Comparative Aspects of BRAF Mutations in Canine Cancers
Abstract
:1. BRAF/MAPK Pathway in Cancer Pathogenesis
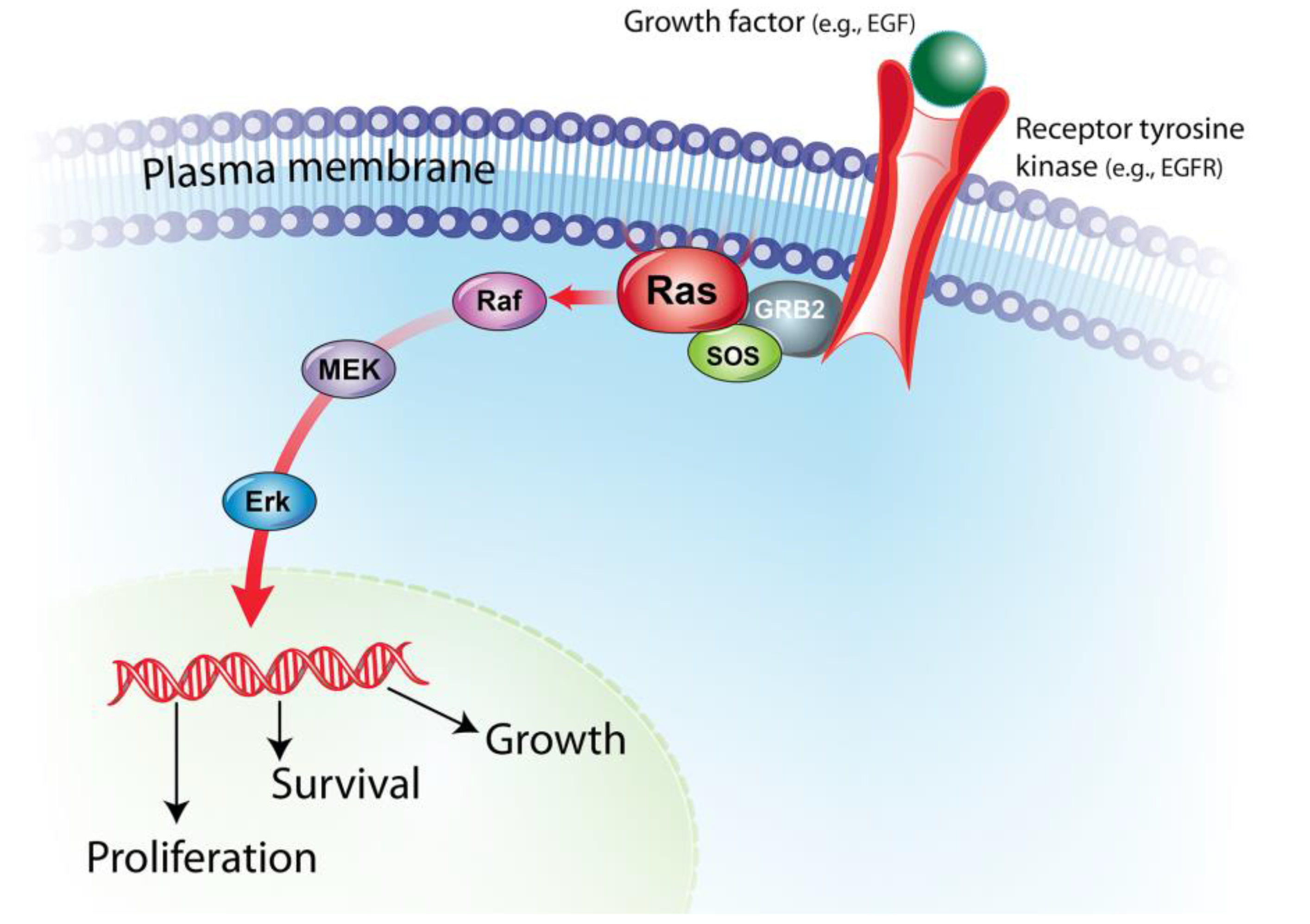
2. BRAF Mutations in Human and Canine Cancers
2.1. Melanocytic Tumors
2.2. Urothelial Carcinoma and Prostatic Carcinoma
2.3. Brain Tumors
2.4. Hematopoietic Tumors
2.5. Thyroid Cancers
2.6. Other Cancers
3. Clinical Implications of BRAF Mutations in Human and Canine Oncology
3.1. BRAF Mutation as a Cancer Marker
3.2. BRAF/MAPK-Targeted Therapy
4. Conclusions
Acknowledgements
Author Contributions
Conflicts of Interest
References
- Dhillon, A.; Hagan, S.; Rath, O.; Kolch, W. Map kinase signalling pathways in cancer. Oncogene 2007, 26, 3279–3290. [Google Scholar] [CrossRef] [PubMed]
- Downward, J. Targeting RAS signalling pathways in cancer therapy. Nat. Rev. Cancer 2003, 3, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Griffey, S.M.; Kraegel, S.A.; Madewell, B.R. Rapid detection of K-ras gene mutations in canine lung cancer using single-strand conformational polymorphism analysis. Carcinogenesis 1998, 19, 959–963. [Google Scholar] [CrossRef] [PubMed]
- Usher, S.G.; Radford, A.D.; Villiers, E.J.; Blackwood, L. RAS, FLT3, and C-KIT mutations in immunophenotyped canine leukemias. Exp. Hematol. 2009, 37, 65–77. [Google Scholar] [CrossRef] [PubMed]
- Campos, M.; Kool, M.M.; Daminet, S.; Ducatelle, R.; Rutteman, G.; Kooistra, H.S.; Galac, S.; Mol, J.A. Upregulation of the PI3K/AKT pathway in the tumorigenesis of canine thyroid carcinoma. J. Vet. Intern. Med. 2014, 28, 1814–1823. [Google Scholar] [CrossRef] [PubMed]
- Mayr, B.; Schaffner, G.; Reifinger, M. K-ras mutations in canine pancreatic cancers. Vet. Rec. 2003, 153, 87–89. [Google Scholar] [CrossRef] [PubMed]
- Richter, A.; Murua Escobar, H.; Gunther, K.; Soller, J.T.; Winkler, S.; Nolte, I.; Bullerdiek, J. RAS gene hot-spot mutations in canine neoplasias. J. Heredity 2005, 96, 764–765. [Google Scholar] [CrossRef] [PubMed]
- Sebolt-Leopold, J.S.; Herrera, R. Targeting the mitogen-activated protein kinase cascade to treat cancer. Nat. Rev. Cancer 2004, 4, 937–947. [Google Scholar] [CrossRef] [PubMed]
- Roskoski, R. RAF protein-serine/threonine kinases: Structure and regulation. Biochem. Biophys. Res. Commun. 2010, 399, 313–317. [Google Scholar] [CrossRef] [PubMed]
- Davies, H.; Bignell, G.R.; Cox, C.; Stephens, P.; Edkins, S.; Clegg, S.; Teague, J.; Woffendin, H.; Garnett, M.J.; Bottomley, W.; et al. Mutations of the BRAF gene in human cancer. Nature 2002, 417, 949–954. [Google Scholar] [CrossRef] [PubMed]
- Mochizuki, H.; Kennedy, K.; Shapiro, S.G.; Breen, M. Braf mutations in canine cancers. PLoS ONE 2015, 10. [Google Scholar] [CrossRef] [PubMed]
