Simple Summary
Worldwide, the animal kingdom contains a largely untapped resource of unknown bacteria that could significantly contribute to the health and safety of human and animal well-being. Specifically, free-ranging species of animals harbor diverse, yet-to-be-discovered bacteria that may have potentially useful applications. Across species, analyzing and categorizing microbes and their genomic components will increase the discovery of potential antibacterial and anti-fungal applications. These applications include use as new probiotics, that is, microbes that contribute to an animal’s gastroenteric health. During this investigation, a member of the Carnobacteria was discovered in the gastrointestinal tract of a North American Gray Wolf. These bacteria have known anti-listeria properties and may help support the gut health of animals. Genotypically, the bacterial isolate was demonstrated to be a unique member of the Carnobacteria. This is the first report of these types of bacteria isolated from any member of the genus Canis, including dogs and their progenitor, the Gray wolf. Because only a small percentage of bacteria have been discovered, this investigation also further supports the vital need to protect the biodiversity of our planet.
Abstract
We hypothesize that bacteria isolated from free-ranging animals could potentially be useful for practical applications. To meet this objective a Gram-positive bacterium was isolated from the gastrointestinal (GI) tract of a Gray Wolf (Canis lupus) using Brucella broth with hemin and vitamin K (BBHK). By small ribosomal RNA (16S) gene sequencing the bacterium was initially identified as a novel Carnobacterium maltaromaticum strain. The bacterium could be propagated both anaerobically and aerobically and was both catalase/oxidase negative and negative by the starch hydrolysis as well as negative using lipase assays. The reference whole genome sequence (WGS) was obtained using both Illumina and Nanopore sequencing. The genome assembly was 3,512,202 bp in length, encoding core bacterial genes with a GC% content of 34.48. No lysogenic bacteriophage genes were detected, although the genome harbors genes for the expression of bacteriocin and other secondary metabolites with potential antimicrobial properties. Multilocus sequence typing (MLST), WGS phylogenetics, average nucleotide identity (ANI), and single nucleotide polymorphism (SNP) analyses of the isolate’s genome indicate this bacterium is a newly identified Carnobacterium maltaromaticum sequence type (ST). Members of the Carnobacteria have anti-listeria activities, highlighting their potential functional properties. Consequently, the isolate could be a potential probiotic for canids and this is the first report on an axenic C. maltaromaticum culture from the genus Canis.
1. Introduction
Lactic acid bacteria (LAB) are a group of Gram-positive, non-sporulating, facultative anaerobic bacteria that include at least 25 genera utilized during food production and preservation [1,2]. The bacteria produce bioactive compounds [3], and many LAB are commonly applied as probiotics to help maintain gastrointestinal equilibrium and health [4,5]. Among the LAB are Carnobacteria, which have potential probiotic and bioprotective attributes encoding antimicrobials active against various pathogens, including Listeria monocytogenes and other pathogenic Gram-positive bacteria [4,6]. Consequently, these bacteria have been investigated for applications such as biopreservatives of foods because they produce antimicrobial peptides such as carnocyclin and piscicolin reviewed in [7,8].
Probiotics are defined by the Food and Agriculture Organization of the United Nations and the WHO (FAO/WHO) as “live microorganisms which when administered in adequate amounts confer a health benefit on the host” [5]. There are a wide variety of microorganisms classified as probiotics that include both bacteria and yeasts. Members of the probiotic bacteria include organisms such as Lactobacillus spp., Lacticaseibacillus spp., Bifidobacterium spp., Enterococcus spp., Streptococcus spp., Bacillus spp., and types of yeast such as Saccharomyces cerevisiae var. boulardii [9,10,11]. Not only have probiotics been used for humans, but they have also been developed for use in companion [12,13,14,15] and free-ranging animals [16]. The development of new probiotic microbial strains includes concerns of safety and efficacy that can potentially be resolved by using whole genome sequencing (WGS) to determine potential virulence and antibiotic resistances along with delineating genus and species identity [17].
Members of the Carnobacterium spp. are found in various ecological niches, including foods, animal organs, feces, and different natural environments [4,6]. One of the first discoveries of Carnobacterium spp. as a potential probiotic was its use to reduce bacterial pathogenesis in rainbow trout [18,19,20]. Using a colorectal epithelial cell line, HT29, it was subsequently demonstrated that bacteriocin-producing Carnobacteria strains reduced Listeria monocytogenes invasion of eukaryotic cells [21]. Although there have been investigations to reduce bacterial disease in aquaculture using Carnobacteria [22,23], very little research has been conducted among monogastric animals other than chickens [24,25]. Our research hypothesis and objectives involve investigating free-ranging animals as a source of new potential probiotic bacteria [26,27,28]. Herein, we describe the isolation and characterization of a Carnobacterium maltaromaticum unique sequence type (ST) from a North American Gray wolf. This is the first report of a Carnobacteria isolated from any member of the genus Canis, and based on genomic analyses, the bacterium may have potential probiotic properties.
2. Materials and Methods
2.1. Isolation of Bacteria and Phenotypic Characterization
Bacterial isolation was made from the ileum of a deceased North American Gray Wolf (Canis lupus) that had been killed when hit by a car (Oregon Veterinary Diagnostic Laboratory, Corvallis, OR; OVDL case 20V15449) [https://vetmed.oregonstate.edu/ovdl] (accessed on 13 November 2024). Briefly, several bacteria were isolated from the ileum of the wolf’s digestive material as described in previous publications [26,28]. Initial bacterial isolations were made using brucella broth agar with hemin and vitamin K (BBHK) at 37 °C in an anaerobe chamber using a Thermo Scientific™ AnaeroPack™ 2.5L Rectangular Container with sachets [29]. Subsequently, a bacterial isolate designated ClWan1 was propagated anaerobically and aerobically on brain heart infusion (BHI) agar. Characterizing the isolate using basic bacteriologic assays such as Gram stains and starch hydrolysis, with catalase, lipase, and oxidase assays were completed via standard techniques [30,31].
2.2. Bacterial Genomic DNA Isolation and Whole Genome Sequencing
The bacterial genomic DNA was purified from ClWan1 3 mL cultures in BHI (Illustra Nucleic Acid PurificationTM system, Cytiva, Marlborough, MA, USA) as described [26,28]. Purified bacterial genomic DNA was quantified by a fluorescence-based Qubit dsDNA system (ThermoFisher, Waltham, MA, USA). Genome sequencing was completed at EzBiome using the NEBNext® Ultra™ II FS DNA library kit for an Illumina library, while the v14 library prep chemistry without fragmentation or size selection for the Nanopore sequencing. Genome sequences were obtained by Illumina NextSeq2000 (2 × 150 bp) and an R10.4.1 flow cell of a Nanopore PromethION (Eugene, OR, USA).
Filtering of sequencing reads was completed using Filtlong v0.2.1 min_length 1000 keep_percent 95 [https://github.com/rrwick/Filtlong] (accessed on 13 November 2024) by removing the 5% worst FASTQ reads. Flye v2.9.2 was used to assemble sequences with Nanopore reads [32]. Draft Illumina reads were aligned [33] to produce polished assemblies [34] with CheckM to detect any contamination [35]. Genomes were annotated with Prokka v1.14.6 [36], and GenoVi v0.4.3 was used to generate circular genome maps [37]. BAGEL4 [38] was used to identify bacteriocin genes and other ribosomally synthesized and post-translationally modified peptides (RiPPs) encoded in the genome. The antiSMASH program [39] was used to search for genes potentially encoding bioactive compounds, such as antimicrobials synthesized by the isolate. The PHAge Search Tool with Enhanced Sequence Translation or PHASTEST [40] was employed to search the genome for putative prophage sequences.
Using a pre-built database [41] composed of NCBI’s National Database of Antibiotic-Resistant Organisms (NDARO, www.ncbi.nlm.nih.gov/pathogens/antimicrobial-resistance/) (accessed on 15 November 2024), reference genes were used to generate a putative antibiotic resistance profile. SAMtools mpileup script [42] was used to calculate the depth and coverage of genes, and virulence factor-encoding genes were identified using a pre-built bowtie2 [43] database composed of reference factors obtained from the Virulence Factors of Pathogenic Bacteria (VFDB) database [44]. Multilocus Sequence Typing (MLST) was conducted using mlst (https://github.com/tseemann/mlst, accessed on 19 December 2024), which relies on the PubMLST website (https://pubmlst.org/, accessed on 19 December 2024) that contains data for diversity among C. maltaromaticum [45].
