.-C.; Chen, Y.; Wang, X.-Y.; Ge, M.-X.; Xue, T.-Y.; Zhang, Q.-Y.; Liu, X.-Y.; Yin, F.-Q.; et al. Gut Microbiota Contributes to High-Altitude Hypoxia Acclimatization of Human Populations. Genome Biol. 2024, 25, 232. [Google Scholar] [CrossRef] [PubMed]
- Bernard, R.; Shilts, M.H.; Strickland, B.A.; Boone, H.H.; Payne, D.C.; Brown, R.F.; Edwards, K.; Das, S.R.; Nicholson, M.R. The Relationship between the Intestinal Microbiome and Body Mass Index in Children with Cystic Fibrosis. J. Cyst. Fibros. 2024, 23, 242–251. [Google Scholar] [CrossRef] [PubMed]
- Luo, T.; Che, Q.; Guo, Z.; Song, T.; Zhao, J.; Xu, D. Modulatory Effects of Traditional Chinese Medicines on Gut Microbiota and the Microbiota-Gut-x Axis. Front. Pharmacol. 2024, 15, 1442854. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Gao, X.; Bai, H.; Ning, K. Traditional Chinese Medicine and Gut Microbiome: Their Respective and Concert Effects on Healthcare. Front. Pharmacol. 2020, 11, 538. [Google Scholar] [CrossRef]
- Fujii, J.; Osaki, T.; Bo, T. Ascorbate Is a Primary Antioxidant in Mammals. Molecules 2022, 27, 6187. [Google Scholar] [CrossRef]
- Kaźmierczak-Barańska, J.; Boguszewska, K.; Adamus-Grabicka, A.; Karwowski, B.T. Two Faces of Vitamin C-Antioxidative and Pro-Oxidative Agent. Nutrients 2020, 12, 1501. [Google Scholar] [CrossRef]
- Mishima, E.; Ito, J.; Wu, Z.; Nakamura, T.; Wahida, A.; Doll, S.; Tonnus, W.; Nepachalovich, P.; Eggenhofer, E.; Aldrovandi, M.; et al. A Non-Canonical Vitamin K Cycle Is a Potent Ferroptosis Suppressor. Nature 2022, 608, 778–783. [Google Scholar] [CrossRef]
- Wei, X.; Wen, Y.; Wei, Y.; Liang, X.; Ma, X.; Zhang, B.; Tang, X. External Therapy of Traditional Chinese Medicine for Treating Irritable Bowel Syndrome with Diarrhea: A Systematic Review and Meta-Analysis. Front. Med. 2022, 9, 940328. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).