- Decker, B.; Parker, H.G.; Dhawan, D.; Kwon, E.M.; Karlins, E.; Davis, B.W.; Ramos-Vara, J.A.; Bonney, P.L.; McNiel, E.A.; Knapp, D.W.; et al. Homologous mutation to human BRAF V600E is common in naturally occurring canine bladder cancer-evidence for a relevant model system and urine-based diagnostic test. Mol. Cancer Res. 2015. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, C.C.; Wu, X.C.; Jemal, A.; Martin, H.J.; Roche, L.M.; Chen, V.W. Incidence of noncutaneous melanomas in the US. Cancer 2005, 103, 1000–1007. [Google Scholar] [CrossRef] [PubMed]
- Chang, A.E.; Karnell, L.H.; Menck, H.R. The national cancer data base report on cutaneous and noncutaneous melanoma. Cancer 1998, 83, 1664–1678. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2015. Ca-A Cancer J. Clin. 2015, 65, 5–29. [Google Scholar] [CrossRef] [PubMed]
- Garbe, C.; Leiter, U. Melanoma epidemiology and trends. Clinics in Dermatology 2009, 27, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Brose, M.S.; Volpe, P.; Feldman, M.; Kumar, M.; Rishi, I.; Gerrero, R.; Einhorn, E.; Herlyn, M.; Minna, J.; Nicholson, A. BRAF and RAS mutations in human lung cancer and melanoma. Cancer. Res. 2002, 62, 6997–7000. [Google Scholar] [PubMed]
- Tsao, H.; Goel, V.; Wu, H.; Yang, G.; Haluska, F.G. Genetic interaction between NRAS and BRAF mutations and PTEN/MMAC1 inactivation in melanoma. J. Invest. Dermatol. 2004, 122, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Goel, V.K.; Lazar, A.J.; Warneke, C.L.; Redston, M.S.; Haluska, F.G. Examination of mutations in BRAF, NRAS, and PTEN in primary cutaneous melanoma. J. Invest. Dermatol. 2006, 126, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Simpson, R.M.; Bastian, B.C.; Michael, H.T.; Webster, J.D.; Prasad, M.L.; Conway, C.M.; Prieto, V.M.; Gary, J.M.; Goldschmidt, M.H.; Esplin, D.G.; et al. Sporadic naturally occurring melanoma in dogs as a preclinical model for human melanoma. Pigm. Cell Melanoma Res. 2014, 27, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Gillard, M.; Cadieu, E.; De Brito, C.; Abadie, J.; Vergier, B.; Devauchelle, P.; Degorce, F.; Dreano, S.; Primot, A.; Dorso, L.; et al. Naturally occurring melanomas in dogs as models for non-uv pathways of human melanomas. Pigm. Cell Melanoma Res. 2014, 27, 90–102. [Google Scholar] [CrossRef] [PubMed]
- Shelly, S.; Chien, M.B.; Yip, B.; Kent, M.S.; Theon, A.P.; McCallan, J.L.; London, C.A. Exon 15 BRAF mutations are uncommon in canine oral malignant melanomas. Mamm Genome 2005, 16, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Fowles, J.; Denton, C.; Gustafson, D. Comparative analysis of MAPK and PI3K/AKT pathway activation and inhibition in human and canine melanoma. Vet. Comp. Oncol. 2013. [Google Scholar] [CrossRef] [PubMed]
