Impact of Enterococcus faecium Kimate-X on Reducing Stress in Dogs Through Gut Microbiota Modulation
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Study on the Alleviation of Oxidative Stress by Enterococcus faecium Kimate-X In Vitro
2.2. Animals and Experimental Design
2.3. Blood Sample Collection and Analysis
2.4. Fecal Sample Collection and Analysis
2.5. DNA Extraction and Shotgun Metagenomic Sequencing
2.6. Sequencing Data Processing
2.7. Microbial Taxonomic and Functional Profiles and Analysis
2.8. Fecal Short-Chain Fatty Acid Analysis
2.9. Statistical Analysis
3. Results
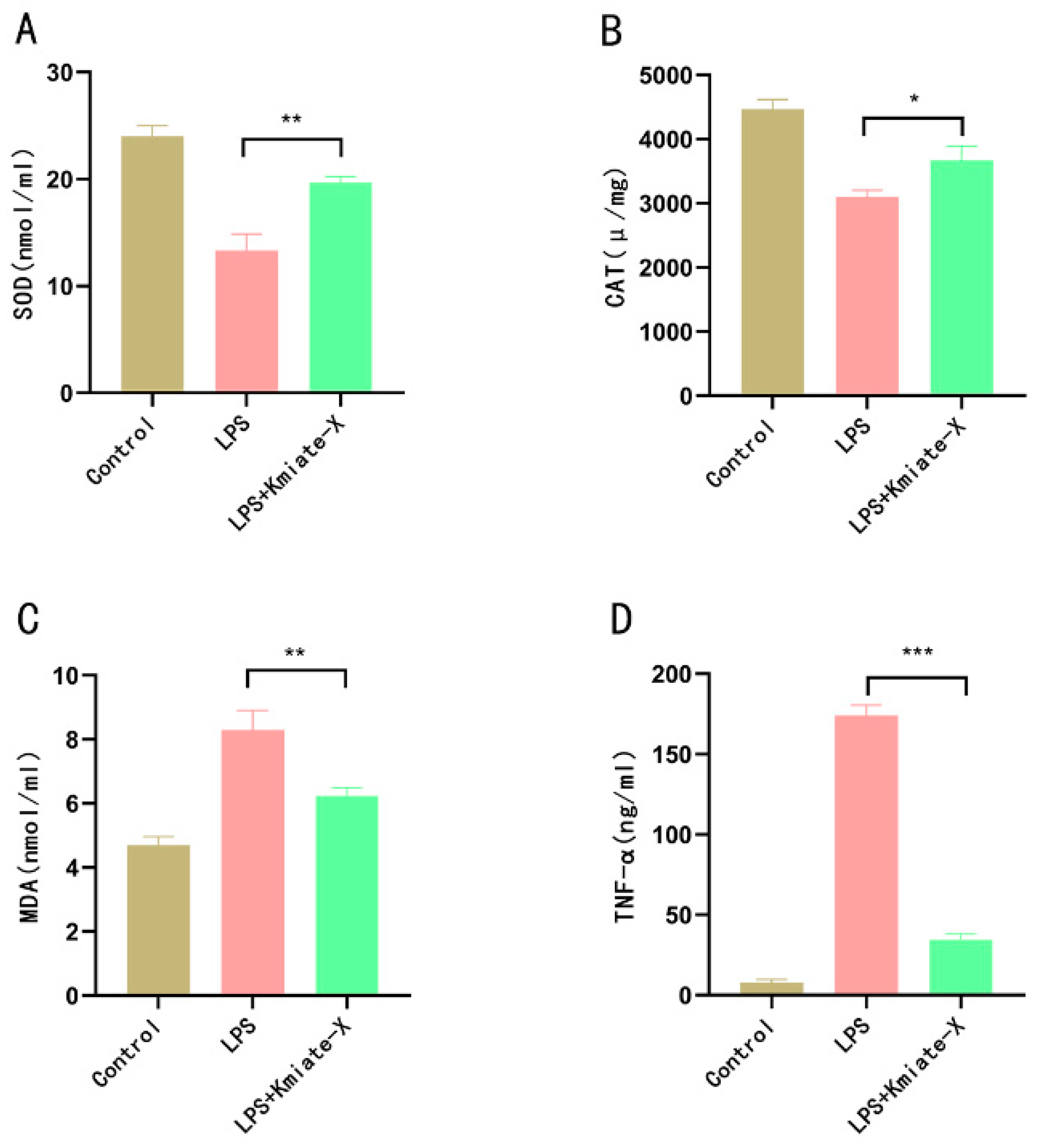
3.1. Effects of Enterococcus faecium Kimate-X on Alleviating Oxidative Stress In Vitro
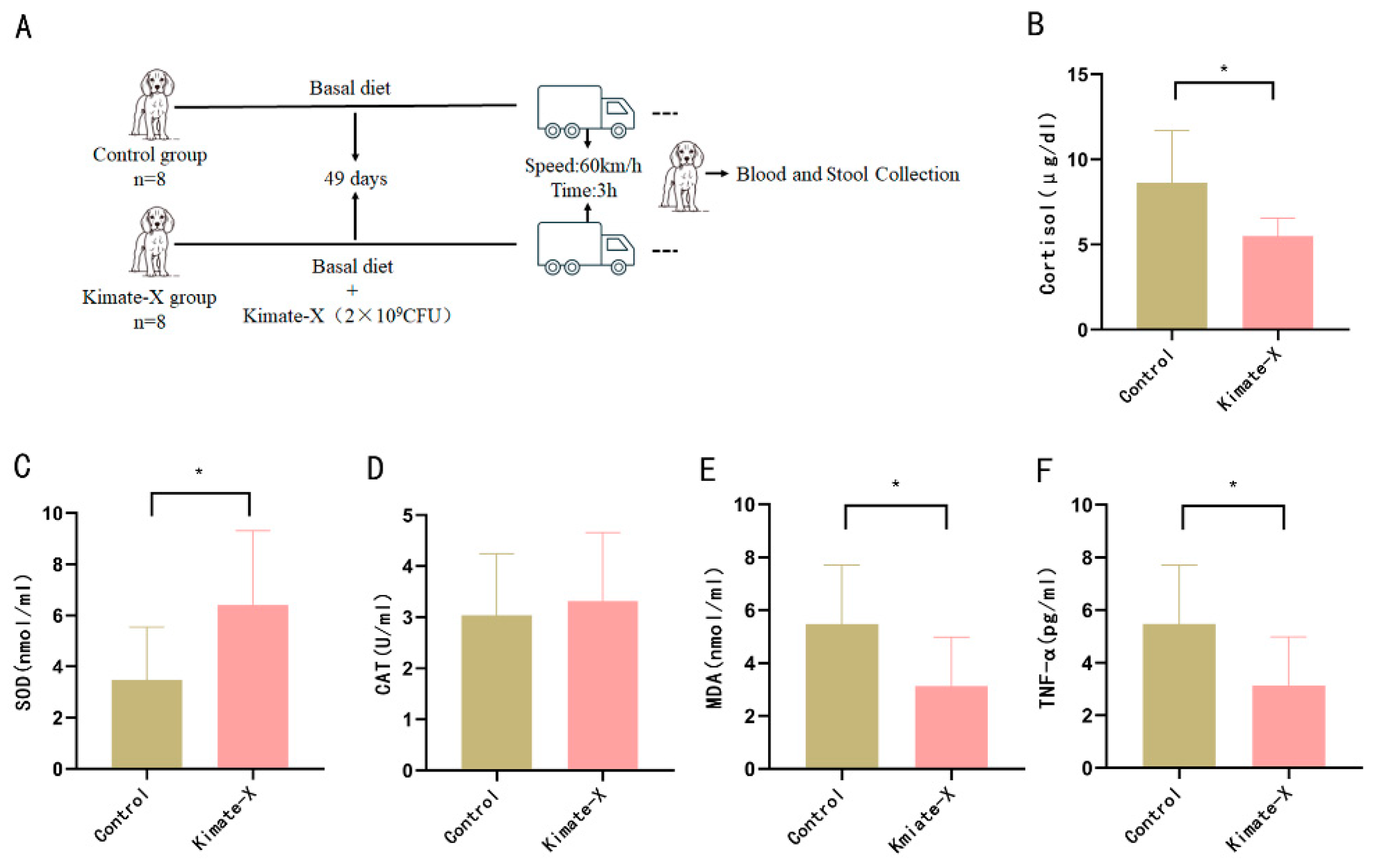
3.2. Effects of Enterococcus faecium Kimate-X on Antioxidant and Inflammatory Markers in Transport-Stressed Dogs
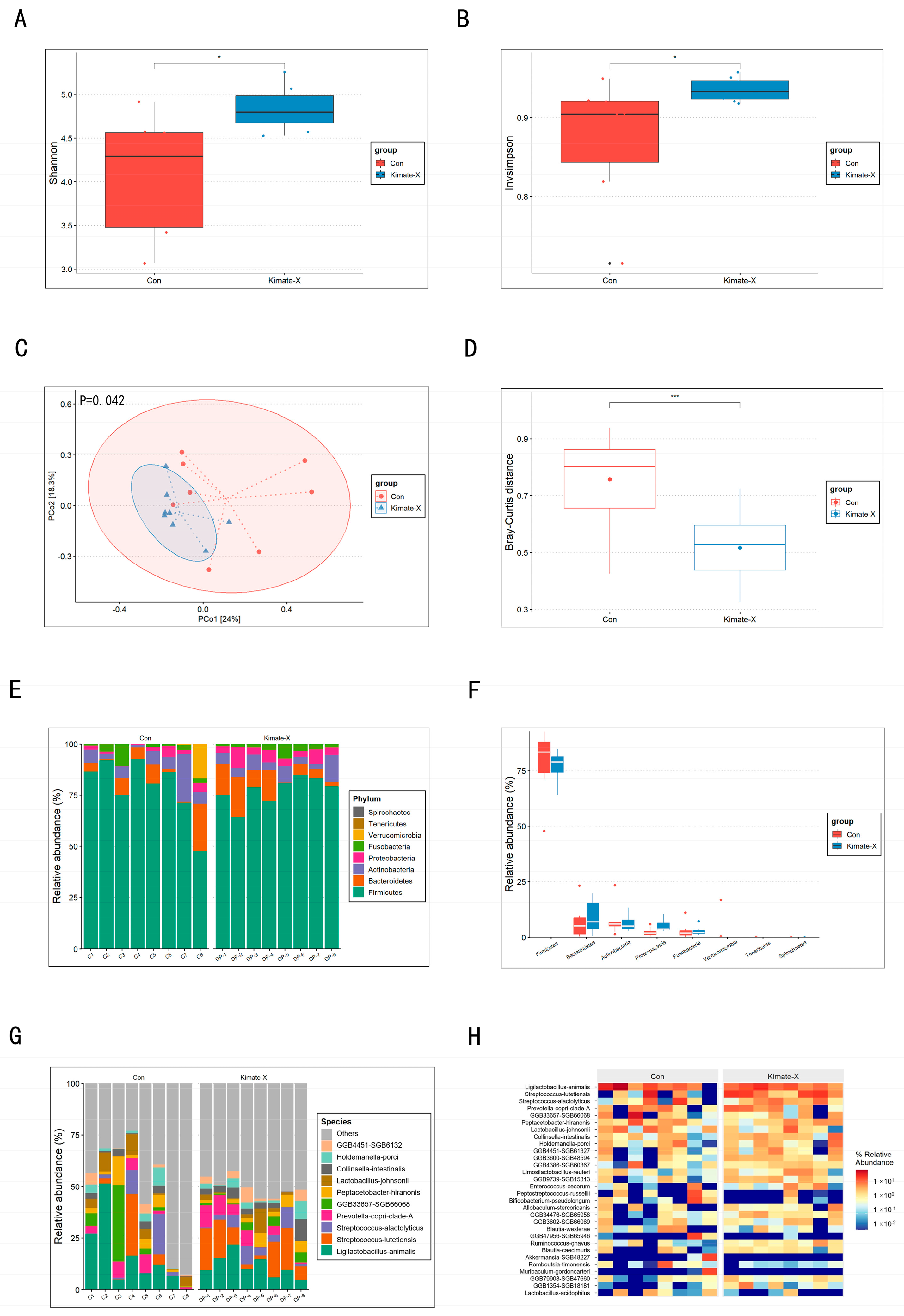
3.3. Results of Fecal Microbiota Composition Analysis
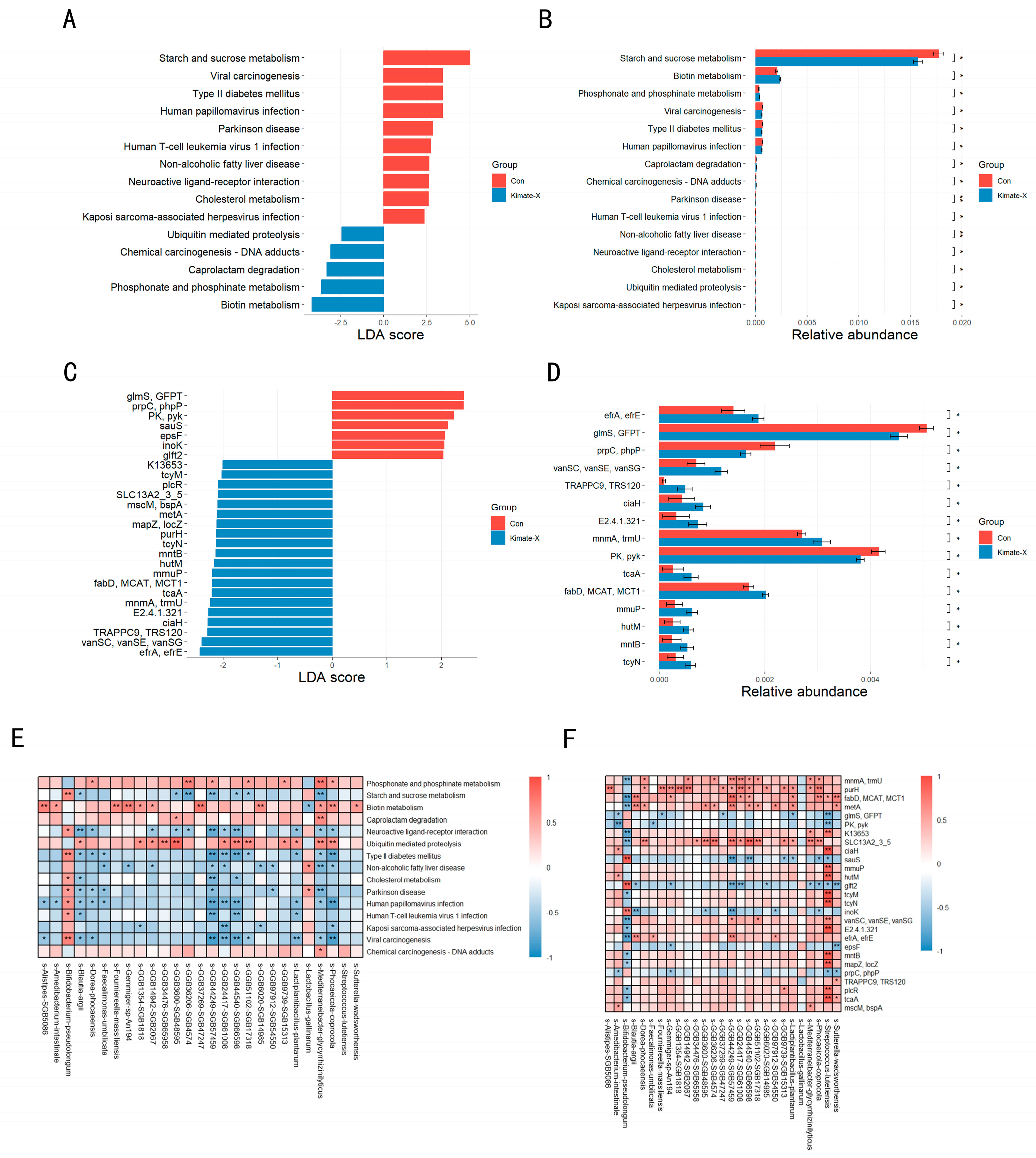
3.4. Metabolic Pathways and Gene Expression in the Gut Microbiota
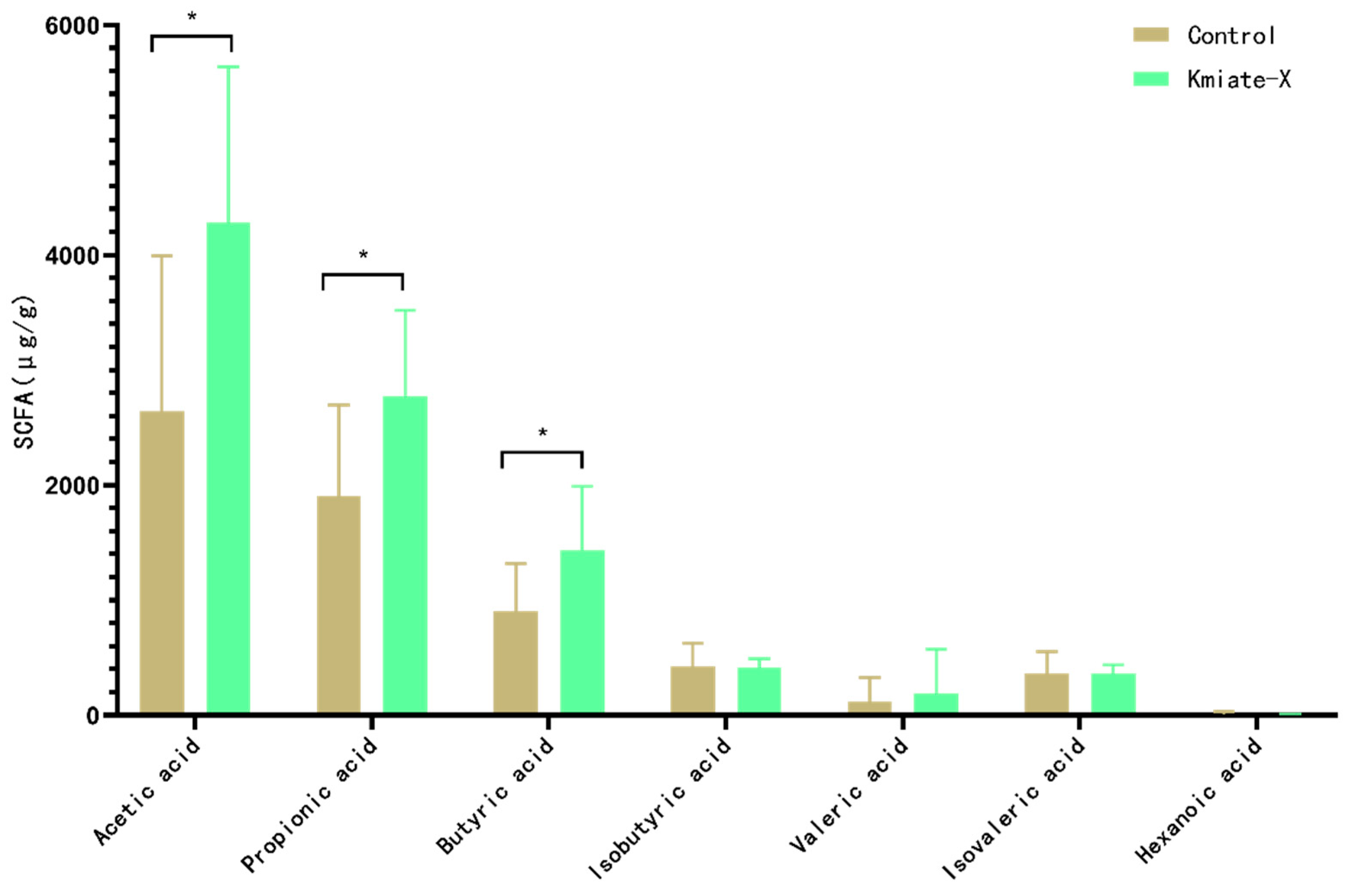
3.5. Results of Fecal SCFA Analysis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Riemer, S. Therapy and Prevention of Noise Fears in Dogs—A Review of the Current Evidence for Practitioners. Animals 2023, 13, 3664. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, C.S.; Vasconcellos, R.S.; Pedreira, R.S.; Silva, F.L.; Sá, F.C.; Kroll, F.S.A.; Maria, A.P.J.; Venturini, K.S.; Carciofi, A.C. Alterations to Oxidative Stress Markers in Dogs after a Short-Term Stress during Transport. J. Nutr. Sci. 2014, 3, e27. [Google Scholar] [CrossRef]
