Review on Usage of Vancomycin in Livestock and Humans: Maintaining Its Efficacy, Prevention of Resistance and Alternative Therapy
Abstract
:1. Introduction
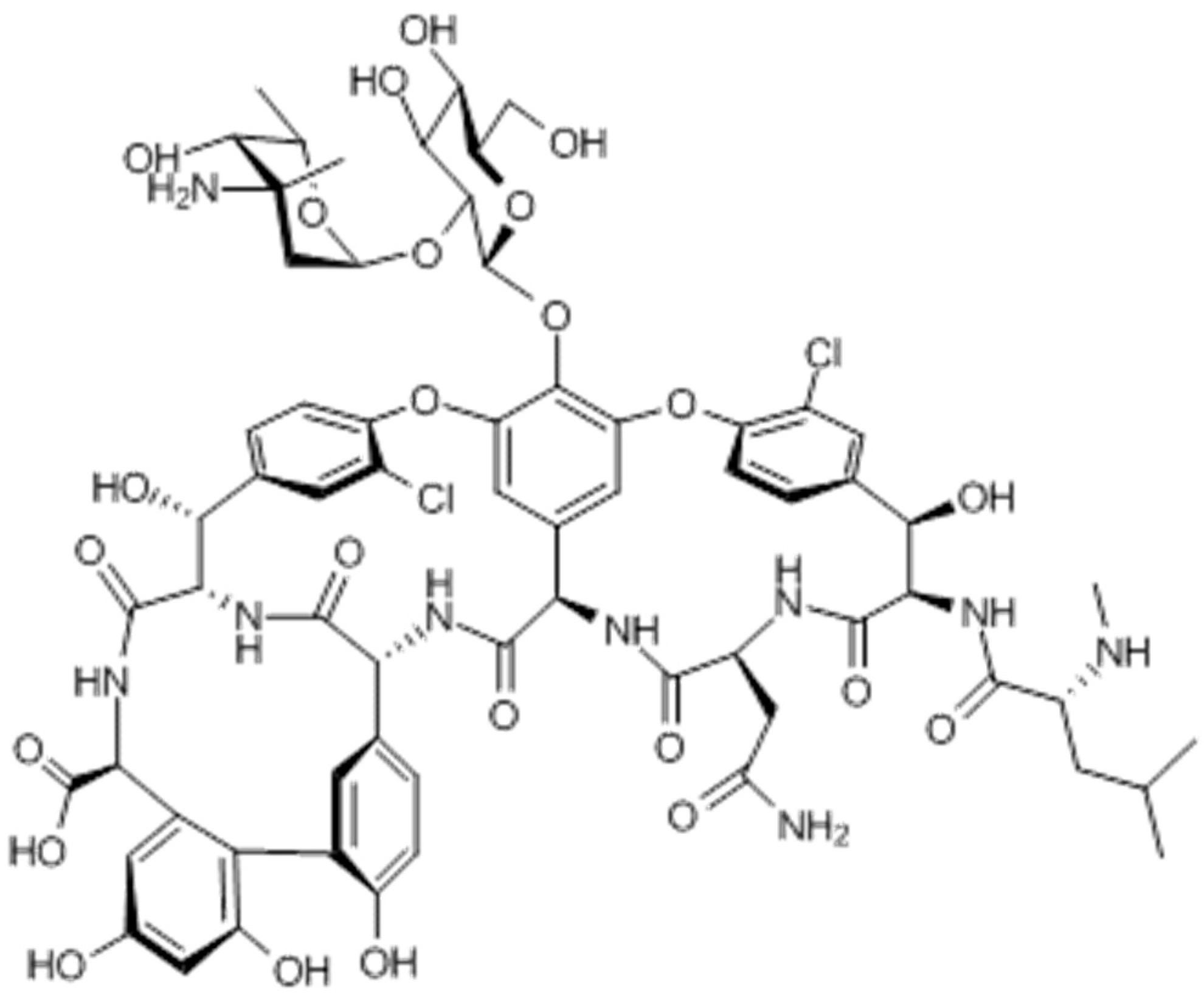
2. Pharmacokinetics and Pharmacodynamics of Vancomycin
3. Therapeutic Indications of Vancomycin
4. Emergence of Resistance to Vancomycin
5. Control of Resistance for Vancomycin
6. Adverse Effect of Vancomycin
7. Alternative Therapy for Vancomycin
8. Conclusions
Author Contributions
Conflicts of Interest
References
- Moellering, R.C., Jr. Vancomycin: A 50-year reassessment. Clin. Infect. Dis. 2006, 42, S3–S4. [Google Scholar] [CrossRef] [PubMed]
- Mark, G.P. Saunders Handbook of Veterinary Drugs Small and Large Animal, 3rd ed.; Saunders: Philadelphia, PA, USA, 2011. [Google Scholar]
- Bassetti, M.; Temperoni, C.; Astilean, A. Review: New antibiotics for bad bugs: Where are we? Ann. Clin. Microbiol. Antimicrob. 2013, 12, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Bennett, P.N.; Brown, M.J. Vancomycin, Clinical Pharmacology, 9th ed.; Churchill Livingstone: London, UK, 2000. [Google Scholar]
- John, F.P.; Baggot, J.D.; Walker, R.D. Glycopeptides: Vancomycin, Teicoplanin, and Avoparcin, Antimicrobial Therapy in Veterinary Medicine, 3rd ed.; Blackwell Publishing Professional: Ames, IA, USA, 2000. [Google Scholar]
- Dana, G.A.; Dowling, M.; Smith, D.A. Vancomycin. In Handbook of Veterinary Drugs, 3rd ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2005. [Google Scholar]
- Cetinkaya, Y.; Falk, P.; Mayhall, C.G. Vancomycin-Resistant Enterococci. Clin. Microbiol. Rev. 2000, 13, 686–707. [Google Scholar] [CrossRef] [PubMed]
- Cynthia, M.K. Vancomycin. In Merck Veterinary Manual, 10th ed.; Merck Sharp & Dohme Corp.: Kenilworth, NJ, USA, 2010; Available online: http://www.merckvetmanual.com/mvm/index.jsp?cfile=htm/bc/191279.htm (accessed on 3 August 2016).
- Gungor, S.; Charro, M.B.D.; Perez, B.R.; Schubert, W.; Isom, P.; Moslemy, P.; Patane, M.A.; Guy, R.H. Trans-scleral iontophoretic delivery of low molecular weight therapeutics. J. Control. Release 2010, 147, 225–231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hubbard, B.K.; Walsh, C.T. Vancomycin Assembly: Nature’s Way. Angew. Chem. 2003, 42, 730–765. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, P.E. Structure, biochemistry and mechanism of action of glycopeptide antibiotics. Eur. J. Clin. Microbiol. Infect. Dis. 1989, 8, 943–950. [Google Scholar] [CrossRef] [PubMed]