- Maldonado, J.L.; Fridlyand, J.; Patel, H.; Jain, A.N.; Busam, K.; Kageshita, T.; Ono, T.; Albertson, D.G.; Pinkel, D.; Bastian, B.C. Determinants of BRAF mutations in primary melanomas. J. Nat. Cancer Inst. 2003, 95, 1878–1890. [Google Scholar] [CrossRef] [PubMed]
- Edwards, R.; Ward, M.; Wu, H.; Medina, C.; Brose, M.; Volpe, P.; Nussen-Lee, S.; Haupt, H.; Martin, A.; Herlyn, M. Absence of BRAF mutations in uv-protected mucosal melanomas. J. Med. Genet 2004, 41, 270–272. [Google Scholar] [CrossRef] [PubMed]
- Pollock, P.M.; Harper, U.L.; Hansen, K.S.; Yudt, L.M.; Stark, M.; Robbins, C.M.; Moses, T.Y.; Hostetter, G.; Wagner, U.; Kakareka, J.; et al. High frequency of BRAF mutations in nevi. Nat. Genet. 2003, 33, 19–20. [Google Scholar] [CrossRef] [PubMed]
- Knowles, M.A.; Hurst, C.D. Molecular biology of bladder cancer: New insights into pathogenesis and clinical diversity. Nat. Rev. Cancer 2015, 15, 25–41. [Google Scholar] [CrossRef] [PubMed]
- Boulalas, L.; Zaravinos, A.; Delakas, D.; Spandidos, D.A. Mutational analysis of the BRAF gene in transitional cell carcinoma of the bladder. Int. J. Biol. Marker 2009, 24, 17–21. [Google Scholar]
- Stoehr, R.; Brinkmann, A.; Filbeck, T.; Gamper, C.; Wild, P.; Blaszyk, H.; Hofstaedter, F.; Knuechel, R.; Hartmann, A. No evidence for mutation of b-raf in urothelial carcinomas of the bladder and upper urinary tract. Oncol. Rep. 2004, 11, 137–141. [Google Scholar] [CrossRef] [PubMed]
- Jebar, A.H.; Hurst, C.D.; Tomlinson, D.C.; Johnston, C.; Taylor, C.F.; Knowles, M.A. FGFR3 and RAS gene mutations are mutually exclusive genetic events in urothelial cell carcinoma. Oncogene 2005, 24, 5218–5225. [Google Scholar] [CrossRef] [PubMed]
- Di Martino, E.; L’Hote, C.G.; Kennedy, W.; Tomlinson, D.C.; Knowles, M.A. Mutant fibroblast growth factor receptor 3 induces intracellular signaling and cellular transformation in a cell type- and mutation-specific manner. Oncogene 2009, 28, 4306–4316. [Google Scholar] [CrossRef] [PubMed]
- Knapp, D.W.; Ramos-Vara, J.A.; Moore, G.E.; Dhawan, D.; Bonney, P.L.; Young, K.E. Urinary bladder cancer in dogs, a naturally occurring model for cancer biology and drug development. ILAR J. 2014, 55, 100–118. [Google Scholar] [CrossRef] [PubMed]
- Knapp, D.W.; Glickman, N.W.; Denicola, D.B.; Bonney, P.L.; Lin, T.L.; Glickman, L.T. Naturally-occurring canine transitional cell carcinoma of the urinary bladder a relevant model of human invasive bladder cancer. Urol. Oncol. 2000, 5, 47–59. [Google Scholar] [CrossRef]
- Kollermann, J.; Albrecht, H.; Schlomm, T.; Huland, H.; Graefen, M.; Bokemeyer, C.; Simon, R.; Sauter, G.; Wilczak, W. Activating braf gene mutations are uncommon in hormone refractory prostate cancer in caucasian patients. Oncol. Lett. 2010, 1, 729–732. [Google Scholar] [PubMed]