- Wang, L.; Ling, Y.Z.; Chuan, X.Y.; Gao, X.X.; Fu, L.D.; Wang, X. Effect of Flight Transport Stress on Blood Parameters in Beagles and the Anti-Stress Effect of Dangshen. Anim. Models Exp. Med. 2018, 1, 162–168. [Google Scholar]
- Mariti, C.; Raspanti, E.; Zilocchi, M.; Carlone, B.; Gazzano, A. The assessment of dog welfare in the waiting room of a veterinary clinic. Anim. Welf. 2015, 24, 299–305. [Google Scholar] [CrossRef]
- Gilbert-Gregory, S.E.; Stull, J.W.; Rice, M.R.; Herron, M.E. Effects of Trazodone on Behavioral Signs of Stress in Hospitalized Dogs. J. Am. Vet. Med. Assoc. 2016, 249, 1281–1291. [Google Scholar] [CrossRef]
- Hong, C.; Huang, Y.; Cao, S.; Wang, L.; Yang, X.; Hu, S.; Gao, K.; Jiang, Z.; Xiao, H. Accurate Models and Nutritional Strategies for Specific Oxidative Stress Factors: Does the Dose Matter in Swine Production? J. Anim. Sci. Biotechnol. 2024, 15, 11. [Google Scholar] [CrossRef]
- Wu, W.L.; Adame, M.D.; Liou, C.W.; Barlow, J.T.; Lai, T.T.; Sharon, G.; Schretter, C.E.; Needham, B.D.; Wang, M.I.; Tang, W.; et al. Microbiota Regulate Social Behaviour via Stress Response Neurons in the Brain. Nature. 2021, 595, 409–414. [Google Scholar] [CrossRef]
- Tristan, D.C.; Diana, L.; Nathalie, F.L.; Tesseraud, S.; Lambert, W.; Bosi, P.; Trevisi, P.; Beaumont, M.; Corrent, E. Functional Amino Acids in Pigs and Chickens: Implication for Gut Health. Front. Vet. Sci. 2021, 8, 663727. [Google Scholar]
