Effects of Chicken Protein Hydrolysate as a Protein Source to Partially Replace Chicken Meal on Gut Health, Gut Microbial Structure, and Metabolite Composition in Cats
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Experimental Treatments
2.3. Molecular Weight Range of Polypeptides
2.4. Fecal Sample Collection
2.5. Fecal Scoring
2.6. Fecal Gases
2.7. Fecal Calprotectin
2.8. Fecal Microbiota Sequencing
2.9. Fecal Non-Targeted Metabolomics
2.10. Statistical Analysis
3. Results
3.1. Molecular Weight Range of Polypeptides
3.2. Fecal Scoring
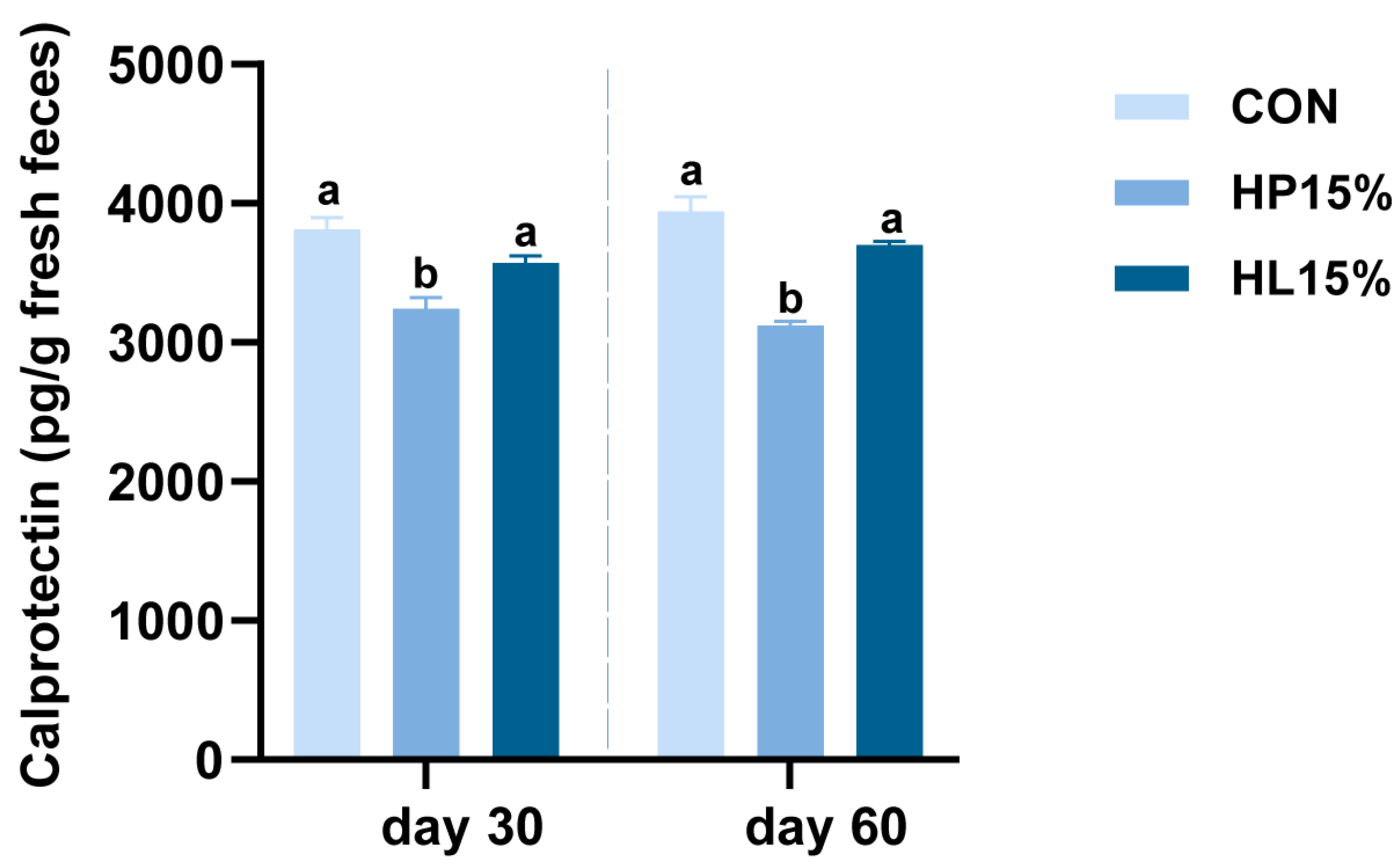
3.3. Fecal Calprotectin
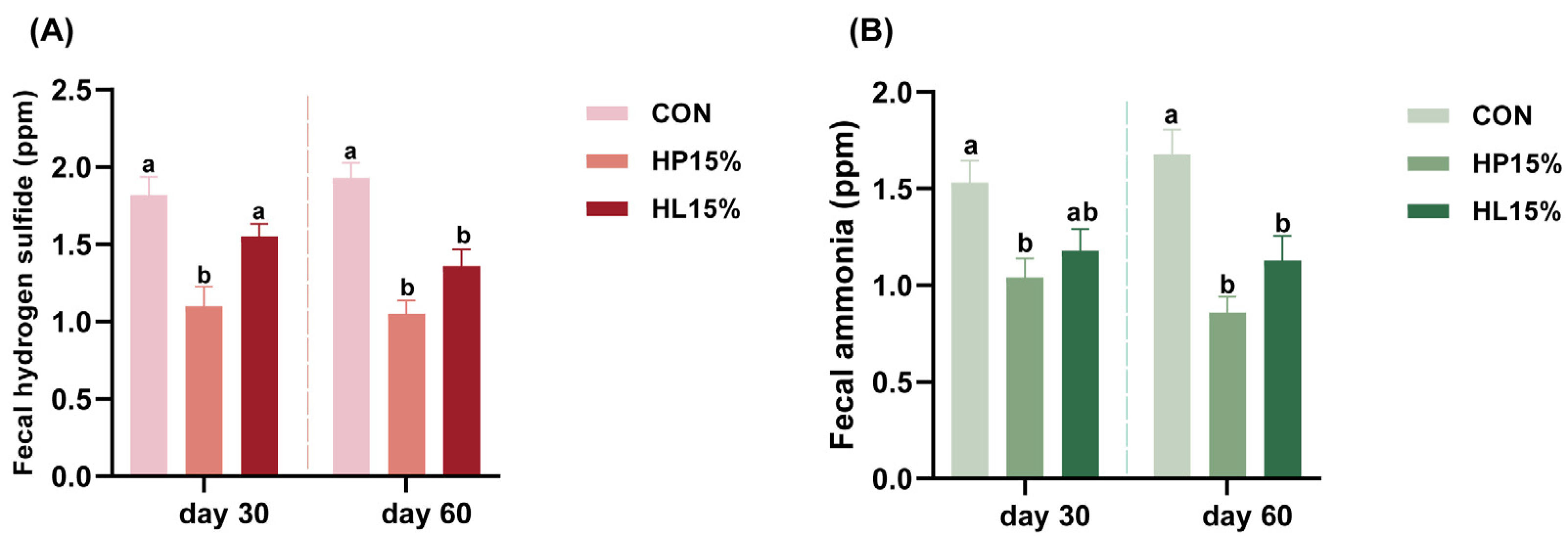
3.4. Fecal Gases Emissions
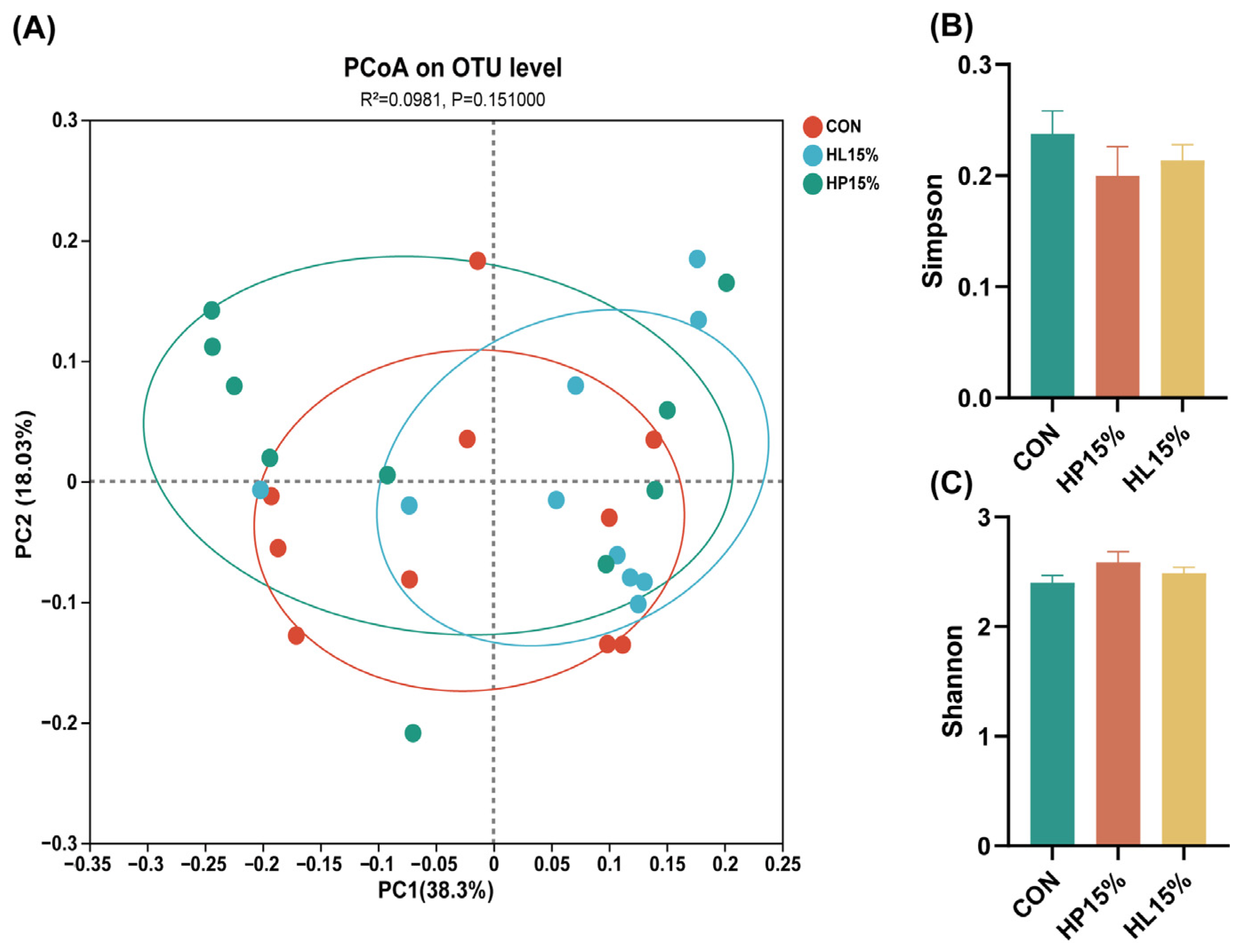
3.5. The Richness and Diversity of Fecal Microbiota
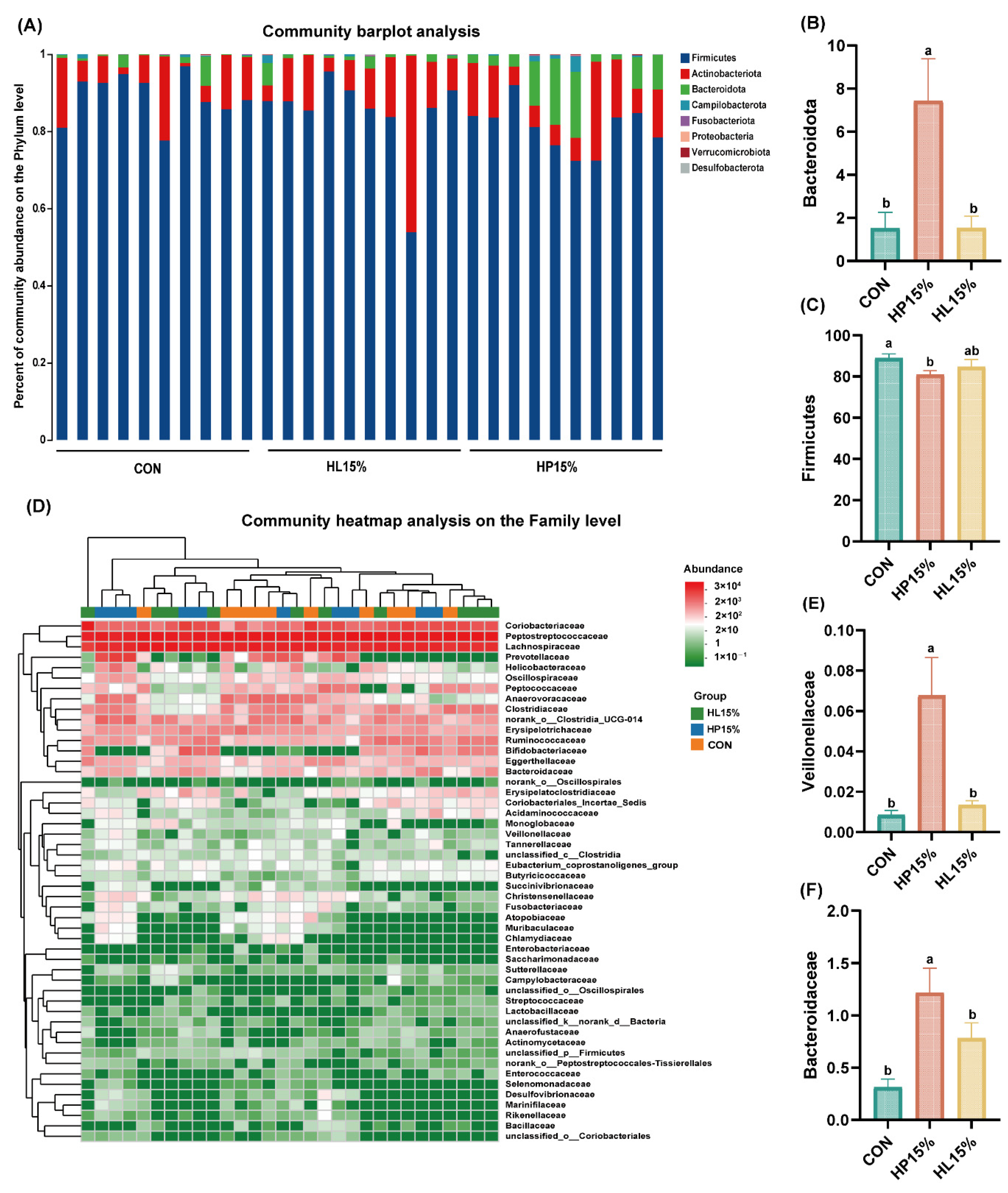
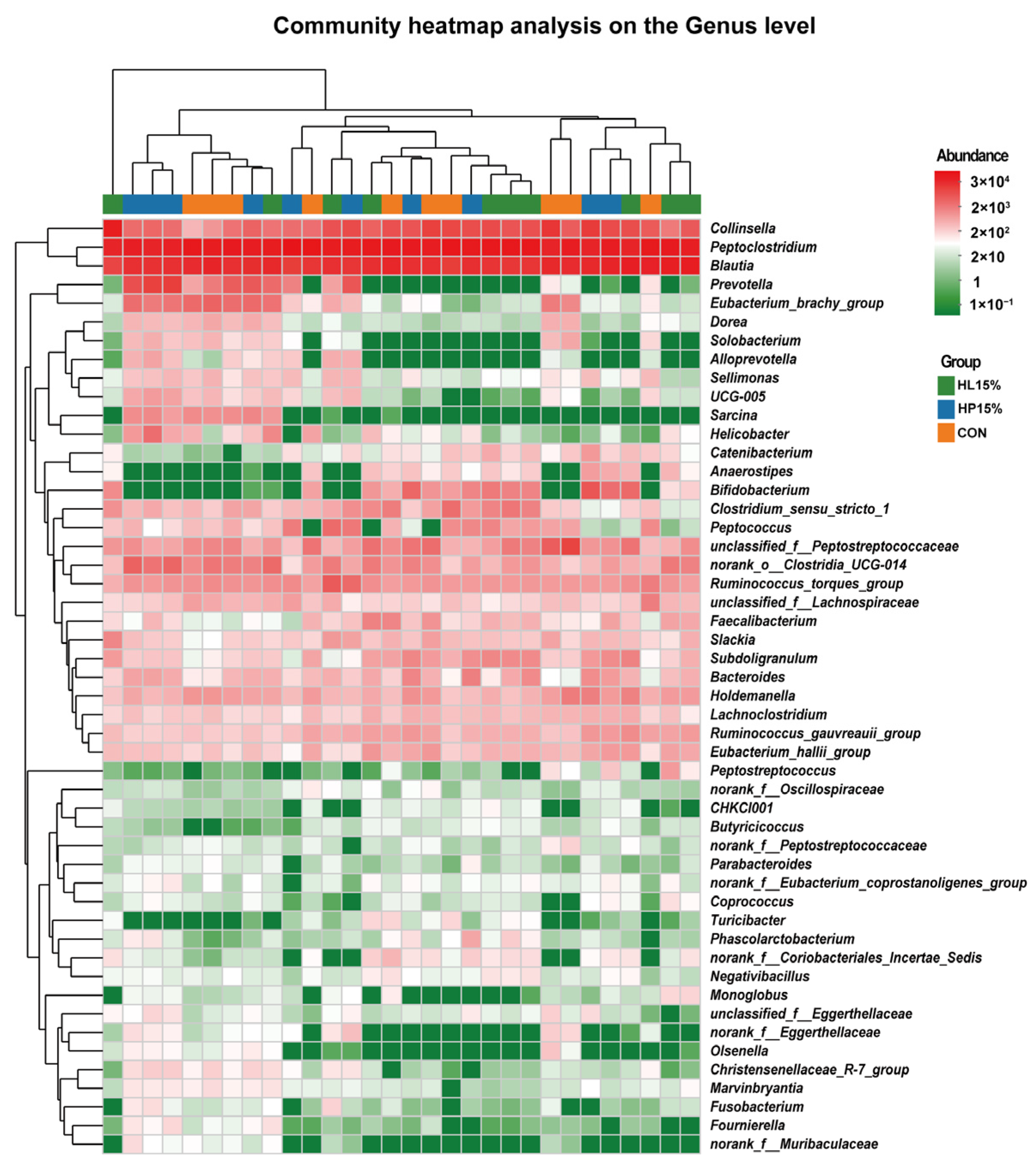
3.6. Fecal Microbiota Composition
3.7. Fecal Metabolomic Composition
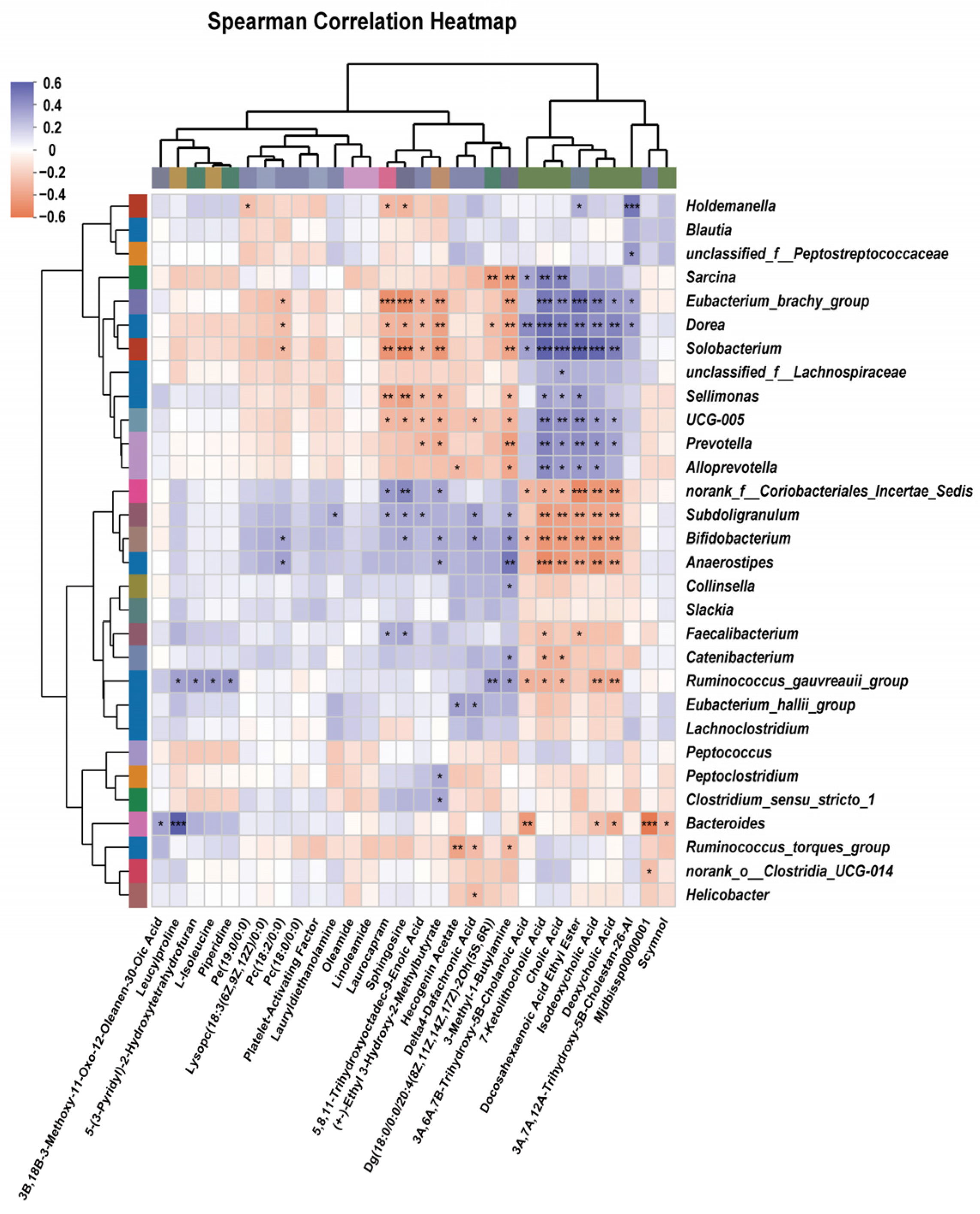
3.8. Correlation Analysis Between Genus-Level Gut Microbiota and Differential Metabolites
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vinassa, M.; Vergnano, D.; Valle, E.; Giribaldi, M.; Nery, J.; Prola, L.; Bergero, D.; Schiavone, A. Profiling Italian Cat and Dog Owners’ Perceptions of Pet Food Quality Traits. BMC Vet. Res. 2020, 16, 131. [Google Scholar] [CrossRef] [PubMed]
- Zha, M.; Zhu, S.; Chen, Y. Probiotics and Cat Health: A Review of Progress and Prospects. Microorganisms 2024, 12, 1080. [Google Scholar] [CrossRef] [PubMed]
- Maturana, M.; Castillejos, L.; Martin-Orue, S.M.; Minel, A.; Chetty, O.; Felix, A.P.; Adib Lesaux, A. Potential Benefits of Yeast Saccharomyces and Their Derivatives in Dogs and Cats: A Review. Front. Vet. Sci. 2023, 10, 1279506. [Google Scholar] [CrossRef] [PubMed]
- Beaubier, S.; Pineda-Vadillo, C.; Mesieres, O.; Framboisier, X.; Galet, O.; Kapel, R. Improving the in Vitro Digestibility of Rapeseed Albumins Resistant to Gastrointestinal Proteolysis While Preserving the Functional Properties Using Enzymatic Hydrolysis. Food Chem. 2023, 407, 135132. [Google Scholar] [CrossRef]
- Casas, G.A.; Jaworski, N.W.; Htoo, J.K.; Stein, H.H. Ileal Digestibility of Amino Acids in Selected Feed Ingredients Fed to Young Growing Pigs. J. Anim. Sci. 2018, 96, 2361–2370. [Google Scholar] [CrossRef]
- Zhang, X.; Li, S.; Li, M.; Hemar, Y. Study of the in Vitro Properties of Oligopeptides from Whey Protein Isolate with High Fisher’s Ratio and Their Ability to Prevent Allergic Response to β-Lactoglobulin in Vivo. Food Chem. 2023, 405, 134841. [Google Scholar] [CrossRef]
- Cole, A.S.; Eastoe, J.E. Chapter 5-Peptides and Proteins. In Biochemistry and Oral Biology, 2nd ed.; Cole, A.S., Eastoe, J.E., Eds.; Butterworth-Heinemann: Oxford, UK, 1988; pp. 44–70. ISBN 978-0-7236-1751-8. [Google Scholar]