- Liu, T.; Willmore-Payne, C.; Layfield, L.J.; Holden, J.A. Lack of BRAF activating mutations in prostate adenocarcinoma: A study of 93 cases. Appl. Immunohistochem. Mol. Morp. 2009, 17, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Palmieri, C.; Lean, F.Z.; Akter, S.H.; Romussi, S.; Grieco, V. A retrospective analysis of 111 canine prostatic samples: Histopathological findings and classification. Res. Vet. Sci. 2014, 97, 568–573. [Google Scholar] [CrossRef] [PubMed]
- Cornell, K.K.; Bostwick, D.G.; Cooley, D.M.; Hall, G.; Harvey, H.J.; Hendrick, M.J.; Pauli, B.U.; Render, J.A.; Stoica, G.; Sweet, D.C.; et al. Clinical and pathologic aspects of spontaneous canine prostate carcinoma: A retrospective analysis of 76 cases. Prostate 2000, 45, 173–183. [Google Scholar] [CrossRef]
- LeRoy, B.E.; Nadella, M.V.; Toribio, R.E.; Leav, I.; Rosol, T.J. Canine prostate carcinomas express markers of urothelial and prostatic differentiation. Vet. Pathol. 2004, 41, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Sorenmo, K.U.; Goldschmidt, M.; Shofer, F.; Goldkamp, C.; Ferracone, J. Immunohistochemical characterization of canine prostatic carcinoma and correlation with castration status and castration time. Vet.Comp. Oncol. 2003, 1, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Louis, D.N.; Ohgaki, H.; Wiestler, O.D.; Cavenee, W.K.; Burger, P.C.; Jouvet, A.; Scheithauer, B.W.; Kleihues, P. The 2007 WHO classification of tumours of the central nervous system. Acta. Neuropathol. 2007, 114, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Schindler, G.; Capper, D.; Meyer, J.; Janzarik, W.; Omran, H.; Herold-Mende, C.; Schmieder, K.; Wesseling, P.; Mawrin, C.; Hasselblatt, M. Analysis of BRAF V600E mutation in 1,320 nervous system tumors reveals high mutation frequencies in pleomorphic xanthoastrocytoma, ganglioglioma and extra-cerebellar pilocytic astrocytoma. Acta. Neuropathol. 2011, 121, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Korshunov, A.; Meyer, J.; Capper, D.; Christians, A.; Remke, M.; Witt, H.; Pfister, S.; von Deimling, A.; Hartmann, C. Combined molecular analysis of BRAF and IDH1 distinguishes pilocytic astrocytoma from diffuse astrocytoma. Acta. Neuropathol. 2009, 118, 401–405. [Google Scholar] [CrossRef] [PubMed]
- Pfister, S.; Janzarik, W.G.; Remke, M.; Ernst, A.; Werft, W.; Becker, N.; Toedt, G.; Wittmann, A.; Kratz, C.; Olbrich, H. BRAF gene duplication constitutes a mechanism of mapk pathway activation in low-grade astrocytomas. J. Clin.Invest. 2008, 118, 1739. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.T.; Kocialkowski, S.; Liu, L.; Pearson, D.M.; Bäcklund, L.M.; Ichimura, K.; Collins, V.P. Tandem duplication producing a novel oncogenic BRAF fusion gene defines the majority of pilocytic astrocytomas. Cancer Res. 2008, 68, 8673–8677. [Google Scholar] [CrossRef] [PubMed]