- Wilson, M.S.; Oba, M.P.; Applegate, C.C.; Samantha, A.K.; Matthew, R.P.; Sharon, A.N.; Swanson, K.S. Effects of a Saccharomyces cerevisiae Fermentation Product-Supplemented Diet on Fecal Characteristics, Oxidative Stress, and Blood Gene Expression of Adult Dogs Undergoing Transport Stress. J. Anim. Sci. 2023, 101, skac378. [Google Scholar] [CrossRef]
- Kang, Y.; Shiyan, J.; Chaoyu, W.; Guo, D.; Liao, P.F.; Wen, J.W.; Kuang, T.; Han, S.; Liu, Q.; Deng, B. Gallnut Tannic Acid Exerts Anti-Stress Effects on Stress-Induced Inflammatory Response, Dysbiotic Gut Microbiota, and Alterations of Serum Metabolic Profile in Beagle Dogs. Front. Nutr. 2022, 9, 847966. [Google Scholar]
- Girault, C.; Priymenko, N.; Helsly, M.; Duranton, C.; Gaunet, F. Dog Behaviours in Veterinary Consultations: Part 1. Effect of the Owner’s Presence or Absence. Vet. J. 2022, 280, 105788. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Liu, B.; Huang, L.; Li, Z.; Cheng, Y.; Tian, Y.; Xie, L. Probiotic Consortia and Their Metabolites Ameliorate the Symptoms of Inflammatory Bowel Diseases in a Colitis Mouse Model. Microbiol. Spectr. 2022, 10, e0065722. [Google Scholar] [CrossRef]
- Plaza-Díaz, J.; Ruiz-Ojeda, J.F.; Vilchez-Padial, M.L.; Angel, G. Evidence of the Anti-Inflammatory Effects of Probiotics and Synbiotics in Intestinal Chronic Diseases. Nutrients 2017, 9, 555. [Google Scholar] [CrossRef]