- Nitanai, Y.; Kikuchi, T.; Kakoi, K.; Hanamaki, S.; Fujisawa, I.; Aoki, K. Crystal structures of the complexes between vancomycin and cell-wall precursor analogs. J. Mol. Biol. 2009, 385, 1422–1432. [Google Scholar] [CrossRef] [PubMed]
- Zaghlol, H.A.; Brown, S.A. Single- and multiple-dose pharmacokinetics of intravenously administered vancomycin in dogs. Am. J. Vet. Res. 1988, 49, 1637–1640. [Google Scholar] [PubMed]
- LaPlante, K.L.; Rybak, M.J. Impact of high-inoculum Staphylococcus aureus on the activities of nafcillin, vancomycin, linezolid, and daptomycin, alone and in combination with gentamicin, in an in vitro pharmacodynamic model. Antimicrob. Agents Chemother. 2004, 48, 4665–4672. [Google Scholar] [CrossRef] [PubMed]
- Levison, M.E.; Levison, J.H. Pharmacokinetics and Pharmacodynamics of Antibacterial Agents. Infect. Dis. Clin. N. Am. 2009, 23, 791–815. [Google Scholar] [CrossRef] [PubMed]
- Cantu, T.G.; Dick, J.D.; Elliott, D.E.; Humphrey, R.L.; Kornhauser, D.M. Protein binding of vancomycin in a patient with immunoglobulin A myeloma. Antimicrob. Agents Chemother. 1990, 34, 1459–1461. [Google Scholar] [CrossRef] [PubMed]
- Micek, S.T. Alternatives to vancomycin for the treatment of methicillin-resistant Staphylococcus aureus infections. Clin. Infect. Dis. 2007, 45, S184–S190. [Google Scholar] [CrossRef] [PubMed]
- Koteva, K.; Hong, H.L.; Wang, X.D.; Nazi, I.; Hughes, D.; Naldrett, M.J.; Buttner, M.J.; Wright, G.D. A vancomycin photoprobe identifies the histidine kinase VanSsc as a vancomycin receptor. Nat. Chem. Biol. 2010, 6, 327–329. [Google Scholar] [CrossRef] [PubMed]
- Albanese, J.; Leone, M.; Bruguerolle, B.; Ayem, M.L.; Lacarelle, B.; Martin, C. Cerebrospinal fluid penetration and pharmacokinetics of vancomycin administered by continuous infusion to mechanically ventilated patients in an intensive care unit. Antimicrob. Agents Chemother. 2000, 44, 1356–1358. [Google Scholar] [CrossRef] [PubMed]
- Nau, R.; Sörgel, F.; Eiffert, H. Penetration of Drugs through the Blood-Cerebrospinal Fluid/Blood-Brain Barrier for Treatment of Central Nervous System Infections. Clin. Microbiol. Rev. 2010, 23, 858–883. [Google Scholar] [CrossRef] [PubMed]