- Kieran, M.W. Targeting BRAF in pediatric brain tumors. Available online: http://europepmc.org/abstract/med/24857135 (accessed on 20 August 2015).
- Dasgupta, T.; Haas-Kogan, D.A. The combination of novel targeted molecular agents and radiation in the treatment of pediatric gliomas. Front. Oncol. 2013, 3. [Google Scholar] [CrossRef] [PubMed]
- Thomas, R.; Duke, S.E.; Wang, H.J.; Breen, T.E.; Higgins, R.J.; Linder, K.E.; Ellis, P.; Langford, C.F.; Dickinson, P.J.; Olby, N.J.; et al. 'Putting our heads together': Insights into genomic conservation between human and canine intracranial tumors. J. Neuro-Oncol. 2009, 94, 333–349. [Google Scholar] [CrossRef] [PubMed]
- Tiacci, E.; Trifonov, V.; Schiavoni, G.; Holmes, A.; Kern, W.; Martelli, M.P.; Pucciarini, A.; Bigerna, B.; Pacini, R.; Wells, V.A.; et al. BRAF mutations in hairy-cell leukemia. N. Engl. J. Med. 2011, 364, 2305–2315. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Yoo, N.; Soung, Y.; Kim, H.; Park, W.; Kim, S.; Lee, J.; Park, J.; Cho, Y.; Kim, C. BRAF mutations in non-hodgkin’s lymphoma. Brit. J. Cancer 2003, 89, 1958–1960. [Google Scholar] [CrossRef] [PubMed]
- Bonello, L.; Voena, C.; Ladetto, M.; Boccadoro, M.; Palestro, G.; Inghirami, G.; Chiarle, R. BRAF gene is not mutated in plasma cell leukemia and multiple myeloma. Leukemia 2003, 17, 2238–2240. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Soung, Y.; Park, W.; Kim, S.; Nam, S.; Min, W.; Lee, J.; Yoo, N.; Lee, S. BRAF mutations in acute leukemias. Leukemia 2004, 18, 170–172. [Google Scholar] [CrossRef] [PubMed]
- Badalian-Very, G.; Vergilio, J.-A.; Degar, B.A.; MacConaill, L.E.; Brandner, B.; Calicchio, M.L.; Kuo, F.C.; Ligon, A.H.; Stevenson, K.E.; Kehoe, S.M. Recurrent BRAF mutations in langerhans cell histiocytosis. Blood 2010, 116, 1919–1923. [Google Scholar] [CrossRef] [PubMed]
- Suter, S.E.; Small, G.W.; Seiser, E.L.; Thomas, R.; Breen, M.; Richards, K.L. FLT3 mutations in canine acute lymphocytic leukemia. BMC Cancer 2011, 11. [Google Scholar] [CrossRef] [PubMed]
- Breen, M.; Modiano, J.F. Evolutionarily conserved cytogenetic changes in hematological malignancies of dogs and humans-man and his best friend share more than companionship. Chromosome Res. 2008, 16, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Longley, B.J.; Wang, X.; Blount, J.L.; Langley, K.; Caughey, G.H. Clustering of activating mutations in c-kit’s juxtamembrane coding region in canine mast cell neoplasms. J. Invest. Dermatol. 1999, 112, 165–170. [Google Scholar] [CrossRef] [PubMed]
- London, C.A.; Galli, S.J.; Yuuki, T.; Hu, Z.Q.; Helfand, S.C.; Geissler, E.N. Spontaneous canine mast cell tumors express tandem duplications in the proto-oncogene c-kit. Exp. Hematol. 1999, 27, 689–697. [Google Scholar] [CrossRef]