- Huang, J.; Xu, Y.; Wang, M.; Yu, S.; Li, Y.; Tian, H.; Zhang, C.; Li, H. Enterococcus faecium R-026 Combined with Bacillus subtilis R-179 Alleviate Hypercholesterolemia and Modulate the Gut Microbiota in C57BL/6 Mice. FEMS Microbiol. Lett. 2023, 370, fnad118. [Google Scholar] [CrossRef] [PubMed]
- Shansong, H.; Kang, Y.; Jiawei, W.; Tao, K.; Zhihao, C. Antimicrobial Peptides Relieve Transportation Stress in Ragdoll Cats by Regulating the Gut Microbiota. Metabolites 2023, 13, 326. [Google Scholar] [CrossRef]
- Jay, S.J.; Matthew, A.A.; Alan, W.D.; Kouassi, R.K. Early Life Thermal Stress: Impact on Future Thermotolerance, Stress Response, Behavior, and Intestinal Morphology in Piglets Exposed to a Heat Stress Challenge during Simulated Transport. J. Anim. Sci. 2018, 96, 1640–1653. [Google Scholar]
- Johannes, H.; Jörg, A.; Camille, G.; Maria, M.; Christine, A. Stress Response of Beagle Dogs to Repeated Short-Distance Road Transport. Animals 2020, 10, 11. [Google Scholar] [CrossRef] [PubMed]
- Qiuye, L.; Jinjiang, D.; Hu, X.; Mingkui, L.; Le, X. Effects of Fecal Microbial Transplantation on Police Performance and Transportation Stress in Kunming Police Dogs. Appl. Microbiol. Biotechnol. 2024, 108, 11–13. [Google Scholar]
- Lee, C.; Kim, W.S.; Verma, R.; Noh, J.; Parket, C.J.; Park, S.; Lee, H.; Park, H.E.; Kim, C.J.; Byun, S.; et al. Probiotic Consortium Confers Synergistic Anti-Inflammatory Effects in Inflammatory Disorders. Nutrients 2024, 16, 790. [Google Scholar] [CrossRef]