2.3. Phylogenetic Analyses of the Wolf Bacterial Isolate
The first assay for determining the ClWan1 species identification was utilizing the 16S rRNA gene via SpeciesFinder 2.0 [46]. Subsequently, bacterial core genes were extracted from the Up-to-date Bacterial Core Genes (UBCG) system [47]. Core genes were concatenated and aligned with MAFFT v7.508 [48] using the G-INS-i strategy, and phylogenies were constructed with 1000 bootstrap replicates using RAxML-NG v. 1.1.0 [49]. OrthoANIu [50] was used to calculate average nucleotide identity (ANI) values, and a neighbor-joining tree was generated from the ANI values using the “ape” R library [51]. Reference and the ClWan1 genomes were analyzed for single nucleotide polymorphism (SNP) comparisons using parsnp [52], and a matrix was produced using a custom script [41]. The genome was also submitted to the Type (Strain) Genome Server (TYGS) in tandem with the List of Prokaryotic names coupled to the Standing in Nomenclature (LPSN) [53], followed by preparing phylogenetic trees in MEGA12 [54] to confirm phylogenetic relationships among other bacteria.
3. Results
3.1. Bacterial Isolation and Phenotypic Characterization
Bacterial isolates were plated on BBHK and then incubated anaerobically for 48 hr. One isolate designated ClWan1 was characterized as a rod-shaped, nonmotile, Gram-positive bacterium (Supplementary Figure S1) that was facultatively anaerobic. Subsequently, ClWan1 was propagated on brain heart infusion (BHI) agar and was cultured aerobically. The isolate was both catalase and oxidase negative and could be propagated on BHI agar media. However, ClWan1 did not metabolize lipids, nor did it hydrolyze starch.
3.2. Whole Genome Sequence Characteristics of Bacterium ClWan1
Sequencing and assembly of the ClWan1 genomic DNA resulted in a whole genome reference sequence with a coverage of 99.45%. The assembly resulted in a single contig that was 3,512,202 base pairs in length with the same N50. The whole genome sequence (WGS) had a GC content of 34.48% (Table 1). This is very similar in size and GC content to the Carnobacterium maltaromaticum SF2022 strain reference genome (e.g., RefSeq: GCF_949790605.1; NZ_OX460976, Ref [55] and reviews [6,7,8]).

Table 1.
Whole Genome Assembly Statistics for Bacterial Isolate ClWan1 and SF2022. Carnobacterium maltaromaticum strain SF2022 isolate SF2022 chromosome SF2022_c1 from locus GenBank NZ_OX460976.
ClWan1 was identified as a novel MLST sequence type with three novel alleles, using the schema corresponding to Carnobacterium maltaromaticum. The results are displayed in Table 2. The ST data is curated by Frédéric Borges [56] on the PubMLST website [45].

Table 2.
PubMLST Sequence Typing of Bacterial Isolate ClWan1. Allele numbers with a * indicate a partial match to the allele with at least 10% coverage of the reference allele length. Allele numbers with a ^ indicate a novel full-length allele.
The MSLT of isolate ClWan1 was most similar to a C. maltaromaticum ST63 with three exact matches found at glpQ, leuS, and pyrE genes. The dapE gene had three sequence differences, including a G → A at position 2559336, an A → G at 2559337, and a T → C at 2559354. The pyc gene had a C → T at position 944654. Isolate ClWan1 had two unique full-length alleles of ddlA and ilvE. These genes encode a D-alanine-D-alanine ligase A and a branched-chain-amino-acid aminotransferase, respectively. Consequently, isolate ClWan1 is potentially a unique ST of the species.
The 3.5 Mb WGS of ClWan1 single contig mapped as a circular genome (Figure 1). The figure also illustrates the clusters of orthologous groups (COG) and gene features, such as coding sequences and GC skew. Using the PHAge Search Tool with Enhanced Sequence Translation (PHASTEST) program [40], no bacteriophage sequences were detected in the ClWan1 genome.

Figure 1.
Genome Map and Genome Composition of Bacterial Isolate ClWan1. The map represents gene content as a circle plot from inner to outer circles: GC skew, GC content, negative sequence gene content, negative coding sequences, tRNA location, positive coding sequences, and positive sequence gene content with genomic coordinates.
The core bacterial genes for ClWan1 are listed in Table 3 and as a GenBank-type file in Supplementary Table S1. The genes are delineated into COG categories, with the transcription category having the most genes. The defense mechanism had a high percentage of genes in its category, and many of these have been studied for their antimicrobial peptides and bacteriocins [57,58,59]. There are 310 carbohydrate transport and metabolism genes, including genes involved in starch digestion that are important to probiotics [3].

Table 3.
Gene Composition of the Bacterial Isolate ClWan1 Genome. Clusters of orthologous groups (COG) and gene function are tabulated by the number of genes and percentage of each genome total in respective columns.
The secondary metabolites biosynthesis, transport, and metabolism gene category has 42 genes. The antiSMASH program, a secondary metabolite gene prediction tool [39], detected five genes associated with secondary metabolism (Supplementary Table S2). Among these were a phytoene synthase, type III polyketide synthase (T3PK), cyclic lactone autoinducer, thiopeptide genes, non-ribosomal peptide synthetase, and a ribosomally synthesized and post-translationally modified peptide-like gene (Ripp-like). This points to the clear capacity of the organism to produce bioactive metabolites that impact its environment.
The bacteriocin gene was also detected using the BAGEL software (http://bagel4.molgenrug.nl/) [38]; it encoded an antimicrobial peptide CarnobacteriocinB1 from position 2791273 to 2791458 that is 100% similar to the class II bacteriocin found among the genus Carnobacterium [WP_010051997]. The peptide contained the characteristic YGNGV motif of the family of class II bacteriocins [59,60]. A putative Carnobacteriocin-BM1 bacteriocin immunity protein gene encoded from positions 2791473 to 2791739 is 99% similar to other members of the genus [WP_010051996]. A lantibiotic dehydratase C-terminal domain-containing protein of Carnobacterium maltaromaticum was detected from positions 2715970 to 2716767 of the ClWan1 genome encoding a peptide that is 98% similar to a previously reported protein [WP_414024519].
Table 4 shows the genome location of several antimicrobial resistance genes. These include genes encoding an ABC-F type ribosomal protection protein, class A beta-lactamase, and a NAD(+)-rifampin ADP-ribosyltransferase.

Table 4.
Antimicrobial Resistance Gene Profile for ClWan1.
3.3. Phylogenetic Analyses of Gray Wolf Isolate ClWan1
Phylogeny utilizing the 16S rRNA gene was used to initially classify isolate ClWan1 as a unique Carnobacterium maltaromaticum. Also, phylogenetics of the 16S rRNA gene using Species Finder [46] resulted in ClWan1 being most closely related to C. maltaromaticum strain S_T_MRS_58 [JX860544]. Subsequently, the WGS was used to confirm this result (Figure 2 and Supplementary Figures S2 and S3). This analysis resulted in ClWan1 being closely related to C. maltaromaticum and C. piscicola. Interestingly, a bacterium originally classified as Lactobacillus carnis strain DSM20722 isolated from vacuum-packaged meat appears to be a Carnobacterium sp. by the TYGS-LPSN analysis [61]. This bacterium was reclassified as a C. maltaromaticum [62], while C. maltaromaticum MX5, originally isolated from milk as Lactobacillus maltaromicus [63], was also reclassified as C. maltaromaticum [62]. These isolates are closely related to ClWan1 isolated from a Gray wolf gastrointestinal tract. The C. maltaromaticum MX5 is considered a type strain (also ATCC® 27865™) for the species [JQMX00000000] and is the nearest neighbor for ClWan1 (Figure 2).

Figure 2.