- Matzneller, P.; Burian, A.; Zeitlinger, M.; Sauermann, R. Understanding the Activity of Antibiotics in Cerebrospinal Fluid in vitro. Pharmacology 2016, 97, 233–244. [Google Scholar] [CrossRef] [PubMed]
- Forouzesh, A.; Moise, P.A.; Sakoulas, G. Vancomycin ototoxicity: A reevaluation in an era of increasing doses. Antimicrob. Agents Chemother. 2009, 53, 2483–2486. [Google Scholar] [CrossRef] [PubMed]
- Chavers, L.S.; Moser, S.A.; Benjamin, W.H.; Banks, S.E.; Steinhauer, J.R.; Smith, A.M.; Johnson, C.N.; Funkhouser, E.; Chavers, L.P.; Stamm, A.M.; et al. Vancomycin-resistant enterococci: 15 years and counting. J. Hosp. Infect. 2003, 53, 159–171. [Google Scholar] [CrossRef] [PubMed]
- Anuradha, G.; Scot, D.E.; Ludek, Z. Dogs Leaving the ICU Carry a Very Large Multi-Drug Resistant Enterococcal Population with Capacity for Biofilm Formation and Horizontal Gene Transfer. PLoS ONE 2011, 6. Available online: http://journals.plos.org/plosone/article/file (accessed on 1 January 2017). [Google Scholar]
- Leekha, S.; Terrell, C.L.; Edson, R.S. General Principles of Antimicrobial Therapy. Mayo. Clin. Proc. 2011, 86, 156–167. [Google Scholar] [CrossRef] [PubMed]
- Cheong, J.Y.; Bakry, M.M.; Lau, C.L.; Rahman, R.A. The relationship between trough concentration of vancomycin and effect on methicillin-resistant Staphylococcus aureus in critically ill patients. S. Afr. Med. J. 2012, 102, 616–619. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Shen, L.; Hsu, L.; Ko, W.; Wu, F.L. Pharmacokinetics of vancomycin in adults receiving extracorporeal membrane oxygenation. J. Formos. Med. Assoc. 2016, 115, 560–570. [Google Scholar] [CrossRef] [PubMed]
- Cisek, A.A.; Rzewuska, M.; Witkowski, W.; Binek, M. Antimicrobial resistance in Rhodococcus equi. Acta Biochim. Pol. 2014, 61, 633–638. [Google Scholar] [PubMed]
- Steer, A.C.; Carapetis, J.R. Acute hematogenous osteomyelitis in children: Recognition and management. Paediatr. Drugs 2004, 6, 333–346. [Google Scholar] [CrossRef] [PubMed]
- Mevius, D.J.; Koene, M.G.J.; Wit, B.; van Pelt, W.; Bondt, N. Monitoring of Antimicrobial Resistance and Antibiotic Usage in Animals in the Netherlands. 2012. Available online: http://www.uu.nl/SiteCollectionImages (accessed on 1 January 2017).