- Apostolopoulos, V.; Bojarska, J.; Chai, T.-T.; Elnagdy, S.; Kaczmarek, K.; Matsoukas, J.; New, R.; Parang, K.; Lopez, O.P.; Parhiz, H.; et al. A Global Review on Short Peptides: Frontiers and Perspectives. Molecules 2021, 26, 430. [Google Scholar] [CrossRef]
- Deb-Choudhury, S.; Bermingham, E.N.; Young, W.; Barnett, M.P.G.; Knowles, S.O.; Harland, D.; Clerens, S.; Dyer, J.M. The Effects of a Wool Hydrolysate on Short-Chain Fatty Acid Production and Fecal Microbial Composition in the Domestic Cat (Felis catus). Food Funct. 2018, 9, 4107–4121. [Google Scholar] [CrossRef]
- Li, Y.; Liu, M.; Wei, Y.; Li, L.; Ma, D.; Weng, Y.; Wang, H.; Xu, X. Influence of a Mixture of Protein Hydrolysate from Black Soldier Fly Larvae and Schizochytrium on Palatability, Plasma Biochemistry, and Antioxidative and Anti-Inflammatory Capacity in Cat Diets. Animals 2024, 14, 751. [Google Scholar] [CrossRef]
- Chelakkot, C.; Ghim, J.; Ryu, S.H. Mechanisms Regulating Intestinal Barrier Integrity and Its Pathological Implications. Exp. Mol. Med. 2018, 50, 1–9. [Google Scholar] [CrossRef]
- Thomas, S.; Izard, J.; Walsh, E.; Batich, K.; Chongsathidkiet, P.; Clarke, G.; Sela, D.A.; Muller, A.J.; Mullin, J.M.; Albert, K.; et al. The Host Microbiome Regulates and Maintains Human Health: A Primer and Perspective for Non-Microbiologists. Cancer Res. 2017, 77, 1783–1812. [Google Scholar] [CrossRef] [PubMed]
- Bengmark, S. Gut Microbiota, Immune Development and Function. Pharmacol. Res. 2013, 69, 87–113. [Google Scholar] [CrossRef]
- Luo, W.; Zhao, M.; Dwidar, M.; Gao, Y.; Xiang, L.; Wu, X.; Medema, M.H.; Xu, S.; Li, X.; Schäfer, H.; et al. Microbial Assimilatory Sulfate Reduction-Mediated H2S: An Overlooked Role in Crohn’s Disease Development. Microbiome 2024, 12, 152. [Google Scholar] [CrossRef] [PubMed]
- Bugrov, N.; Rudenko, P.; Lutsay, V.; Gurina, R.; Zharov, A.; Khairova, N.; Molchanova, M.; Krotova, E.; Shopinskaya, M.; Bolshakova, M.; et al. Fecal Microbiota Analysis in Cats with Intestinal Dysbiosis of Varying Severity. Pathogens 2022, 11, 234. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Li, D.; Zhang, M.; Liu, P.; Wang, H.; Li, Y.; Wu, Y. The Effects of Dietary Saccharomyces Cerevisiae Supplementation on Gut Microbiota Composition and Gut Health in Aged Labrador Retrievers. Animals 2024, 14, 1713. [Google Scholar] [CrossRef]
- Albenberg, L.G.; Wu, G.D. Diet and the Intestinal Microbiome: Associations, Functions, and Implications for Health and Disease. Gastroenterology 2014, 146, 1564–1572. [Google Scholar] [CrossRef]
- Hsu, C.; Marx, F.; Guldenpfennig, R.; Valizadegan, N.; de Godoy, M.R.C. The Effects of Hydrolyzed Protein on Macronutrient Digestibility, Fecal Metabolites and Microbiota, Oxidative Stress and Inflammatory Biomarkers, and Skin and Coat Quality in Adult Dogs. J. Anim. Sci. 2024, 102, skae057. [Google Scholar] [CrossRef]