Phylogeny of Isolate ClWan1 Based on Whole Genome Sequences. Whole genome sequences were submitted to the type strain genome server (TYGS). The output was also saved as a Newick file and then analyzed using the molecular evolutionary genetics analysis (MEGA) program described in the methods (Supplementary Figure S3). The branch lengths in substitutions per site, and bootstrap confidences are above the lines of interest in blue.
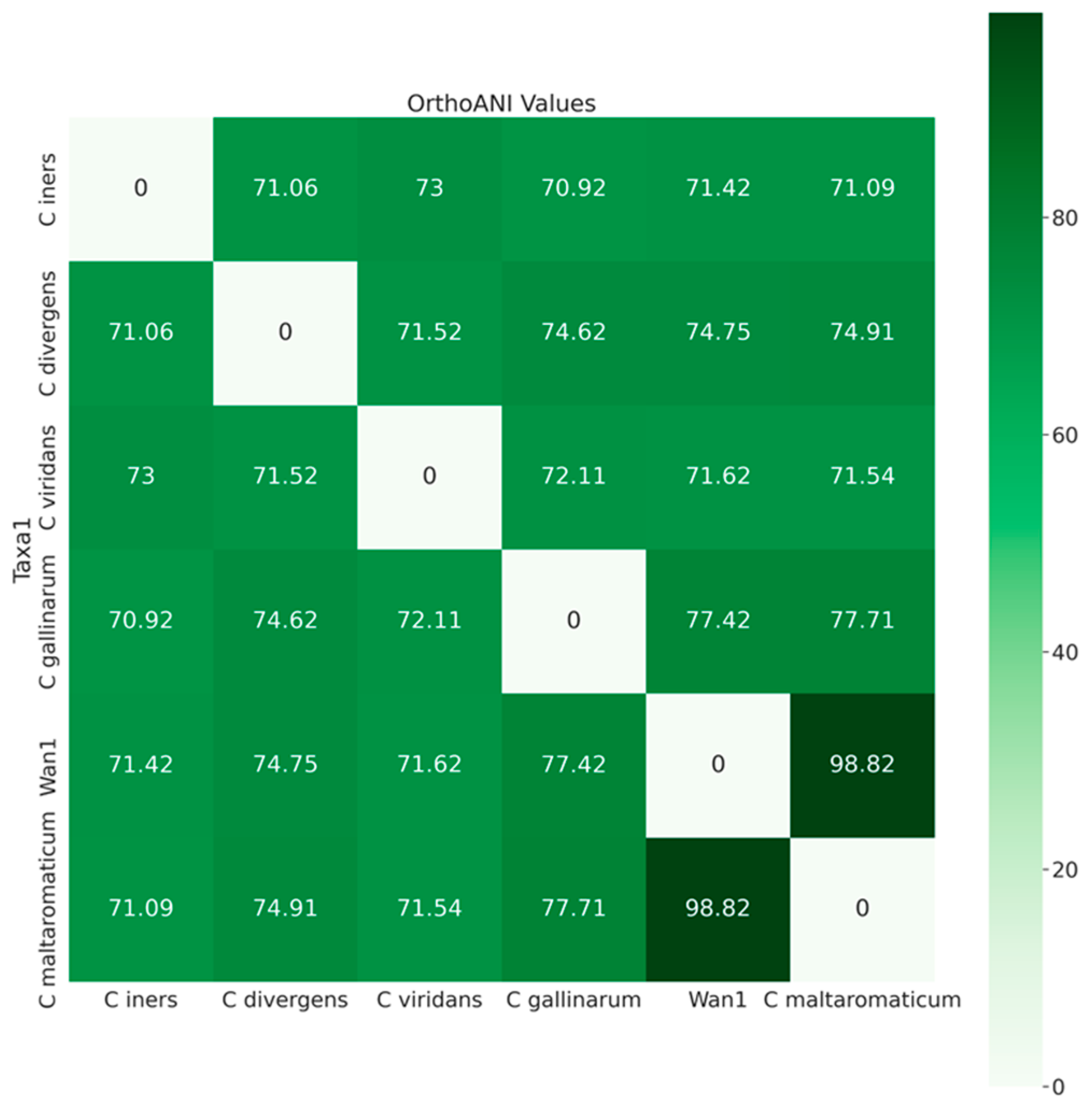
Further analysis utilizing average nucleotide identities (orthoANI) classified isolate ClWan1 as a C. maltaromaticum (Figure 3). The ANI of ClWan1 was 98.82% similar to C. maltaromaticum and only 77.71% similar to its nearest neighbor species, a C. gallinarum isolate.
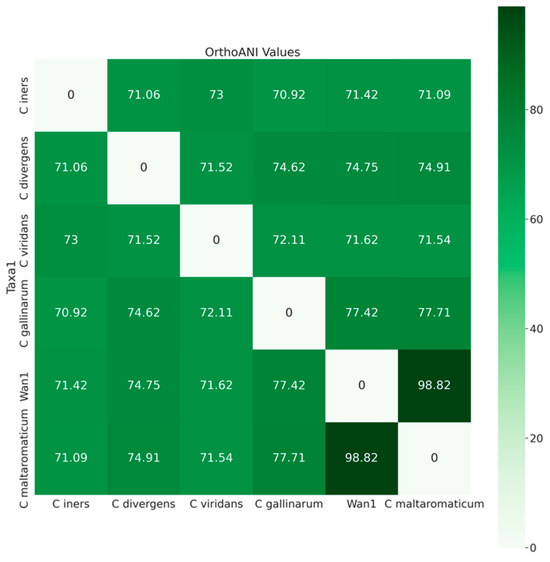
Figure 3.
Average Nucleotide Identities Among Carnobacterium spp. Average nucleotide identity (ANI) values, query, and reference coverage values were calculated with OrthoANIu, and a neighbor-joining tree was constructed from the ANI values using the ‘ape’ R library functions described in the methods.
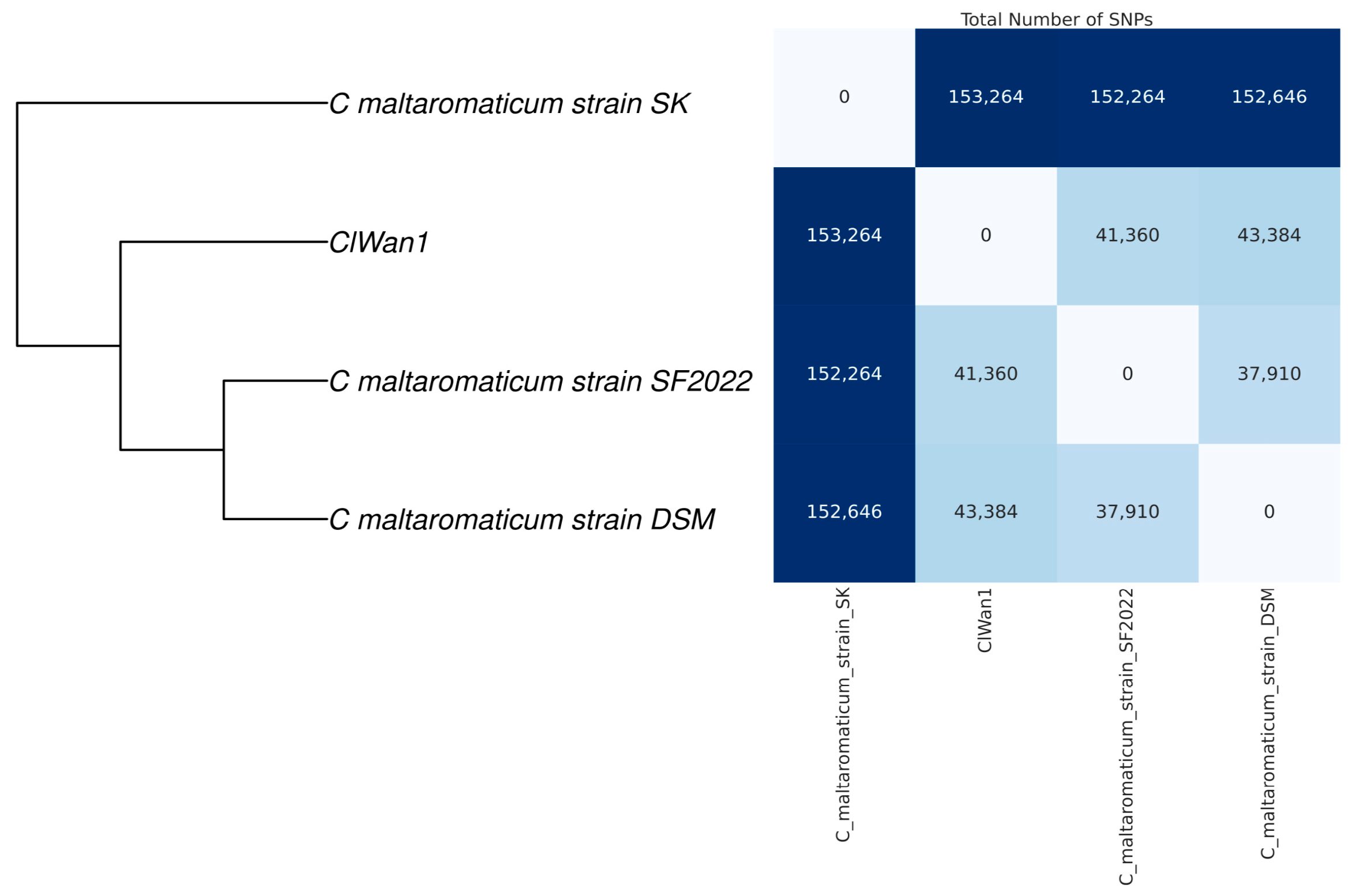
Using single nucleotide polymorphism (SNP) analysis of the core genome, it was determined that ClWan1 was a unique ST among C. maltaromaticum isolates (Figure 4). The core genome SNP analysis demonstrated that ClWan1 had 41,360 SNPs from its nearest neighbor C. maltaromaticum strain SF2022.