- Nikiforov, Y.E.; Nikiforova, M.N. Molecular genetics and diagnosis of thyroid cancer. Nat. Rev. Endocrinol. 2011, 7, 569–580. [Google Scholar] [CrossRef] [PubMed]
- Xing, M. BRAF mutation in thyroid cancer. Endocr-Related Cancer. 2005, 12, 245–262. [Google Scholar] [CrossRef] [PubMed]
- Wucherer, K.L.; Wilke, V. Thyroid cancer in dogs: An update based on 638 cases (1995–2005). J. Amer. Anim. Hosp. Assn. 2010, 46, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Vara, J.A.; Miller, M.A.; Johnson, G.C.; Pace, L.W. Immunohistochemical detection of thyroid transcription factor-1, thyroglobulin, and calcitonin in canine normal, hyperplastic, and neoplastic thyroid gland. Vet. Pathol. 2002, 39, 480–487. [Google Scholar] [CrossRef] [PubMed]
- Cardarella, S.; Ogino, A.; Nishino, M.; Butaney, M.; Shen, J.; Lydon, C.; Yeap, B.Y.; Sholl, L.M.; Johnson, B.E.; Jänne, P.A. Clinical, pathologic, and biologic features associated with BRAF mutations in non–small cell lung cancer. Clin. Cancer Res. 2013, 19, 4532–4540. [Google Scholar] [CrossRef] [PubMed]
- Marchetti, A.; Felicioni, L.; Malatesta, S.; Sciarrotta, M.G.; Guetti, L.; Chella, A.; Viola, P.; Pullara, C.; Mucilli, F.; Buttitta, F. Clinical features and outcome of patients with non–small-cell lung cancer harboring BRAF mutations. J. Clin. Oncol. 2011, 29, 3574–3579. [Google Scholar] [CrossRef] [PubMed]
- Hegedüs, L. The thyroid nodule. N.Engl.J. Med. 2004, 351, 1764–1771. [Google Scholar] [CrossRef] [PubMed]
- Nikiforov, Y.E.; Steward, D.L.; Robinson-Smith, T.M.; Haugen, B.R.; Klopper, J.P.; Zhu, Z.; Fagin, J.A.; Falciglia, M.; Weber, K.; Nikiforova, M.N. Molecular testing for mutations in improving the fine-needle aspiration diagnosis of thyroid nodules. J. Clin. Endocrinol. Metab. 2009, 94, 2092–2098. [Google Scholar] [CrossRef] [PubMed]
- Crowley, E.; Di Nicolantonio, F.; Loupakis, F.; Bardelli, A. Liquid biopsy: Monitoring cancer-genetics in the blood. Nat. Rev. Clin. Oncol. 2013, 10, 472–484. [Google Scholar] [CrossRef] [PubMed]
- Sanmamed, M.F.; Fernandez-Landazuri, S.; Rodriguez, C.; Zarate, R.; Lozano, M.D.; Zubiri, L.; Perez-Gracia, J.L.; Martin-Algarra, S.; Gonzalez, A. Quantitative cell-free circulating BRAFV600E mutation analysis by use of droplet digital PCR in the follow-up of patients with melanoma being treated with BRAF inhibitors. Clin. Chem. 2015, 61, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Shinozaki, M.; O'Day, S.J.; Kitago, M.; Amersi, F.; Kuo, C.; Kim, J.; Wang, H.J.; Hoon, D.S. Utility of circulating B-RAF DNA mutation in serum for monitoring melanoma patients receiving biochemotherapy. Clin. Cancer Res. 2007, 13, 2068–2074. [Google Scholar] [CrossRef] [PubMed]