- Bai, M.; Liu, H.; Yan, Y.; Duan, S.; Szeto, I.M.-Y.; He, J.; Hu, J.; Fu, Y.; Xu, K.; Xiong, X. Hydrolyzed Protein Formula Improves the Nutritional Tolerance by Increasing Intestinal Development and Altering Cecal Microbiota in Low-Birth-Weight Piglets. Front. Nutr. 2024, 11, 1439110. [Google Scholar] [CrossRef]
- Wang, X.; Lu, S.; Fang, Z.; Wang, H.; Zhu, J.; Zhao, J.; Zhang, H.; Hong, K.; Lu, W.; Chen, W. A Recommended Amount of Hydrolyzed Protein Improves Physiological Function by Regulating Gut Microbiota in Aged Mice. Food Res. Int. 2022, 154, 110970. [Google Scholar] [CrossRef]
- Zou, F.; Wang, X.; Glitza Oliva, I.C.; McQuade, J.L.; Wang, J.; Zhang, H.C.; Thompson, J.A.; Thomas, A.S.; Wang, Y. Fecal Calprotectin Concentration to Assess Endoscopic and Histologic Remission in Patients with Cancer with Immune-Mediated Diarrhea and Colitis. J. Immunother. Cancer 2021, 9, e002058. [Google Scholar] [CrossRef]
- Grellet, A.; Heilmann, R.M.; Lecoindre, P.; Feugier, A.; Day, M.J.; Peeters, D.; Freiche, V.; Hernandez, J.; Grandjean, D.; Suchodolski, J.S.; et al. Fecal Calprotectin Concentrations in Adult Dogs with Chronic Diarrhea. Am. J. Vet. Res. 2013, 74, 706–711. [Google Scholar] [CrossRef] [PubMed]
- Deng, P.; Swanson, K.S. Gut Microbiota of Humans, Dogs and Cats: Current Knowledge and Future Opportunities and Challenges. Br. J. Nutr. 2015, 113, S6–S17. [Google Scholar] [CrossRef] [PubMed]
- Atuahene, D.; Costale, A.; Martello, E.; Mannelli, A.; Radice, E.; Ribaldone, D.G.; Chiofalo, B.; Stefanon, B.; Meineri, G. A Supplement with Bromelain, Lentinula Edodes, and Quercetin: Antioxidant Capacity and Effects on Morphofunctional and Fecal Parameters (Calprotectin, Cortisol, and Intestinal Fermentation Products) in Kennel Dogs. Vet. Sci. 2023, 10, 486. [Google Scholar] [CrossRef]
- Riggers, D.S.; Xenoulis, P.G.; Karra, D.A.; Enderle, L.L.; Köller, G.; Böttcher, D.; Steiner, J.M.; Heilmann, R.M. Fecal Calprotectin Concentrations in Cats with Chronic Enteropathies. Vet. Sci. 2023, 10, 419. [Google Scholar] [CrossRef]
- Su, Q.; Tun, H.M.; Liu, Q.; Yeoh, Y.K.; Mak, J.W.Y.; Chan, F.K.; Ng, S.C. Gut Microbiome Signatures Reflect Different Subtypes of Irritable Bowel Syndrome. Gut Microbes 2023, 15, 2157697. [Google Scholar] [CrossRef] [PubMed]
- Pitcher, M.C.; Cummings, J.H. Hydrogen Sulphide: A Bacterial Toxin in Ulcerative Colitis? Gut 1996, 39, 1–4. [Google Scholar] [CrossRef]
- Arriaga, H.; Salcedo, G.; Martínez-Suller, L.; Calsamiglia, S.; Merino, P. Effect of Dietary Crude Protein Modification on Ammonia and Nitrous Oxide Concentration on a Tie-Stall Dairy Barn Floor. J. Dairy Sci. 2010, 93, 3158–3165. [Google Scholar] [CrossRef]
- Wang, H.; Shen, J.; Pi, Y.; Gao, K.; Zhu, W. Low-Protein Diets Supplemented with Casein Hydrolysate Favor the Microbiota and Enhance the Mucosal Humoral Immunity in the Colon of Pigs. J. Anim. Sci. Biotechnol. 2019, 10, 79. [Google Scholar] [CrossRef]