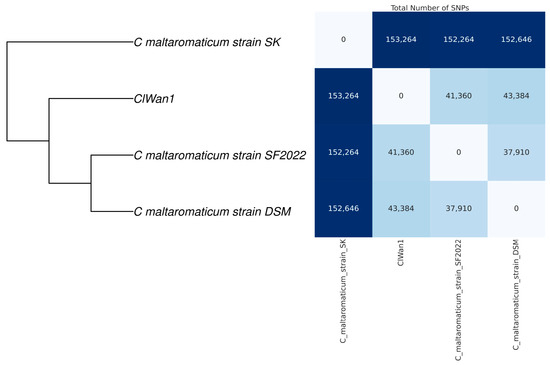
Figure 4.
Single Nucleotide Polymorphism Matrix Among Carnobacterium maltaromaticum Isolates. Reference and the ClWan1 genomes were analyzed for single nucleotide polymorphisms (SNP) comparisons using parsnp with default parameters. The matrix was produced using a custom script described in the methods.
Digital DNA-DNA hybridization (dDDH) using the TYGS and LPSN database ([53]; Supplementary Table S3) resulted in the ClWan1 genome being 90.6% similar to C. maltaromaticum MX5 [Accession GCA_000744945.1] and 90.6% to the misnamed L. carnis DSM 20722, now C. maltaromaticum DSM 20722 [Accession GCA_001437035.1]. The G+C content difference was 0.05% with C. maltaromaticum MX5 and 0.17% with L. carnis DSM 20722. The results indicate that the ClWan1 from a North American Gray wolf isolate is a unique ST among the C. maltaromaticum isolates reported in GenBank. Also of interest is a bacterium reported as Lactobacillus carnis isolate that phylogenetically groups with the Carnobacterium spp.
4. Discussion
Carnobacteria are a group of Lactic acid bacteria that reportedly have anti-listerial and other probiotic properties [4,6,7,8]. Carnobacterium spp. whether they are or are not known bacteriocin producers, reduced eukaryotic cell invasion by Listeria monocytogenes [21]. L. monocytogenes is found in waterways and can colonize in the intestines of mammals, including ruminants [64,65,66], which wolves are known to consume alongside the rest of the carcass, except the rumen and possibly abomasa contents [67]. Thus, C. maltaromaticum, which has anti-listeria properties in vitro [21], is likely to confer increased resistance to listeriosis in the animal, in vivo. Furthermore, should this pattern hold, C. maltaromaticum might show potential benefit as a probiotic for other, similar, omnivorous monogastric animals that interface with humans and potentially human waste, such as dogs [68,69,70].
In studies with rats performed by Li, et al. [71], it was demonstrated that C. maltaromaticum could also stimulate Vitamin D production in mice intestines, which reduces the rate of colorectal cancer. This type of cancer is also common in domestic dogs and humans and can be fatal to the animal [72]. With further research, it is possible to determine if this mechanism is also functional in dogs and thus a potential form of preventive veterinary healthcare. It is possible to characterize, extract, isolate, purify, and concentrate the extracellular secretion of these, and related, strains for use as an anti-listeria treatment [7,8]. Given the tendency for L. monocytogenes to develop unique ribotypes [65], which are associated with listeria outbreaks among livestock, a strong anti-listeria treatment is valuable, especially as ampicillin and broader antibiotic resistances become more prevalent in the wild-type strains of L. monocytogenes [73,74,75].
Several other bioactive compounds identified in the ClWan1 genome include thiopeptides, a diverse class of secondary metabolites with broad bioactivity [76]. While the large number of hypothetical genes clustered among the thiopeptide biosynthetic genes makes predicting the exact structure difficult, the top two cluster similarity scores in the MIBiG analysis (an optional antiSMASH 4.0 output), are for cutimycin and microcin P1, which respectively have Staphylococcus spp. and L. monocytogenes, inhibiting activity [77,78]. Also encoded was a single non-ribosomal peptide synthetase gene with two synthetic modules predicted to generate a tyrosine-threonine dipeptide product. As with thiopeptides, cyclic dipeptides are widespread and possess diverse biological activities [79]. A BLAST (Version 2.13.0) search of this NRPS yields distantly related proteins with at most 41% amino acid identity to the S. aureus NRPS [HDP5831075.1]. Among the similar, albeit distantly related clusters in the MIBiG analysis are the genes encoding the biosynthesis of the aureusimines, tyrosine-valine cyclic dipeptides conserved among S. aureus strains, which are thought to play a role in virulence and host colonization [80,81]. A lantibiotic dehydratase was detected in the genome, and this enzyme participates in the biosynthesis of lantibiotics, a class of peptide antibiotics that contain one or more thioether bonds [82]. The biosynthetic gene clusters (BGCs) were previously inferred by WGS analyses in the Carnobacteria [7,8] and were detected in the ClWan1 genome. Potential antibiotic resistance genes were detected in the genome of the C. maltaromaticum isolate reported herein. Previous resistance to antibiotics has been reported in other isolates of the species but retained probiotic properties [83].
Carnobacteria are found in diverse environments, with C. maltaromaticum followed by C. divergens being the most common members of the genus. Genes found among these species are primarily adapted to the gastrointestinal environment of animals, specifically the resistance of C. maltaromaticum to bile [55]. Furthermore, as noted, these bacteria can be “mined” by genomics techniques to discover new natural products with practical applications [7,8]. The isolate from a Gray wolf described herein encodes several bioactive compounds, such as a bacteriocin that potentially has anti-listerial properties. The earliest domesticated animals were dogs (Canis familiaris) that are descendants of the Gray wolf (Canis lupus) that coevolved with humans (reviewed in [84]). Therefore, it could be argued that a dog diet might be enhanced by utilizing useful bacteria from free-ranging wolves [26,28]. Consequently, it is important to protect worldwide biodiversity for many reasons, including improving our understanding of microbial health and disease among free-ranging and domestic species [85].