- Bell, F.W.; Klausner, J.S.; Hayden, D.W.; Feeney, D.A.; Johnston, S.D. Clinical and pathologic features of prostatic adenocarcinoma in sexually intact and castrated dogs: 31 cases (1970–1987). J. Amer. Vet. Med. Assoc. 1991, 199, 1623–1630. [Google Scholar]
- Childress, M.O.; Adams, L.G.; Ramos-Vara, J.A.; Freeman, L.J.; He, S.; Constable, P.D.; Knapp, D.W. Results of biopsy via transurethral cystoscopy and cystotomy for diagnosis of transitional cell carcinoma of the urinary bladder and urethra in dogs: 92 cases (2003–2008). J. Amer. Vet. Med. Assoc. 2011, 239, 350–356. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, S.G.; Raghunath, S.; Williams, C.; Motsinger-Reif, A.A.; Cullen, J.M.; Liu, T.; Albertson, D.; Ruvolo, M.; Bergstrom Lucas, A.; Jin, J.; et al. Canine urothelial carcinoma: Genomically aberrant and comparatively relevant. Chromosome Res. 2015, 23, 311–331. [Google Scholar] [CrossRef] [PubMed]
- Powe, J.R.; Canfield, P.J.; Martin, P.A. Evaluation of the cytologic diagnosis of canine prostatic disorders. Vet. Clin .Path. 2004, 33, 150–154. [Google Scholar] [CrossRef]
- Smith, J. Canine prostatic disease: A review of anatomy, pathology, diagnosis, and treatment. Theriogenology 2008, 70, 375–383. [Google Scholar] [CrossRef] [PubMed]
- Huggett, J.F.; Novak, T.; Garson, J.A.; Green, C.; Morris-Jones, S.D.; Miller, R.F.; Zumla, A. Differential susceptibility of pcr reactions to inhibitors: An important and unrecognised phenomenon. BMC Rese.Notes 2008, 1. [Google Scholar] [CrossRef] [PubMed]
- Toye, B.; Woods, W.; Bobrowska, M.; Ramotar, K. Inhibition of pcr in genital and urine specimens submitted for chlamydia trachomatis testing. J. Clin. Microbiol. 1998, 36, 2356–2358. [Google Scholar] [PubMed]
- Huggett, J.F.; Whale, A. Digital pcr as a novel technology and its potential implications for molecular diagnostics. Clin.Chem. 2013, 59, 1691–1693. [Google Scholar] [CrossRef] [PubMed]
- Balch, C.M.; Gershenwald, J.E.; Soong, S.J.; Thompson, J.F.; Atkins, M.B.; Byrd, D.R.; Buzaid, A.C.; Cochran, A.J.; Coit, D.G.; Ding, S.; et al. Final version of 2009 AJCC melanoma staging and classification. J. Clin.Oncol. 2009, 27, 6199–6206. [Google Scholar] [CrossRef] [PubMed]
- Chapman, P.B.; Hauschild, A.; Robert, C.; Haanen, J.B.; Ascierto, P.; Larkin, J.; Dummer, R.; Garbe, C.; Testori, A.; Maio, M. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N. Eng. J. Med. 2011, 364, 2507–2516. [Google Scholar] [CrossRef] [PubMed]
- Robert, C.; Karaszewska, B.; Schachter, J.; Rutkowski, P.; Mackiewicz, A.; Stroiakovski, D.; Lichinitser, M.; Dummer, R.; Grange, F.; Mortier, L. Improved overall survival in melanoma with combined dabrafenib and trametinib. N. Eng. J. Med. 2015, 372, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Hauschild, A.; Grob, J.-J.; Demidov, L.V.; Jouary, T.; Gutzmer, R.; Millward, M.; Rutkowski, P.; Blank, C.U.; Miller, W.H.; Kaempgen, E. Dabrafenib in BRAF-mutated metastatic melanoma: A multicentre, open-label, phase 3 randomised controlled trial. Lancet 2012, 380, 358–365. [Google Scholar] [CrossRef]