- Jakhar, D.; Sarin, S.K.; Kaur, S. Gut Microbiota and Dynamics of Ammonia Metabolism in Liver Disease. npj Gut Liver 2024, 1, 11. [Google Scholar] [CrossRef]
- Birg, A.; Lin, H.C. The Role of Bacteria-Derived Hydrogen Sulfide in Multiple Axes of Disease. Int. J. Mol. Sci. 2025, 26, 3340. [Google Scholar] [CrossRef]
- Wernimont, S.M.; Radosevich, J.; Jackson, M.I.; Ephraim, E.; Badri, D.V.; MacLeay, J.M.; Jewell, D.E.; Suchodolski, J.S. The Effects of Nutrition on the Gastrointestinal Microbiome of Cats and Dogs: Impact on Health and Disease. Front. Microbiol. 2020, 11, 1266. [Google Scholar] [CrossRef] [PubMed]
- Drut, A.; Mkaouar, H.; Kriaa, A.; Mariaule, V.; Akermi, N.; Méric, T.; Sénécat, O.; Maguin, E.; Hernandez, J.; Rhimi, M. Gut Microbiota in Cats with Inflammatory Bowel Disease and Low-Grade Intestinal T-Cell Lymphoma. Front. Microbiol. 2024, 15, 1346639. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, M.L.; Rogers, Q.R.; Morris, J.G. Nutrition of the Domestic Cat, a Mammalian Carnivore. Annu. Rev. Nutr. 1984, 4, 521–562. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Mei, X.; Wang, Q.; Zhao, P.; Zhou, Y.; Tang, L.; Wang, B.; Xu, S.; Li, X.; Jin, Q.; et al. Compound Bacillus Alleviates Diarrhea by Regulating Gut Microbes, Metabolites, and Inflammatory Responses in Pet Cats. Anim. Microbiome 2023, 5, 49. [Google Scholar] [CrossRef]
- Bai, H.; Liu, T.; Wang, S.; Gong, W.; Shen, L.; Zhang, S.; Wang, Z. Identification of Gut Microbiome and Metabolites Associated with Acute Diarrhea in Cats. Microbiol. Spectr. 2023, 11, e0059023. [Google Scholar] [CrossRef]
- Miller, J.; Żebrowska-Różańska, P.; Czajkowska, A.; Szponar, B.; Kumala-Ćwikła, A.; Chmielarz, M.; Łaczmański, Ł. Faecal Microbiota and Fatty Acids in Feline Chronic Enteropathy. BMC Vet. Res. 2023, 19, 281. [Google Scholar] [CrossRef]
- Cui, Y.; Zhang, M.; Wang, H.; Yu, T.; Zhang, A.; Lin, G.; Guo, Y.; Wu, Y. Organic Trace Minerals Enhance the Gut Health of British Shorthair Cats by Regulating the Structure of Intestinal Microbiota. Metabolites 2024, 14, 494. [Google Scholar] [CrossRef]
- Roediger, W.E. Utilization of Nutrients by Isolated Epithelial Cells of the Rat Colon. Gastroenterology 1982, 83, 424–429. [Google Scholar] [CrossRef]
- Verbrugghe, A.; Hesta, M.; Daminet, S.; Polis, I.; Holst, J.J.; Buyse, J.; Wuyts, B.; Janssens, G.P.J. Propionate Absorbed from the Colon Acts as Gluconeogenic Substrate in a Strict Carnivore, the Domestic Cat (Felis catus). J. Anim. Physiol. Anim. Nutr. 2012, 96, 1054–1064. [Google Scholar] [CrossRef]
- Verbrugghe, A.; Hesta, M.; Gommeren, K.; Daminet, S.; Wuyts, B.; Buyse, J.; Janssens, G.P.J. Oligofructose and Inulin Modulate Glucose and Amino Acid Metabolism through Propionate Production in Normal-Weight and Obese Cats. Br. J. Nutr. 2009, 102, 694–702. [Google Scholar] [CrossRef]