5. Conclusions
Isolate ClWan1 was identified as a Carnobacterium maltaromaticum with a genomic length of 3.51 Mbp, consistent with other species of this genus with genome sizes ranging between 3.33 to 3.87 Mbp [8,55,56]. MSLT analysis, WGS phylogeny, SNP, and ANI comparisons confirmed that isolate ClWan1 is a unique ST of the species. This investigation represents the first reported isolation and axenic culture of a C. maltaromaticum from a free-ranging Gray wolf (Canis lupus). This expands the understanding of its ecological presence and potential beneficial applications for the gut health of animals. There is a knowledge gap in the wolf enteric microbiome that is not fully known and is compelling compared to domesticated dogs and other carnivores [86,87]. Additional investigations could be conducted to determine how widespread this organism is in wolves and dogs to understand if colonization is affected by age, sex, and diet.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/vetsci12050410/s1, Figure S1: Culture of Bacterial Isolate ClWan1 and Gram-stain. Ileal contents from a North American Gray Wolf were cultured on Brucella broth with Hemin and Vitamin K agar (A). Subsequently, the isolate was Gram-stained following culture on brain-heart infusion agar (B). Figure S2: Initial Whole Genome Sequence Phylogenetics Analysis of the ClWan1 genome. The UBCG: Up-to-date bacterial core gene: 92 core bacteria genes was used to classify the isolate ClWan1 [https://www.ezbiocloud.net/tools/ubcg], accessed on 13 October 2024. Figure S3: Phylogeny of Isolate ClWan1 Based on Whole Genome Sequences. Whole genome sequences were submitted to the type strain genome server (TYGS). The output was saved as a Newick file and then analyzed using the molecular evolutionary genetics analysis (MEGA) program described in the methods [54]. The scale bar indicates branch lengths in substitutions per site, and bootstrap confidences are above each line. Tables S1 and S2: Results from the antiSMASH Bacterial Version for the ClWan1 Genome. Table S3: Type Strain Genome Server.
Author Contributions
Conceptualization, B.S.S. and P.N.B.; methodology, J.M., J.L.B., C.C.K., A.N.A., T.W.C., M.B.-D., M.R.A., B.S.S. and P.N.B.; software, J.L.B., J.M., C.C.K., P.A.J., M.N.J., N.A.H., B.S.S. and E.S.F.; validation, B.S.S. and P.N.B.; formal analysis, C.C.K., J.M., J.L.B., T.W.C., M.N.J., N.A.H. and B.S.S.; Data curation, J.L.B., J.M., C.C.K., M.N.J., N.A.H. and B.S.S.; Writing—original draft preparation, B.S.S., C.C.K., J.L.B., J.M., M.N.J., N.A.H. and P.A.J.; Review and editing, C.C.K., J.M., J.L.B., M.N.J., N.A.H., P.A.J., P.N.B., E.S.F. and B.S.S.; Supervision and Project Administration, P.N.B., E.S.F. and B.S.S.; Funding acquisition, B.S.S., P.N.B. and E.S.F.; C.C.K., J.L.B. and J.M. contributed equally to the preparation of this manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
Research funding support was provided to PNB as a start-up grant from the OSU Faculty Innovation Committee, the Gaskins Fund, and an OSU AID grant #M6008M Org: 192520 “Animal Probiotics Discovery” and the Women’s Giving Circle “Undergraduate Bacteriology and Molecular Biology Research at Oregon State University-Cascades” 4110-863100 along with Professional Development Funds to PNB and BSS. The project was also partially supported by a National Science Foundation grant (IOS-2114641) awarded to ESF.
Institutional Review Board Statement
The Oregon State University Institutional Biosafety Committee has approved the research reported herein as IBC Proposal #3923.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data are retrievable at the National Center for Biotechnology Information (NCBI) as BioProject PRJNA1125382 Carnobacterium maltaromaticum ClWan1 with BioSample accession SAMN38498379. The Sequence Read Archive (SRA) is SRS19889843 with Illumina reads as SRX22921895 and nanopore reads as SRX22921894. The genome submission is ID SUB14546156 with a temporary Accession CP158642.
Acknowledgments
Funding was provided to T.C. as a Layman and URSA Fellow and to J.M. as a Layman Fellow, all at Oregon State University Cascades. The results reported are part of ongoing undergraduate research at Oregon State University-Cascades and have been presented at the annual Cascades Research and Scholarship Symposium: https://osucascades.edu/research/students/cascades-research-and-scholarship-symposium, accessed on 13 October 2024.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in designing the study; in the collection, analysis, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results. No author has received funding from commercial sources or companies that sell drugs or medical devices or provide medical services. No author has received honoraria for speaking at any symposia related to this research, nor do they hold a position on an advisory board related to the research. No author is an inventor of any planned, pending, or awarded patent on this work.
References
- Zheng, J.; Wittouck, S.; Salvetti, E.; Franz, C.M.A.P.; Harris, H.M.B.; Mattarelli, P.; O’Toole, P.W.; Pot, B.; Vandamme, P.; Walter, J.; et al. A Taxonomic Note on the Genus Lactobacillus: Description of 23 Novel Genera, Emended Description of the Genus Lactobacillus Beijerinck 1901, and Union of Lactobacillaceae and Leuconostocaceae. Int. J. Syst. Evol. Microbiol. 2020, 70, 2782–2858. [Google Scholar] [CrossRef] [PubMed]
- Ayed, L.; M’hir, S.; Nuzzolese, D.; Di Cagno, R.; Filannino, P. Harnessing the Health and Techno-Functional Potential of Lactic Acid Bacteria: A Comprehensive Review. Foods 2024, 13, 1538. [Google Scholar] [CrossRef]
- Abdul Hakim, B.N.; Xuan, N.J.; Oslan, S.N.H. A Comprehensive Review of Bioactive Compounds from Lactic Acid Bacteria: Potential Functions as Functional Food in Dietetics and the Food Industry. Foods 2023, 12, 2850. [Google Scholar] [CrossRef] [PubMed]
- Leisner, J.J.; Laursen, B.G.; Prévost, H.; Drider, D.; Dalgaard, P. Carnobacterium: Positive and Negative Effects in the Environment and in Foods. FEMS Microbiol. Rev. 2007, 31, 592–613. [Google Scholar] [CrossRef]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. The International Scientific Association for Probiotics and Prebiotics Consensus Statement on the Scope and Appropriate Use of the Term Probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Evangelista, A.G.; Danielski, G.M.; Corrêa, J.A.F.; Cavalari, C.M.A.; Souza, I.R.; Luciano, F.B.; Macedo, R.E.F. Carnobacterium as a bioprotective and potential probiotic culture to improve food quality, food safety, and human health—A scoping review. Crit. Rev. Food Sci. Nutr. 2023, 63, 6946–6959. [Google Scholar] [CrossRef]