- Behzad, Z.; Ali, S.; Nazli, N.; Bagher, L.; Leila, A. The Effects of Supplementation with Probiotic on Biomarkers of Oxidative Stress in Adult Subjects: A Systematic Review and Meta-Analysis of Randomized Trials. Probiotics Antimicrob. Proteins 2020, 12, 102–111. [Google Scholar]
- Staudacher, H.M.; Black, C.J.; Teasdale, S.B.; Mikocka-Walus, A.; Keefer, L. Irritable Bowel Syndrome and Mental Health Comorbidity—Approach to Multidisciplinary Management. Nat. Rev. Gastroenterol. Hepatol. 2023, 20, 582–596. [Google Scholar] [CrossRef] [PubMed]
- Lijin, S.; Qinghua, S.; Haonan, Z.; Yiming, Z.; Yujing, W. Roseburia hominis Alleviates Neuroinflammation via Short-Chain Fatty Acids through Histone Deacetylase Inhibition. Mol. Nutr. Food Res. 2022, 66, e2200164. [Google Scholar]
- Villena, J.; Villena, J.; Villena, J.; Kitazawa, H.; Kitazawa, H. Editorial: Immunobiotics—Interactions of Beneficial Microbes with the Immune System. Front. Immunol. 2017, 8, 1580. [Google Scholar] [CrossRef] [PubMed]
- Xinzhou, W.; Peng, Z.; Xin, Z. Probiotics Regulate Gut Microbiota: An Effective Method to Improve Immunity. Molecules 2021, 26, 6076. [Google Scholar] [CrossRef]
- Yiting, Y.; Yu, L.; Ying, Q.; Ting, Z. Bacteroides uniformis-Induced Perturbations in Colonic Microbiota and Bile Acid Levels Inhibit TH17 Differentiation and Ameliorate Colitis Developments. npj Biofilms Microbiomes 2023, 9, 56. [Google Scholar]