- Garcia-Mazcorro, J.F.; Barcenas-Walls, J.R.; Suchodolski, J.S.; Steiner, J.M. Molecular Assessment of the Fecal Microbiota in Healthy Cats and Dogs before and during Supplementation with Fructo-Oligosaccharides (FOS) and Inulin Using High-Throughput 454-Pyrosequencing. PeerJ 2017, 5, e3184. [Google Scholar] [CrossRef] [PubMed]
- Ephraim, E.; Jewell, D.E. Effect of Nutrition on Age-Related Metabolic Markers and the Gut Microbiota in Cats. Microorganisms 2021, 9, 2430. [Google Scholar] [CrossRef]
- Suchodolski, J.S.; Foster, M.L.; Sohail, M.U.; Leutenegger, C.; Queen, E.V.; Steiner, J.M.; Marks, S.L. The Fecal Microbiome in Cats with Diarrhea. PLoS ONE 2015, 10, e0127378. [Google Scholar] [CrossRef] [PubMed]
- Ye, S.; Yan, F.; Wang, H.; Mo, X.; Liu, J.; Zhang, Y.; Li, H.; Chen, D. Diversity Analysis of Gut Microbiota between Healthy Controls and Those with Atopic Dermatitis in a Chinese Population. J. Dermatol. 2021, 48, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Luise, D.; Le Sciellour, M.; Buchet, A.; Resmond, R.; Clement, C.; Rossignol, M.-N.; Jardet, D.; Zemb, O.; Belloc, C.; Merlot, E. The Fecal Microbiota of Piglets during Weaning Transition and Its Association with Piglet Growth across Various Farm Environments. PLoS ONE 2021, 16, e0250655. [Google Scholar] [CrossRef]
- Zhu, S.; Guo, Z.; Liu, L.; Gao, Y.; Bai, L.; Chen, Y.; Zha, M. Complex Probiotics Relieve Constipation Through Regulation of the Intestinal Microbiota in Kittens. Microorganisms 2025, 13, 563. [Google Scholar] [CrossRef]
- Mohebali, N.; Weigel, M.; Hain, T.; Sütel, M.; Bull, J.; Kreikemeyer, B.; Breitrück, A. Faecalibacterium Prausnitzii, Bacteroides Faecis and Roseburia Intestinalis Attenuate Clinical Symptoms of Experimental Colitis by Regulating Treg/Th17 Cell Balance and Intestinal Barrier Integrity. Biomed. Pharmacother. 2023, 167, 115568. [Google Scholar] [CrossRef]
- Honneffer, J.B.; Minamoto, Y.; Suchodolski, J.S. Microbiota Alterations in Acute and Chronic Gastrointestinal Inflammation of Cats and Dogs. World J. Gastroenterol. 2014, 20, 16489–16497. [Google Scholar] [CrossRef]
- Schroeder, B.O.; Birchenough, G.M.H.; Ståhlman, M.; Arike, L.; Johansson, M.E.V.; Hansson, G.C.; Bäckhed, F. Bifidobacteria or Fiber Protect against Diet-Induced Microbiota-Mediated Colonic Mucus Deterioration. Cell Host Microbe 2018, 23, 27–40.e7. [Google Scholar] [CrossRef]
- Summers, S.; Quimby, J.; Gagné, J.; Lappin, M. The Effect of Dietary Protein Concentration on the Fecal Microbiome and Serum Concentrations of Gut-Derived Uremic Toxins in Healthy Adult Cats. Vet. Sci. 2023, 10, 497. [Google Scholar] [CrossRef]
- Brennan, L.; de Roos, B. Role of Metabolomics in the Delivery of Precision Nutrition. Redox Biol. 2023, 65, 102808. [Google Scholar] [CrossRef] [PubMed]