- Begrem, S.; Ivaniuk, F.; Gigout-Chevalier, F.; Kolypczuk, L.; Bonnetot, S.; Leroi, F.; Grovel, O.; Delbarre-Ladrat, C.; Passerini, D. New Insight into Antimicrobial Compounds from Food and Marine-Sourced Carnobacterium Species through Phenotype and Genome Analyses. Microorganisms 2020, 8, 1093. [Google Scholar] [CrossRef]
- Gontijo, M.T.P.; Ramia, N.E.; Dijamentiuk, A.; Elfassy, A.; Taha, S.; Mangavel, C.; Revol-Junelles, A.M.; Borges, F. Mining Biosynthetic Gene Clusters in Carnobacterium maltaromaticum by Interference Competition Network and Genome Analysis. Microorganisms 2022, 10, 1794. [Google Scholar] [CrossRef]
- Cunningham, M.; Azcarate-Peril, M.A.; Barnard, A.; Benoit, V.; Grimaldi, R.; Guyonnet, D.; Holscher, H.D.; Hunter, K.; Manurung, S.; Obis, D.; et al. Shaping the Future of Probiotics and Prebiotics. Trends Microbiol. 2021, 29, 667–685. [Google Scholar] [CrossRef]
- Latif, A.; Shehzad, A.; Niazi, S.; Zahid, A.; Ashraf, W.; Iqbal, M.W.; Rehman, A.; Riaz, T.; Aadil, R.M.; Khan, I.M.; et al. Probiotics: Mechanism of Action, Health Benefits and their Application in Food Industries. Front. Microbiol. 2023, 1, 1216674. [Google Scholar] [CrossRef]
- Gul, S.; Durante-Mangoni, E. Unraveling the Puzzle: Health Benefits of Probiotics-A Comprehensive Review. J. Clin. Med. 2024, 13, 1436. [Google Scholar] [CrossRef]
- Markowiak, P.; Śliżewska, K. The Role of Probiotics, Prebiotics, and Synbiotics in Animal Nutrition. Gut Pathog. 2018, 10, 21. [Google Scholar] [CrossRef] [PubMed]
- Pilla, R.; Suchodolski, J.S. The Gut Microbiome of Dogs and Cats, and the Influence of Diet. Vet. Clin. N. Am. Small Anim. Pract. 2021, 51, 605–621. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, S.S. Evidence-based Use of Biotics in the Management of Gastrointestinal Disorders in Dogs and Cats. Vet. Rec. 2024, 195, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Wilson, S.M.; Swanson, K.S. The Influence of ‘Biotics’ on the Gut Microbiome of Dogs and Cats. Vet. Rec. 2024, 195, 2–12. [Google Scholar] [CrossRef]
- Garcias-Bonet, N.; Roik, A.; Tierney, B.; García, F.C.; Villela, H.D.M.; Dungan, A.M.; Quigley, K.M.; Sweet, M.; Berg, G.; Gram, L.; et al. Horizon Scanning the Application of Probiotics for Wildlife. Trends Microbiol. 2024, 32, 252–269. [Google Scholar] [CrossRef]
- Merenstein, D.; Pot, B.; Leyer, G.; Ouwehand, A.C.; Preidis, G.A.; Elkins, C.A.; Hill, C.; Lewis, Z.T.; Shane, A.L.; Zmora, N.; et al. Emerging Issues in Probiotic Safety: 2023 Perspectives. Gut Microbes 2023, 15, 2185034. [Google Scholar] [CrossRef]
- Spanggaard, B.; Huber, I.; Nielsen, J.; Sick, E.B.; Pipper, C.B.; Martinussen, T.; Slierendrecht, W.J.; Gram, L. The Probiotic Potential against Vibriosis of the Indigenous Microflora of Rainbow Trout. Environ. Microbiol. 2001, 3, 755–765. [Google Scholar] [CrossRef]
- Kim, D.H.; Austin, B. Innate Immune Responses in Rainbow Trout (Oncorhynchus mykiss, Walbaum) Induced by Probiotics. Fish Shellfish Immunol. 2006, 21, 513–524. [Google Scholar] [CrossRef]
- Kim, D.H.; Austin, B. Characterization of Probiotic Carnobacteria Isolated from Rainbow Trout (Oncorhynchus mykiss) intestine. Lett. Appl. Microbiol. 2008, 47, 141–147. [Google Scholar] [CrossRef]
- Pilchová, T.; Pilet, M.F.; Cappelier, J.M.; Pazlarová, J.; Tresse, O. Protective Effect of Carnobacterium spp. against Listeria monocytogenes during Host Cell Invasion Using In vitro HT29 Model. Front. Cell Infect. Microbiol. 2016, 6, 88. [Google Scholar] [CrossRef] [PubMed]
- Nayak, S.K. Probiotics and Immunity: A Fish Perspective. Fish Shellfish Immunol. 2010, 29, 2–14. [Google Scholar] [CrossRef]
- Martínez Cruz, P.; Ibáñez, A.L.; Monroy Hermosillo, O.A.; Ramírez Saad, H.C. Use of probiotics in aquaculture. ISRN Microbiol. 2012, 2012, 916845. [Google Scholar] [CrossRef]
- Smialek, M.; Burchardt, S.; Koncicki, A. The influence of Probiotic Supplementation in Broiler Chickens on Population and Carcass Contamination with Campylobacter spp.—Field study. Res. Vet. Sci. 2018, 118, 312–316. [Google Scholar] [CrossRef]
- Bogucka, J.; Ribeiro, D.M.; Bogusławska-Tryk, M.; Dankowiakowska, A.; da Costa, R.P.R.; Bednarczyk, M. Microstructure of the Small Intestine in Broiler Chickens Fed a Diet with Probiotic or Synbiotic Supplementation. J. Anim. Physiol. Anim. Nutr. 2019, 103, 1785–1791. [Google Scholar] [CrossRef]
- McCabe, J.; Bryant, J.L.; Klews, C.C.; Johnson, M.; Atchley, A.N.; Cousins, T.W.; Dominguez, A.; Gabriel, M.; Middleton, K.; Bowles, N.A.; et al. Phenotypic and Draft Genome Sequence Analyses of a Paenibacillus sp. Isolated from the Gastrointestinal Tract of a North American Gray Wolf (Canis lupus). Appl. Microbiol. 2023, 3, 1120–1129. [Google Scholar] [CrossRef]
- Keshri, J.; Smith, K.M.; Svendsen, M.K.; Keillor, H.R.; Moss, M.L.; Jordan, H.J.; Larkin, A.M.; Garrish, J.K.; Line, J.E.; Ball, P.N.; et al. Phenotypic Characterization and Draft Genome Sequence Analyses of Two Novel Endospore-Forming Sporosarcina spp. Isolated from Canada Goose (Branta canadensis) Feces. Microorganisms 2024, 12, 70. [Google Scholar] [CrossRef]
- Bryant, J.L.; McCabe, J.; Klews, C.C.; Johnson, M.; Atchley, A.N.; Cousins, T.W.; Barnard-Davidson, M.; Smith, K.M.; Ackermann, M.R.; Netherland, M., Jr.; et al. Phenotypic and Complete Reference Whole Genome Sequence Analyses of Two Paenibacillus spp. Isolates from a Gray Wolf (Canis lupus) Gastrointestinal Tract. Vet. Sci. 2025, 12, 51. [Google Scholar] [CrossRef]
- Berry, P.L.; Taylor, E.; Phillips, I. The Use of an Anaerobic Incubator for the Isolation of Anaerobes from Clinical Samples. J. Clin. Pathol. 1982, 35, 1158–1162. [Google Scholar] [CrossRef]
- Chapin, K.; Murray, P. Manual of Clinical Microbiology, 7th ed.; Murray, P.R., Baron, E.J., Pfaller, M.A., Tenover, F.C., Yolken, R.H., Eds.; American Society for Microbiology: Washington, DC, USA, 1999. [Google Scholar]
- Mahon, C.R.; Lehman, D.C.; Manuselis, G. Textbook of Diagnostic Microbiology, 5th ed.; W. B Saunders Co.: Philadelphia, PA, USA, 2014. [Google Scholar]
- Kolmogorov, M.; Yuan, J.; Lin, Y.; Pevzner, P.A. Assembly of Long, Error-prone Reads Using Repeat Graphs. Nat. Biotechnol. 2019, 37, 540–546. [Google Scholar] [CrossRef]
- Li, H. Toward a Better Understanding of Artifacts in Variant Calling from High-coverage Samples. Bioinformatics 2014, 30, 2843–2851. [Google Scholar] [CrossRef]