- Matsumoto, M.; Inoue, R.; Tsukahara, T.; Ushida, K.; Chiji, H. Voluntary Running Exercise Alters Microbiota Composition and Increases n-Butyrate Concentration in the Rat Cecum. Biosci. Biotechnol. Biochem. 2008, 72, 572–576. [Google Scholar] [CrossRef]
- Kang, S.S.; Jeraldo, P.R.; Kurti, A.; Miller, M.E.B.; Cook, M.D.; Whitlock, K.; Goldenfeld, N.; Woods, J.A.; White, B.A.; Chia, N.; et al. Diet and Exercise Orthogonally Alter the Gut Microbiome and Reveal Independent Associations with Anxiety and Cognition. Mol. Neurodegener. 2014, 9, 36. [Google Scholar] [CrossRef]
- Kazemi, A.; Noorbala, A.A.; Azam, K.; Djafarian, K. Effect of Prebiotic and Probiotic Supplementation on Circulating Pro-Inflammatory Cytokines and Urinary Cortisol Levels in Patients with Major Depressive Disorder: A Double-Blind, Placebo-Controlled Randomized Clinical Trial. J. Funct. Foods 2019, 52, 596–602. [Google Scholar] [CrossRef]
- Sapolsky, R.M.; Romero, L.M.; Munck, A.U. How Do Glucocorticoids Influence Stress Responses? Integrating Permissive, Suppressive, Stimulatory, and Preparative Actions. Endocr. Rev. 2000, 21, 55–89. [Google Scholar]
- Zhicong, F.; Zhaowei, B.; Hongcan, H.; Tingting, L.; Ruiti, R. Dietary Strategies for Relieving Stress in Pet Dogs and Cats. Antioxidants 2023, 12, 545. [Google Scholar] [CrossRef]
- Muhammad, A.; Farhan, S.; Abbas, Y.S.; Muzzamal, H.; Roshina, R. Human Gut Microbiota in Health and Disease: Unveiling the Relationship. Front. Microbiol. 2022, 13, 999001. [Google Scholar]