- Rael, L.T.; Bar-Or, R.; Banton, K.L.; Mains, C.W.; Roshon, M.; Tanner, A.H.; Lieser, M.J.; Acuna, D.L.; Bar-Or, D. The Anti-Inflammatory Effect of LMWF5A and N-Acetyl Kynurenine on Macrophages: Involvement of Aryl Hydrocarbon Receptor in Mechanism of Action. Biochem. Biophys. Rep. 2018, 15, 61–67. [Google Scholar] [CrossRef]
- Purushothaman, A.; Sheeja, A.A.; Janardanan, D. Hydroxyl Radical Scavenging Activity of Melatonin and Its Related Indolamines. Free Radic. Res. 2020, 54, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Martin, F.-P.; Cominetti, O.; Berger, B.; Combremont, S.; Marquis, J.; Xie, G.; Jia, W.; Pinto-Sanchez, M.I.; Bercik, P.; Bergonzelli, G. Metabolome-Associated Psychological Comorbidities Improvement in Irritable Bowel Syndrome Patients Receiving a Probiotic. Gut Microbes 2024, 16, 2347715. [Google Scholar] [CrossRef]
- Aust, A.-C.; Vidova, V.; Coufalikova, K.; Smetanova, S.; Kozeluhova, K.; Micenkova, L.; Videnska, P.; Smatana, S.; Budinska, E.; Borek, I.; et al. Fecal Tryptophan Metabolite Profiling in Newborns in Relation to Microbiota and Antibiotic Treatment. Appl. Microbiol. Biotechnol. 2024, 108, 504. [Google Scholar] [CrossRef]
- Dahabiyeh, L.A.; Nimer, R.M.; Wells, J.D.; Abu-Rish, E.Y.; Fiehn, O. Diagnosing Parkinson’s Disease and Monitoring Its Progression: Biomarkers from Combined GC-TOF MS and LC-MS/MS Untargeted Metabolomics. Heliyon 2024, 10, e30452. [Google Scholar] [CrossRef]
- Chiang, J.Y.L.; Ferrell, J.M. Up to Date on Cholesterol 7 Alpha-Hydroxylase (CYP7A1) in Bile Acid Synthesis. Liver Res. 2020, 4, 47–63. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Jiao, T.; Liu, W.; Luo, Y.; Wang, J.; Guo, X.; Tong, X.; Lin, Z.; Sun, C.; Wang, K.; et al. Hepatic Cytochrome P450 8B1 and Cholic Acid Potentiate Intestinal Epithelial Injury in Colitis by Suppressing Intestinal Stem Cell Renewal. Cell Stem Cell 2022, 29, 1366–1381. [Google Scholar] [CrossRef]
- Sung, C.-H.; Pilla, R.; Marsilio, S.; Chow, B.; Zornow, K.A.; Slovak, J.E.; Lidbury, J.A.; Steiner, J.M.; Hill, S.L.; Suchodolski, J.S. Fecal Concentrations of Long-Chain Fatty Acids, Sterols, and Unconjugated Bile Acids in Cats with Chronic Enteropathy. Animals 2023, 13, 2753. [Google Scholar] [CrossRef]
- Guo, Q.; Chen, L.; Liu, Z.; Zheng, B. Chlorogenic Acid/Linoleic Acid-Fortified Wheat-Resistant Starch Ameliorates High-Fat Diet-Induced Gut Barrier Damage by Modulating Gut Metabolism. J. Agric. Food Chem. 2024, 72, 11759–11772. [Google Scholar] [CrossRef]
- Chen, C.; Xu, L.; Xia, W.; Qi, C.; Liu, J.; Hua, B.; Zhang, Z.; Qi, X.; Miao, M. Metabolomics and Transcription Profiling of Pumpkin Fruit Reveals Enhanced Bioactive Flavonoids and Coumarins in Giant Pumpkin (Cucurbita maxima). J. Agric. Food Chem. 2023, 71, 10459–10469. [Google Scholar] [CrossRef]