- Follows, G.A.; Sims, H.; Bloxham, D.M.; Zenz, T.; Hopper, M.A.; Liu, H.; Bench, A.; Wright, P.; van't Veer, M.B.; Scott, M.A. Rapid response of biallelic BRAF V600E mutated hairy cell leukaemia to low dose vemurafenib. Brit. J. Haematol. 2013, 161, 150–153. [Google Scholar] [CrossRef] [PubMed]
- Haroche, J.; Cohen-Aubart, F.; Emile, J.-F.; Arnaud, L.; Maksud, P.; Charlotte, F.; Cluzel, P.; Drier, A.; Hervier, B.; Benameur, N. Dramatic efficacy of vemurafenib in both multisystemic and refractory erdheim-chester disease and langerhans cell histiocytosis harboring the BRAF V600E mutation. Blood 2013, 121, 1495–1500. [Google Scholar] [CrossRef] [PubMed]
- Munoz, J.; Schlette, E.; Kurzrock, R. Rapid response to vemurafenib in a heavily pretreated patient with hairy cell leukemia and a BRAF mutation. J. Clin.Oncol. 2013, 31, e351–e352. [Google Scholar] [CrossRef] [PubMed]
- Holderfield, M.; Deuker, M.M.; McCormick, F.; McMahon, M. Targeting RAF kinases for cancer therapy: BRAF-mutated melanoma and beyond. Nat. Rev. Cancer 2014, 14, 455–467. [Google Scholar] [CrossRef] [PubMed]
- Kopetz, S.; Desai, J.; Chan, E.; Hecht, J.; O'dwyer, P.; Lee, R.; Nolop, K.; Saltz, L. PLX4032 in metastatic colorectal cancer patients with mutant BRAF tumors. In Proceedings of ASCO Annual Meeting Proceedings, Chicago, USA, 4–8 June 2010; p. 3534.
- Prahallad, A.; Sun, C.; Huang, S.; di Nicolantonio, F.; Salazar, R.; Zecchin, D.; Beijersbergen, R.L.; Bardelli, A.; Bernards, R. Unresponsiveness of colon cancer to BRAF (V600E) inhibition through feedback activation of EGFR. Nature 2012, 483, 100–103. [Google Scholar] [CrossRef] [PubMed]
- Montero-Conde, C.; Ruiz-Llorente, S.; Dominguez, J.M.; Knauf, J.A.; Viale, A.; Sherman, E.J.; Ryder, M.; Ghossein, R.A.; Rosen, N.; Fagin, J.A. Relief of feedback inhibition of HER3 transcription by RAF and MEK inhibitors attenuates their antitumor effects in BRAF-mutant thyroid carcinomas. Cancer Disc. 2013, 3, 520–533. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mochizuki, H.; Breen, M. Comparative Aspects of BRAF Mutations in Canine Cancers. Vet. Sci. 2015, 2, 231-245. https://doi.org/10.3390/vetsci2030231
Mochizuki H, Breen M. Comparative Aspects of BRAF Mutations in Canine Cancers. Veterinary Sciences. 2015; 2(3):231-245. https://doi.org/10.3390/vetsci2030231
Chicago/Turabian StyleMochizuki, Hiroyuki, and Matthew Breen. 2015. "Comparative Aspects of BRAF Mutations in Canine Cancers" Veterinary Sciences 2, no. 3: 231-245. https://doi.org/10.3390/vetsci2030231