- Diesal, A.; Moriello, K.A. A busy clinician’s review of cyclosporine. Vet. Med. 2008, 103, 266. Available online: http://veterinarymedicine.dvm360.com/busy-clinicians-review-cyclosporine (accessed on 2 January 2017). [Google Scholar]
- Nath, S.R.; Mathew, A.P.; Mohan, A.; Anila, K.R. Rhodococcusequi granulomatous mastitis in an immuno-competent patient—A case report. J. Med. Microbiol. 2013, 62, 1253–1255. [Google Scholar] [CrossRef] [PubMed]
- Orsini, J.A.; Parsons, C.S.; Stine, L.; Haddock, M.; Ramberg, C.F.; Benson, C.E.; Nunamaker, D.M. Vancomycin for the treatment of methicillin-resistant staphylococcal and enterococcal infections in 15 horses. Can. J. Vet. Res. 2005, 69, 278–286. [Google Scholar] [PubMed]
- Matsumoto, K.; Watanabe, E.; Kanazawa, N.; Fukamizu, T.; Shiemi, A.; Yokoyama, Y.; Ikawa, K.; Morikawa, N.; Takeda, Y. Pharmacokinetic/pharmacodynamic analysis of teicoplanin in patients with MRSA infections. Clin. Pharmacol. 2016, 8, 15–18. [Google Scholar] [PubMed]
- Svetitsky, S.; Leibovici, L.; Paul, M. Comparative efficacy and safety of vancomycin versus teicoplanin: Systematic review and meta-analysis. Antimicrob. Agents Chemother. 2009, 53, 4069–4079. [Google Scholar] [CrossRef] [PubMed]
- Martinez, L.M.; Sanroman, J.L.; Cruz, A.M.; Tendillo, F.; Rioja, E.; Roman, F. Evaluation of safety and pharmacokinetics of vancomycin after intraosseous regional limb perfusion and comparison of results with those obtained after intravenous regional limb perfusion in horses. Am. J. Vet. Res. 2006, 67, 1701–1707. [Google Scholar] [CrossRef] [PubMed]
- Slovis, N.M.; Elam, J.; Estrada, M.; Leutenegger, C.M. Infectious agents associated with diarrhoea in neonatal foals in central Kentucky: A comprehensive molecular study. Equine. Vet. J. 2014, 46, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Bandelj, P.; Briski, F.; Frlic, O.; Rataj, A.V.; Rupnik, M.; Ocepek, M.; Vengust, M. Identification of risk factors influencing Clostridium difficile prevalence in middle-size dairy farms. BMC Vet. Res. 2016, 47, 41. [Google Scholar] [CrossRef] [PubMed]
- Keel, K.; Brazier, J.S.; Post, K.W.; Weese, S.; Songer, G. Prevalence of PCR Ribotypes among Clostridium difficile Isolates from Pigs, Calves, and Other Species. J. Clin. Microbiol. 2007, 45, 1963–1964. [Google Scholar] [CrossRef] [PubMed]
- Boss, S.M.; Gries, C.L.; Kirchner, B.K.; Smith, G.D.; Francis, P.C. Use of vancomycin hydrochloride for treatment of Clostridium difficile enteritis in Syrian hamsters. J. Am. Assoc. Lab. Anim. Sci. 1994, 44, 31–37. [Google Scholar]
- Fish, R.; Nipah, R.; Jones, C.; Finney, H.; Fan, S.L. Intraperitoneal Vancomycin Concentrations during Peritoneal Dialysis–Associated Peritonitis: Correlation with Serum Levels. Perit. Dial. Int. 2012, 32, 332–338. [Google Scholar] [CrossRef] [PubMed]
- British Medical Association. Vancomycin. In British National Formulary, 68th ed.; British National Formulary Publications, Royal Pharmaceutical Society of Great Britain: London, UK, 2015; Available online: http://www.bnf.org/bnf/index.htm (accessed on 8 October 2016).