- Baldi, S.; Tristán Asensi, M.; Pallecchi, M.; Sofi, F.; Bartolucci, G.; Amedei, A. Interplay between Lignans and Gut Microbiota: Nutritional, Functional and Methodological Aspects. Molecules 2023, 28, 343. [Google Scholar] [CrossRef]
- Adolphe, J.L.; Whiting, S.J.; Juurlink, B.H.J.; Thorpe, L.U.; Alcorn, J. Health Effects with Consumption of the Flax Lignan Secoisolariciresinol Diglucoside. Br. J. Nutr. 2010, 103, 929–938. [Google Scholar] [CrossRef]
- Liu, G.; Li, J.; Li, Y.; Hu, Y.; Franke, A.A.; Liang, L.; Hu, F.B.; Chan, A.T.; Mukamal, K.J.; Rimm, E.B.; et al. Gut Microbiota-Derived Metabolites and Risk of Coronary Artery Disease: A Prospective Study among US Men and Women. Am. J. Clin. Nutr. 2021, 114, 238–247. [Google Scholar] [CrossRef] [PubMed]
- Mali, A.V.; Padhye, S.B.; Anant, S.; Hegde, M.V.; Kadam, S.S. Anticancer and Antimetastatic Potential of Enterolactone: Clinical, Preclinical and Mechanistic Perspectives. Eur. J. Pharmacol. 2019, 852, 107–124. [Google Scholar] [CrossRef] [PubMed]
- Petrenko, V.; Sinturel, F.; Loizides-Mangold, U.; Montoya, J.P.; Chera, S.; Riezman, H.; Dibner, C. Type 2 Diabetes Disrupts Circadian Orchestration of Lipid Metabolism and Membrane Fluidity in Human Pancreatic Islets. PLoS Biol. 2022, 20, e3001725. [Google Scholar] [CrossRef] [PubMed]
- Petrenko, V.; Sinturel, F.; Riezman, H.; Dibner, C. Lipid Metabolism around the Body Clocks. Prog. Lipid Res. 2023, 91, 101235. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Cheng, X.; Zhen, W.; Zeng, D.; Qu, L.; Wang, Z.; Ning, Z. Yeast Culture Improves Egg Quality and Reproductive Performance of Aged Breeder Layers by Regulating Gut Microbes. Front. Microbiol. 2021, 12, 633276. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, L.; Guo, F.; Liu, X.; Li, X.; Han, Z.; Li, X.; Shi, X.; Wen, L.; Wang, J. Metabolomic and Transcriptomic Analysis of Effects of Three MUFA-Rich Oils on Hepatic Glucose and Lipid Metabolism in Mice. Mol. Nutr. Food Res. 2023, 67, 2300398. [Google Scholar] [CrossRef]
- Membrez, M.; Migliavacca, E.; Christen, S.; Yaku, K.; Trieu, J.; Lee, A.K.; Morandini, F.; Giner, M.P.; Stiner, J.; Makarov, M.V.; et al. Trigonelline Is an NAD+Precursor That Improves Muscle Function during Ageing and Is Reduced in Human Sarcopenia. Nat. Metab. 2024, 6, 433. [Google Scholar] [CrossRef] [PubMed]
- Olofinsan, K.; Abrahamse, H.; George, B.P. Therapeutic Role of Alkaloids and Alkaloid Derivatives in Cancer Management. Molecules 2023, 28, 5578. [Google Scholar] [CrossRef] [PubMed]
- Kelly, B.; Pearce, E.L. Amino Assets: How Amino Acids Support Immunity. Cell Metab. 2020, 32, 154–175. [Google Scholar] [CrossRef] [PubMed]