- Wick, R.R.; Holt, K.E. Polypolish: Short-read Polishing of Long-read Bacterial Genome Assemblies. PLoS Comput. Biol. 2022, 18, e1009802. [Google Scholar] [CrossRef]
- Parks, D.H.; Imelfort, M.; Skennerton, C.T.; Hugenholtz, P.; Tyson, G.W. CheckM: Assessing the Quality of Microbial Genomes Recovered from Isolates, Single Cells, and Metagenomes. Genome Res. 2015, 25, 1043–1055. [Google Scholar] [CrossRef] [PubMed]
- Seemann, T. Prokka: Rapid Prokaryotic Genome Annotation. Bioinformatics 2014, 30, 2068–2069. [Google Scholar] [CrossRef] [PubMed]
- Cumsille, A.; Durán, R.E.; Rodríguez-Delherbe, A.; Saona-Urmeneta, V.; Cámara, B.; Seeger, M.; Araya, M.; Jara, N.; Buil-Aranda, C. GenoVi, an Open-source Automated Circular Genome Visualizer for Bacteria and Archaea. PLoS Comput. Biol. 2023, 19, e1010998. [Google Scholar] [CrossRef] [PubMed]
- van Heel, A.J.; de Jong, A.; Song, C.; Viel, J.H.; Kok, J.; Kuipers, O.P. BAGEL4: A User-Friendly Web Server to Thoroughly Mine RiPPs and Bacteriocins. Nucleic Acids Res. 2018, 46, W278–W281. [Google Scholar] [CrossRef]
- Blin, K.; Shaw, S.; Medema, M.H.; Weber, T. The antiSMASH Database Version 4: Additional Genomes and BGCs, New Sequence-based Searches and More. Nucleic Acids Res. 2024, 52, D586–D589. [Google Scholar] [CrossRef] [PubMed]
- Wishart, D.S.; Han, S.; Saha, S.; Oler, E.; Peters, H.; Grant, J.R.; Stothard, P.; Gautam, V. PHASTEST: Faster than PHASTER, Better than PHAST. Nucleic Acids Res. 2023, 51, W443–W450. [Google Scholar] [CrossRef]
- Chalita, M.; Kim, Y.O.; Park, S.; Oh, H.S.; Cho, J.H.; Moon, J.; Baek, N.; Moon, C.; Lee, K.; Yang, J.; et al. EzBioCloud: A Genome-driven Database and Platform for Microbiome Identification and Discovery. Int. J. Syst. Evol. Microbiol. 2024, 74, 006421. [Google Scholar] [CrossRef]
- Li, H.; Handsaker, B.; Wysoker, A.; Fennell, T.; Ruan, J.; Homer, N.; Marth, G.; Abecasis, G.; Durbin, R. 1000 Genome Project Data Processing Subgroup. The Sequence Alignment/Map format and SAMtools. Bioinformatics 2009, 25, 2078–2079. [Google Scholar] [CrossRef]
- Langmead, B.; Salzberg, S.L. Fast Gapped-read Alignment with Bowtie 2. Nat. Methods 2012, 9, 357–359. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Zheng, D.; Zhou, S.; Chen, L.; Yang, J. VFDB 2022: A General Classification Scheme for Bacterial Virulence Factors. Nucleic Acids Res. 2022, 50, D912–D917. [Google Scholar] [CrossRef]
- Jolley, K.A.; Bray, J.E.; Maiden, M.C.J. Open-access Bacterial Population Genomics: BIGSdb Software, the PubMLST.org Website and Their Applications. Wellcome Open Res. 2018, 3, 124. [Google Scholar] [CrossRef] [PubMed]
- Larsen, M.V.; Cosentino, S.; Lukjancenko, O.; Saputra, D.; Rasmussen, S.; Hasman, H.; Sicheritz-Pontén, T.; Aarestrup, F.M.; Ussery, D.W.; Lund, O. Benchmarking of Methods for Genomic Taxonomy. J. Clin. Microbiol. 2014, 52, 1529–1539. [Google Scholar] [CrossRef]
- Kim, J.; Na, S.I.; Kim, D.; Chun, J. UBCG2: Up-to-Date Bacterial Core Genes and Pipeline for Phylogenomic Analysis. J. Microbiol. 2021, 59, 609–615. [Google Scholar] [CrossRef] [PubMed]
- Katoh, K.; Standley, D.M. MAFFT Multiple Sequence Alignment Software Version 7: Improvements in Performance and Usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef]
- Kozlov, A.M.; Darriba, D.; Flouri, T.; Morel, B.; Stamatakis, A. RAxML-NG: A Fast, Scalable and User-friendly Tool for Maximum Likelihood Phylogenetic Inference. Bioinformatics 2019, 35, 4453–4455. [Google Scholar] [CrossRef]
- Yoon, S.H.; Ha, S.M.; Lim, J.; Kwon, S.; Chun, J. A Large-scale Evaluation of Algorithms to Calculate Average Nucleotide Identity. Antonie Van Leeuwenhoek 2017, 110, 1281–1286. [Google Scholar] [CrossRef]
- Paradis, E.; Schliep, K. ape 5.0: An Environment for Modern Phylogenetics and Evolutionary Analyses in R. Bioinformatics 2019, 35, 526–528. [Google Scholar] [CrossRef]
- Treangen, T.J.; Ondov, B.D.; Koren, S.; Phillippy, A.M. The Harvest Suite for Rapid Core-genome Alignment and Visualization of Thousands of Intraspecific Microbial Genomes. Genome Biol. 2014, 15, 524. [Google Scholar] [CrossRef]
- Meier-Kolthoff, J.P.; Carbasse, J.S.; Peinado-Olarte, R.L.; Göker, M. TYGS and LPSN: A Database Tandem for Fast and Reliable Genome-based Classification and Nomenclature of Prokaryotes. Nucleic Acids Res. 2022, 7, D801–D807. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Suleski, M.; Sanderford, M.; Sharma, S.; Tamura, K. MEGA12: Molecular Evolutionary Genetic Analysis Version 12 for Adaptive and Green Computing. Mol. Biol. Evol. 2024, 41, msae263. [Google Scholar] [CrossRef]
- Iskandar, C.F.; Borges, F.; Taminiau, B.; Daube, G.; Zagorec, M.; Remenant, B.; Leisner, J.J.; Hansen, M.A.; Sørensen, S.J.; Mangavel, C.; et al. Comparative Genomic Analysis Reveals Ecological Differentiation in the Genus Carnobacterium. Front Microbiol. 2017, 8, 357. [Google Scholar] [CrossRef]
- Borges, F. Carnobacterium maltaromaticum. Available online: https://pubmlst.org/organisms/carnobacterium-maltaromaticum (accessed on 19 December 2024).
- Gradisteanu Pircalabioru, G.; Popa, L.I.; Marutescu, L.; Gheorghe, I.; Popa, M.; Czobor Barbu, I.; Cristescu, R.; Chifiriuc, M.C. Bacteriocins in the Era of Antibiotic Resistance: Rising to the Challenge. Pharmaceutics 2021, 13, 196. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Ramos, A.; Madi-Moussa, D.; Coucheney, F.; Drider, D. Current Knowledge of the Mode of Action and Immunity Mechanisms of LAB-Bacteriocins. Microorganisms 2021, 9, 2107. [Google Scholar] [CrossRef]
- González-Gragera, E.; García-López, J.D.; Teso-Pérez, C.; Jiménez-Hernández, I.; Peralta-Sánchez, J.M.; Valdivia, E.; Montalban-Lopez, M.; Martín-Platero, A.M.; Baños, A.; Martínez-Bueno, M. Genomic Characterization of Piscicolin CM22 Produced by Carnobacterium maltaromaticum CM22 Strain Isolated from Salmon (Salmo salar). Probiotics Antimicrob Proteins 2024. [Google Scholar] [CrossRef]
- Quadri, L.; Sailer, M.; Roy, K.L.; Vederas, J.C.; Stiles, M.E. Chemical and Genetic Characterization of Bacteriocins Produced by Carnobacterium piscicola LV17B. J. Biol. Chem. 1994, 269, 12204–12211. [Google Scholar] [CrossRef]
- Shaw, B.G.; Harding, C.D. Atypical Lactobacilli from Vacuum-packaged Meats: Comparison by DNA Hybridization, Cell Composition and Biochemical Tests with a Description of Lactobacillus carnis sp. nov. Syst. Appl. Microbiol. 1985, 6, 291297. [Google Scholar] [CrossRef]