- Zakaria, E.G. Human Gut Microbiota/Microbiome in Health and Diseases: A Review. Antonie Van Leeuwenhoek 2020, 113, 2019–2040. [Google Scholar]
- Chen, P.; Xu, H.; Tang, H.; Zhao, F.; Yang, C.; Kwok, L.; Cong, C.; Wu, Y.; Zhang, W.; Zhou, X.; et al. Modulation of Gut Mucosal Microbiota as a Mechanism of Probiotics-Based Adjunctive Therapy for Ulcerative Colitis. Microb. Biotechnol. 2020, 13, 2032–2043. [Google Scholar] [CrossRef]
- Evans, C.C.; LePard, J.K.; Kwak, W.J.; Stancukas, M.C.; Laskowski, S.; Dougherty, J.; Moulton, L.; Glawe, A.; Wang, Y.; Leone, V.; et al. Exercise Prevents Weight Gain and Alters the Gut Microbiota in a Mouse Model of High Fat Diet-Induced Obesity. PLoS ONE 2017, 9, e92193. [Google Scholar] [CrossRef]
- Facchin, S.; Bertin, L.; Bonazzi, E.; Lorenzon, G.; Barba, C.; Barberio, B.; Zingone, F.; Maniero, D.; Scarpa, M.; Ruffolo, C.; et al. Short-Chain Fatty Acids and Human Health: From Metabolic Pathways to Current Therapeutic Implications. Life 2024, 14, 559. [Google Scholar] [CrossRef]
- Virginie, A.M.; Yann, R. Short Chain Fatty Acids: The Messengers from Down Below. Front. Neurosci. 2023, 17, 197759. [Google Scholar]
- Dan, Z.; Ping, Y.J.; Ning, Y.Z.; Yao, L.; Ting, G.L. Short-Chain Fatty Acids in Diseases. Cell Commun. Signal. 2023, 21, 212. [Google Scholar]
- Chaima, E.; Léa, L.; Nadine, M.; Christine, B.; Pierre, D. Fatty Acids Produced by the Gut Microbiota Dampens Host Inflammatory Responses by Modulating Intestinal SUMOylation. Gut Microbes 2022, 14, 2108280. [Google Scholar]