- Arias, C.A.; Singh, K.V.; Panesso, D.; Murray, B.E. Time-Kill and Synergism Studies of Ceftobiprole against Enterococcus faecalis, Including β-Lactamase-Producing and Vancomycin-Resistant Isolates. Antimicrob. Agents Chemother. 2007, 51, 2043–2047. [Google Scholar] [CrossRef] [PubMed]
- Azimian, A.; Havaei, S.A.; Fazeli, H.; Naderi, M.; Ghazvini, K.; Samiee, S.M.; Soleimani, M.; Peerayeh, S.N. Genetic Characterization of a Vancomycin-Resistant Staphylococcus aureus Isolate from the Respiratory Tract of a Patient in a University Hospital in Northeastern Iran. J. Clin. Microbiol. 2012, 50, 3581–3585. [Google Scholar] [CrossRef] [PubMed]
- Climo, M.W.; Patron, R.L.; Archer, G. Combinations of Vancomycin and β-Lactams are synergistic against Staphylococci with reduced susceptibilities to Vancomycin. Antimicrob. Agents Chemother. 1999, 43, 1747–1753. [Google Scholar] [PubMed]
- Chen, C.Y.; Huang, Y.C. Review: New epidemiology of Staphylococcus aureus infection in Asia. Clin. Microbiol. Infect. 2014, 20, 605–623. [Google Scholar] [CrossRef] [PubMed]
- Tenover, F.C., Jr.; Moellering, R.C. The rationale for revising the Clinical and Laboratory Standards Institute vancomycin minimal inhibitory concentration interpretive criteria for Staphylococcus aureus. Clin. Infect. Dis. 2007, 44, 1208–1215. [Google Scholar] [CrossRef] [PubMed]
- Lodise, T.P.; Miller, C.D.; Graves, J.; Evans, A.; Graffunder, E.; Helmecke, M.; Stellrecht, K. Predictors of high vancomycin MIC values among patients with methicillin-resistant Staphylococcus aureus bacteremia. J. Antimicrob. Chemother. 2008, 62, 1138–1141. [Google Scholar] [CrossRef] [PubMed]
- Rybak, M.; Lomaestro, B., Jr.; Moellering, R.; Craig, W.; Billeter, M.; Dalovisio, J.R.; Dalovisio, J.R.; Levine, D.P. Therapeutic Monitoring of Vancomycin in Adults. Am. J. Health Syst. Pharm. 2009, 66, 82–98. [Google Scholar] [CrossRef] [PubMed]
- Soriano, A.; Marco, F.; Martínez, J.A.; Pisos, E.; Almela, M.; Dimova, V.P.; Alamo, D.; Ortega, M.; Lopez, J.; Mensa, J. Influence of vancomycin minimum inhibitory concentration on the treatment of methicillin-resistant Staphylococcus aureus bacteremia. Clin. Infect. Dis. 2008, 46, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Bogaard, A.E.V.D.; Stobberingh, E.E. Epidemiology of resistance to antibiotics links between animals and humans. Int. J. Antimicrob. Agents 2000, 14, 327–335. [Google Scholar] [CrossRef]
- Devriese, L.A.; Ieven, M.; Goossens, H.; Vandamme, P.; Pot, B.; Hommez, J.; Haesebrouck, F. Presence of vancomycin-resistant enterococci in farm and pet animals. Antimicrob. Agents Chemother. 1996, 40, 2285–2287. [Google Scholar] [PubMed]
- Nilsson, O. Vancomycin resistant enterococci in farm animals—Occurrence and importance. Infect. Ecol. Epidermiol. 2012, 2. [Google Scholar] [CrossRef] [PubMed]
- Bogaard, A.E.V.D.; Bruinsma, N.; Stobberingh, E.E. The effect of banning avoparcin on VRE carriage in The Netherlands. J. Antimicrob. Chemother. 2000, 46, 146–148. [Google Scholar] [CrossRef] [PubMed]
- Guillemot, D. Antibiotic use in humans and bacterial resistance. Curr. Opin. Microbiol. 1999, 2, 494–498. [Google Scholar] [CrossRef]