- Mora, D.; Scarpellini, M.; Franzetti, L.; Colombo, S.; Galli, A. Reclassification of Lactobacillus maltaromicus (Miller et al. 1974) DSM 20342T and DSM 20344 and Carnobacterium piscicola (Collins et al. 1987) DSM 20730T and DSM 20722 as Carnobacterium maltaromaticum comb. nov. Int. J. Syst. Evol. Microbiol. 2003, 53, 675–678. [Google Scholar] [CrossRef]
- Miller, A., III; Morgan, M.E.; Libbey, L.M. Lactobacillus maltaromicus, a new species producing a malty aroma. Int. J. Syst. Bacteriol. 1974, 24, 346–354. [Google Scholar] [CrossRef]
- Wiedmann, M.; Bruce, J.L.; Knorr, R.; Bodis, M.; Cole, E.M.; McDowell, C.I.; McDonough, P.L.; Batt, C.A. Ribotype Diversity of Listeria monocytogenes Strains Associated with Outbreaks of Listeriosis in Ruminants. J. Clin. Microbiol. 1996, 34, 1086–1090. [Google Scholar] [CrossRef] [PubMed]
- Schlech, W.F. Epidemiology and Clinical Manifestations of Listeria monocytogenes Infection. Microbiol. Spectr. 2019, 7, 3. [Google Scholar] [CrossRef]
- Weyna, A.A.W.; Niedringhaus, K.D.; Kunkel, M.R.; Fenton, H.M.A.; Keel, M.K.; Webb, A.H.; Bahnson, C.; Radisic, R.; Munk, B.; Sánchez, S.; et al. Listeriosis with Viral Coinfections in 8 Gray Foxes, 8 Wild Turkeys, and 2 Young Cervids in the Southeastern United States. J. Vet. Diagn. Investig. 2022, 34, 654–661. [Google Scholar] [CrossRef] [PubMed]
- Stahler, D.R.; Smith, D.W.; Guernsey, D.S. Foraging and Feeding Ecology of the Gray Wolf (Canis lupus): Lessons from Yellowstone National Park, Wyoming, USA. J. Nutr. 2006, 136, 1923S–1926S. [Google Scholar] [CrossRef] [PubMed]
- Lyu, T.; Liu, G.; Zhang, H.; Wang, L.; Zhou, S.; Dou, H.; Pang, B.; Sha, W.; Zhang, H. Changes in Feeding Habits Promoted the Differentiation of the Composition and Function of Gut Microbiotas Between Domestic Dogs (Canis lupus familiaris) and Gray Wolves (Canis lupus). AMB Express. 2018, 8, 123. [Google Scholar] [CrossRef]
- Wetzels, S.U.; Strachan, C.R.; Conrady, B.; Wagner, M.; Burgener, I.A.; Virányi, Z.; Selberherr, E. Wolves, Dogs and Humans in Regular Contact Can Mutually Impact Each Other’s Skin Microbiota. Sci. Rep. 2021, 11, 17106. [Google Scholar] [CrossRef]
- Podar, N.A.; Carrell, A.A.; Cassidy, K.A.; Klingeman, D.M.; Yang, Z.; Stahler, E.A.; Smith, D.W.; Stahler, D.R.; Podar, M. From Wolves to Humans: Oral Microbiome Resistance to Transfer Across Mammalian Hosts. mBio 2024, 15, e0334223. [Google Scholar] [CrossRef]
- Li, Q.; Chan, H.; Liu, W.X.; Liu, C.A.; Zhou, Y.; Huang, D.; Wang, X.; Li, X.; Xie, C.; Liu, W.Y.; et al. Carnobacterium maltaromaticum Boosts Intestinal Vitamin D Production to Suppress Colorectal Cancer in Female Mice. Cancer Cell. 2023, 41, 1450–1465.e8. [Google Scholar] [CrossRef]
- Wang, J.; Wang, T.; Sun, Y.; Feng, Y.; Kisseberth, W.C.; Henry, C.J.; Mok, I.; Lana, S.E.; Dobbin, K.; Northrup, N.; et al. Proliferative and Invasive Colorectal Tumors in Pet Dogs Provide Unique Insights into Human Colorectal Cancer. Cancers 2018, 10, 330. [Google Scholar] [CrossRef]
- Olaimat, A.N.; Al-Holy, M.A.; Shahbaz, H.M.; Al-Nabulsi, A.A.; Abu Ghoush, M.H.; Osaili, T.M.; Ayyash, M.M.; Holley, R.A. Emergence of Antibiotic Resistance in Listeria monocytogenes Isolated from Food Products: A Comprehensive Review. Compr. Rev. Food Sci. Food Saf. 2018, 17, 1277–1292. [Google Scholar] [CrossRef]
- Baquero, F.; Lanza, V.F.; Duval, M.; Coque, T.M. Ecogenetics of Antibiotic Resistance in Listeria monocytogenes. Mol. Microbiol. 2020, 113, 570–579. [Google Scholar] [CrossRef]
- Díaz-Martínez, C.; Bolívar, A.; Mercanoglu Taban, B.; Kanca, N.; Pérez-Rodríguez, F. Exploring the Antibiotic Resistance of Listeria monocytogenes in Food Environments—A Review. Crit. Rev. Microbiol. 2024, 15, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Hwang, H.-J.; Ciufolini, M.A. Therapies from Thiopeptides. Molecules 2023, 28, 7579. [Google Scholar] [CrossRef] [PubMed]
- Claesen, J.; Spagnolo, J.B.; Ramos, S.F.; Kurita, K.L.; Byrd, A.L.; Aksenov, A.A.; Melnik, A.V.; Wong, W.R.; Wang, S.; Hernandez, R.D.; et al. A Cutibacterium acnes Antibiotic Modulates Human Skin Microbiota Composition in Hair Follicles. Sci Transl Med. 2020, 12, eaay5445. [Google Scholar] [CrossRef] [PubMed]
- Carnio, M.C.; Stachelhaus, T.; Francis, K.P.; Scherer, S. Pyridinyl Polythiazole Class Peptide Antibiotic Micrococcin P1, Secreted by Foodborne Staphylococcus equorum WS2733, is Biosynthesized Nonribosomally. Eur J Biochem. 2001, 268, 6390–6401. [Google Scholar] [CrossRef]
- Widodo, W.S.; Billerbeck, S. Natural and Engineered Cyclodipeptides: Biosynthesis, Chemical Diversity, and Engineering Strategies for Diversification and High-yield Bioproduction. Eng. Microbiol. 2022, 3, 100067. [Google Scholar] [CrossRef]
- Wyatt, M.A.; Wang, W.; Roux, C.M.; Beasley, F.C.; Heinrichs, D.E.; Dunman, P.M.; Magarvey, N.A. Staphylococcus aureus Nonribosomal Peptide Secondary Metabolites Regulate Virulence. Science 2010, 29, 294–296. [Google Scholar] [CrossRef]
- Zimmermann, M.; Fischbach, M.A. A Family of Pyrazinone Natural Products from a Conserved Nonribosomal Peptide Synthetase in Staphylococcus aureus. Chem. Biol. 2010, 17, 925–930. [Google Scholar] [CrossRef]
- Chen, J.; Kuipers, O.P. Isolation and Analysis of the Nisin Biosynthesis Complex NisBTC: Further Insights into Their Cooperative Action. mBio 2021, 12, e0258521. [Google Scholar] [CrossRef]
- Vargas-González, A.; Barajas, M.; Pérez-Sánchez, T. Isolation of Lactic Acid Bacteria (LAB) from Salmonids for Potential Use as Probiotics: In Vitro Assays and Toxicity Assessment of Salmo trutta Embryonated Eggs. Animals 2024, 14, 200. [Google Scholar] [CrossRef]
- Elzinga, D.C.; Kulwicki, R.; Iselin, S.; Spence, L.; Capaldi, A. Rapid Evolution of Prehistoric Dogs from Wolves by Natural and Sexual Selection Emerges from an Agent-based Model. Proc. Biol. Sci. 2025, 292, 20242646. [Google Scholar] [CrossRef] [PubMed]
- Bornbusch, S.L.; Power, M.L.; Schulkin, J.; Drea, C.M.; Maslanka, M.T.; Muletz-Wolz, C.R. Integrating Microbiome Science and Evolutionary Medicine into Animal Health and Conservation. Biol. Rev. Camb. Philos. Soc. 2024, 99, 458–477. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, B.; Liu, C.; Li, X.; Li, X.; Zhang, X.; Irwin, D.M.; Wu, Z.; Chen, Z.; Jin, Q.; et al. Differences in the gut microbiomes of dogs and wolves: Roles of antibiotics and starch. BMC Vet Res. 2021, 17, 112. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Sun, M.; Xu, D.; Gao, Z.; Shi, Y.; Wang, S.; Zhou, Y. Gut microbiome of captive wolves is more similar to domestic dogs than wild wolves indicated by metagenomics study. Front Microbiol. 2022, 13, 1027188. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).