- Ravindran, R.; Loebbermann, J.; Nakaya, H.I.; Khan, N.; Ma, H.; Gama, L.; Machiah, D.K.; Lawson, B.; Hakimpour, P.; Wang, Y.-C.; et al. The Amino Acid Sensor GCN2 Controls Gut Inflammation by Inhibiting Inflammasome Activation. Nature 2016, 531, 523–527. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Li, L.; Lai, P.; Wei, Y.; Lai, C.; Liu, Y.; Yang, M.; Zhou, S.; Chen, J.; Sun, J. Effects of Different Drying Methods on Physicochemical Properties and Nutritional Quality of Abalone Bioactive Peptides. Molecules 2025, 30, 1516. [Google Scholar] [CrossRef]


| Items | CON Group | HP15% | HL15% |
|---|---|---|---|
| Ingredient, % | |||
| Chicken meal | 22.86 | 10.93 | 9.94 |
| Chicken hydrolyzed liquid | — | — | 15.00 |
| Chicken hydrolyzed powder | — | 15.00 | — |
| Duck meal | 15.90 | 15.90 | 15.90 |
| Rice | 21.86 | 20.87 | 21.86 |
| Peeled pea | 19.87 | 19.87 | 19.87 |
| Premix 1 | 6.59 | 6.59 | 6.59 |
| Flavor enhancers 2 | 2.98 | 2.98 | 2.98 |
| Chicken fat | 9.94 | 7.95 | 7.95 |
| Total | 100 | 100 | 100 |
| Nutrient level, % | |||
| Dry matter | 91.62 | 91.79 | 91.24 |
| Crude fat | 15.57 | 16.37 | 15.68 |
| Crude ash | 4.82 | 4.42 | 4.39 |
| Crude protein | 37.55 | 37.40 | 37.09 |
| Crude fiber | 2.67 | 2.60 | 2.63 |
| Groups | >10 kda (%) | Mn | Mw |
|---|---|---|---|
| Chicken meal | 47.37 | 16,748 | 17,605 |
| HP | 3.04 | 14,079 | 15,056 |
| HL | 10.21 | 14,207 | 14,997 |
| Sampling Date | CON | HP15% | HL15% | SEM | p-Value |
|---|---|---|---|---|---|
| Day 0 | 2.55 | 2.30 | 2.35 | 0.13 | 0.63 |
| Day 30 | 2.70 | 2.40 | 2.35 | 0.10 | 0.39 |
| Day 60 | 2.70 | 2.55 | 2.20 | 0.12 | 0.26 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, T.; Humbert, F.; Li, D.; Savarin, A.; Zhang, M.; Cui, Y.; Wang, H.; Dong, T.; Wu, Y. Effects of Chicken Protein Hydrolysate as a Protein Source to Partially Replace Chicken Meal on Gut Health, Gut Microbial Structure, and Metabolite Composition in Cats. Vet. Sci. 2025, 12, 388. https://doi.org/10.3390/vetsci12040388
Yu T, Humbert F, Li D, Savarin A, Zhang M, Cui Y, Wang H, Dong T, Wu Y. Effects of Chicken Protein Hydrolysate as a Protein Source to Partially Replace Chicken Meal on Gut Health, Gut Microbial Structure, and Metabolite Composition in Cats. Veterinary Sciences. 2025; 12(4):388. https://doi.org/10.3390/vetsci12040388
Chicago/Turabian StyleYu, Tong, Fabian Humbert, Dan Li, Arnaud Savarin, Mingrui Zhang, Yingyue Cui, Haotian Wang, Tianyu Dong, and Yi Wu. 2025. "Effects of Chicken Protein Hydrolysate as a Protein Source to Partially Replace Chicken Meal on Gut Health, Gut Microbial Structure, and Metabolite Composition in Cats" Veterinary Sciences 12, no. 4: 388. https://doi.org/10.3390/vetsci12040388
APA StyleYu, T., Humbert, F., Li, D., Savarin, A., Zhang, M., Cui, Y., Wang, H., Dong, T., & Wu, Y. (2025). Effects of Chicken Protein Hydrolysate as a Protein Source to Partially Replace Chicken Meal on Gut Health, Gut Microbial Structure, and Metabolite Composition in Cats. Veterinary Sciences, 12(4), 388. https://doi.org/10.3390/vetsci12040388