- Lathers, C.M. Role of veterinary medicine in public health: Antibiotic use in food animals and humans and the effect on evolution of antibacterial resistance. J. Clin. Pharmacol. 2001, 41, 595–599. [Google Scholar] [CrossRef] [PubMed]
- Hidayat, L.K.; Hsu, D.I.; Quist, R.; Shriner, K.A.; Beringer, W. High-dose vancomycin therapy for methicillin-resistant Staphylococcus aureus infections: Efficacy and toxicity. Arch. Intern. Med. 2006, 166, 2138–2144. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.N. Microbiological Features of Vancomycin in the 21st Century: Minimum Inhibitory Concentration Creep, Bactericidal/Static Activity, and Applied Breakpoints to Predict Clinical Outcomes or Detect Resistant Strains. Clin. Infect. Dis. 2006, 42 (Suppl. 1), S13–S24. [Google Scholar] [CrossRef] [PubMed]
- Giuliano, C.; Haase, K.K.; Hall, R. Use of vancomycin pharmacokinetic—Pharmacodynamic properties in the treatment of MRSA infections. Expert Rev. Anti-Infect. Ther. 2010, 8, 95–106. [Google Scholar] [CrossRef] [PubMed]
- Brummett, R.E.; Fox, K.E. Vancomycin- and erythromycin-induced hearing loss in humans. Antimicrob. Agents Chemother. 1989, 33, 791–796. [Google Scholar] [CrossRef] [PubMed]
- Elting, L.S.; Rubenstein, E.B.; Kurtin, D.; Rolston, K.V.; Fangtang, J.; Martin, C.G.; Raad, I.I.; Whimbey, E.E.; Manzullo, E.; Bodey, G.P. Mississippi mud in the 1990s: Risks and outcomes of vancomycin-associated toxicity in general oncology practice. Cancer 1998, 83, 2597–2607. [Google Scholar] [PubMed]
- Stanley, D.; McGrath, B.J.; Lamp, K.C.; Rybak, M.J. Effect of human serum on killing activity of vancomycin and teicoplanin against Staphylococcus aureus. Pharmacotherapy 1994, 14, 35–39. [Google Scholar]
- Tange, R.A.; Kieviet, H.L.; Marle, J.V.; Sjoback, D.B.; Ring, W. An experimental study of vancomycin-induced cochlear damage. Arch. Otorhinolaryngol. 1989, 246, 67–70. [Google Scholar] [CrossRef] [PubMed]
- Pai, M.P.; Mercier, R.C.; Koster, S.A. Epidemiology of vancomycin-induced neutropenia in patients receiving home intravenous infusion therapy. Ann. Pharmacother. 2006, 40, 224–228. [Google Scholar] [CrossRef] [PubMed]
- Bishop, T. Vancomycin. In The Veterinary Formulary, 6th ed.; Pharmaceutical Press: London, UK, 2005. [Google Scholar]
- Ray, A.S.; Haikal, A.; Hammoud, K.A.; Yu, S.L. Vancomycin and the Risk of AKI: A Systematic Review and Meta-Analysis. CJASN 2016, 11, 2132–2140. [Google Scholar]
- Bailie, G.R.; Neal, D. Vancomycin ototoxicity and nephrotoxicity. Med. Toxicol. Advers. Drug Exp. 1988, 3, 376–386. [Google Scholar] [CrossRef]
- Drygalski, A.V.; Curtis, B.R.; Bougie, D.W.; McFarland, J.G.; Ahl, S.; Limbu, I.; Baker, K.R.; Aster, R.H. Vancomycin-induced immune thrombocytopenia. N. Engl. J. Med. 2007, 356, 904–910. [Google Scholar] [CrossRef] [PubMed]
- Healy, D.P.; Sahai, J.V.; Fuller, S.H.; Polk, R.E. Vancomycin-induced histamine release and “red man syndrome”: Comparison of 1-and 2-hour infusions. Antimicrob. Agents Chemother. 1990, 34, 550–554. [Google Scholar] [CrossRef] [PubMed]
- Michael, J.R. The Pharmacokinetic and Pharmacodynamic Properties of Vancomycin. Clin. Infect. Dis. 2006, 42, S35–S39. [Google Scholar]
- Glycopetides. Available online: http://www.merckmanuals.com/vet/search.html (accessed on 3 November 2016).