- Haro, C.; Rangel-Zúñiga, O.A.; Alcalá-Díaz, J.F.; Gómez-Delgado, F.; Pérez-Martínez, P.; Delgado-Lista, J.; Quintana-Navarro, G.M.; Landa, B.B.; Navas-Cortés, J.A.; Tena-Sempere, M.; et al. Intestinal Microbiota Is Influenced by Gender and Body Mass Index. PLoS ONE 2016, 11, e0154090. [Google Scholar] [CrossRef]
- O’Toole, W.P.; Claesson, J.M. Gut microbiota: Changes throughout the lifespan from infancy to elderly. Int. Dairy J. 2010, 20, 281–291. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, R.; Hu, W.; Zhong, S.; Chen, W.; Chen, M.; Yu, Q. Impact of Enterococcus faecium Kimate-X on Reducing Stress in Dogs Through Gut Microbiota Modulation. Vet. Sci. 2025, 12, 412. https://doi.org/10.3390/vetsci12050412
Zhang R, Hu W, Zhong S, Chen W, Chen M, Yu Q. Impact of Enterococcus faecium Kimate-X on Reducing Stress in Dogs Through Gut Microbiota Modulation. Veterinary Sciences. 2025; 12(5):412. https://doi.org/10.3390/vetsci12050412
Chicago/Turabian StyleZhang, Rui, Wanjin Hu, Saiwei Zhong, Weiyang Chen, Meiru Chen, and Qinghua Yu. 2025. "Impact of Enterococcus faecium Kimate-X on Reducing Stress in Dogs Through Gut Microbiota Modulation" Veterinary Sciences 12, no. 5: 412. https://doi.org/10.3390/vetsci12050412
APA StyleZhang, R., Hu, W., Zhong, S., Chen, W., Chen, M., & Yu, Q. (2025). Impact of Enterococcus faecium Kimate-X on Reducing Stress in Dogs Through Gut Microbiota Modulation. Veterinary Sciences, 12(5), 412. https://doi.org/10.3390/vetsci12050412