- Radostits, O.M.; Gay, C.C.; Blood, D.C.; Hinchcliff, K.W. Veterinary Medicine: A Textbook of the Diseases of Cattle, Horses, Sheep, Pigs and Goats, 9th ed.; W B Saunders: Philadelphia, PA, USA, 2000. [Google Scholar]
- Ruben, D. Cisplatin for Dogs. 2015. Available online: http://www.petplace.com/article/drug-library/library (accessed on 1 January 2017).
- Cynamon, M.H.; Klemens, S.P.; Sharpe, C.A.; Chase, S. Activities of several novel oxazolidinones against Mycobacterium tuberculosis in a murine model. Antimicrob. Agents Chemother. 1999, 43, 1189–1191. [Google Scholar] [PubMed]
- Slatter, J.G.; Adams, L.A.; Bush, C.E.; Chiba, K.; Yates, P.T.D.; Feenstra, K.L.; Koike, S.; Ozawa, N.; Peng, G.W.; Sams, J.P.; et al. Pharmacokinetics, toxicokinetics, distribution, metabolism and excretion of linezolid in mouse, rat and dog. Xenobiotica 2002, 32, 907–924. [Google Scholar] [CrossRef] [PubMed]
- Vergidis, P.; Rouse, M.S.; Euba, G.; Karau, M.J.; Schmidt, S.M.; Mandrekar, J.N.; Steckelberg, J.M.; Patel, R. Treatment with Linezolid or Vancomycin in Combination with Rifampin Is Effective in an Animal Model of Methicillin-Resistant Staphylococcus aureus Foreign Body Osteomyelitis. Antimicrob. Agents Chemother. 2011, 55, 1182–1186. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, A.B.; Pedrera, M.I.; Barriga, C. In vivo effect of teicoplanin and vancomycin upon haemolytic and bactericidal activity of serum against Staphylococcus aureus. Comp. Immunol. Microbiol. Infect. Dis. 1996, 19, 283–288. [Google Scholar] [CrossRef]
- Sakai, Y.; Tsukahara, T.; Bukawa, W.; Matsubara, N.; Ushida, K. Cell Preparation of Enterococcus faecalis Strain EC-12 Prevents Vancomycin-Resistant Enterococci Colonization in the Cecum of Newly Hatched Chicks. Poultry. Sci. J. 2006, 85, 273–277. [Google Scholar] [CrossRef]
© 2017 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wijesekara, P.N.K.; Kumbukgolla, W.W.; Jayaweera, J.A.A.S.; Rawat, D. Review on Usage of Vancomycin in Livestock and Humans: Maintaining Its Efficacy, Prevention of Resistance and Alternative Therapy. Vet. Sci. 2017, 4, 6. https://doi.org/10.3390/vetsci4010006
Wijesekara PNK, Kumbukgolla WW, Jayaweera JAAS, Rawat D. Review on Usage of Vancomycin in Livestock and Humans: Maintaining Its Efficacy, Prevention of Resistance and Alternative Therapy. Veterinary Sciences. 2017; 4(1):6. https://doi.org/10.3390/vetsci4010006
Chicago/Turabian StyleWijesekara, Panditharathnalage Nishantha Kumara, Wikum Widuranga Kumbukgolla, Jayaweera Arachchige Asela Sampath Jayaweera, and Diwan Rawat. 2017. "Review on Usage of Vancomycin in Livestock and Humans: Maintaining Its Efficacy, Prevention of Resistance and Alternative Therapy" Veterinary Sciences 4, no. 1: 6. https://doi.org/10.3390/vetsci4010006
APA StyleWijesekara, P. N. K., Kumbukgolla, W. W., Jayaweera, J. A. A. S., & Rawat, D. (2017). Review on Usage of Vancomycin in Livestock and Humans: Maintaining Its Efficacy, Prevention of Resistance and Alternative Therapy. Veterinary Sciences, 4(1), 6. https://doi.org/10.3390/vetsci4010006




