Long-Term Effects of Dietary Olive Oil and Hydrogenated Vegetable Oil on Expression of Lipogenic Genes in Subcutaneous Adipose Tissue of Dairy Cows
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals, Experimental Diets and Tissue Sampling
2.2. Biopsies, RNA Extraction, and Reverse Transcription Quantitative Polymerase Chain Reaction (RTqPCR)
2.3. Statistical Analysis
3. Results
3.1. Lipogenic Genes Affected by OO or HVO
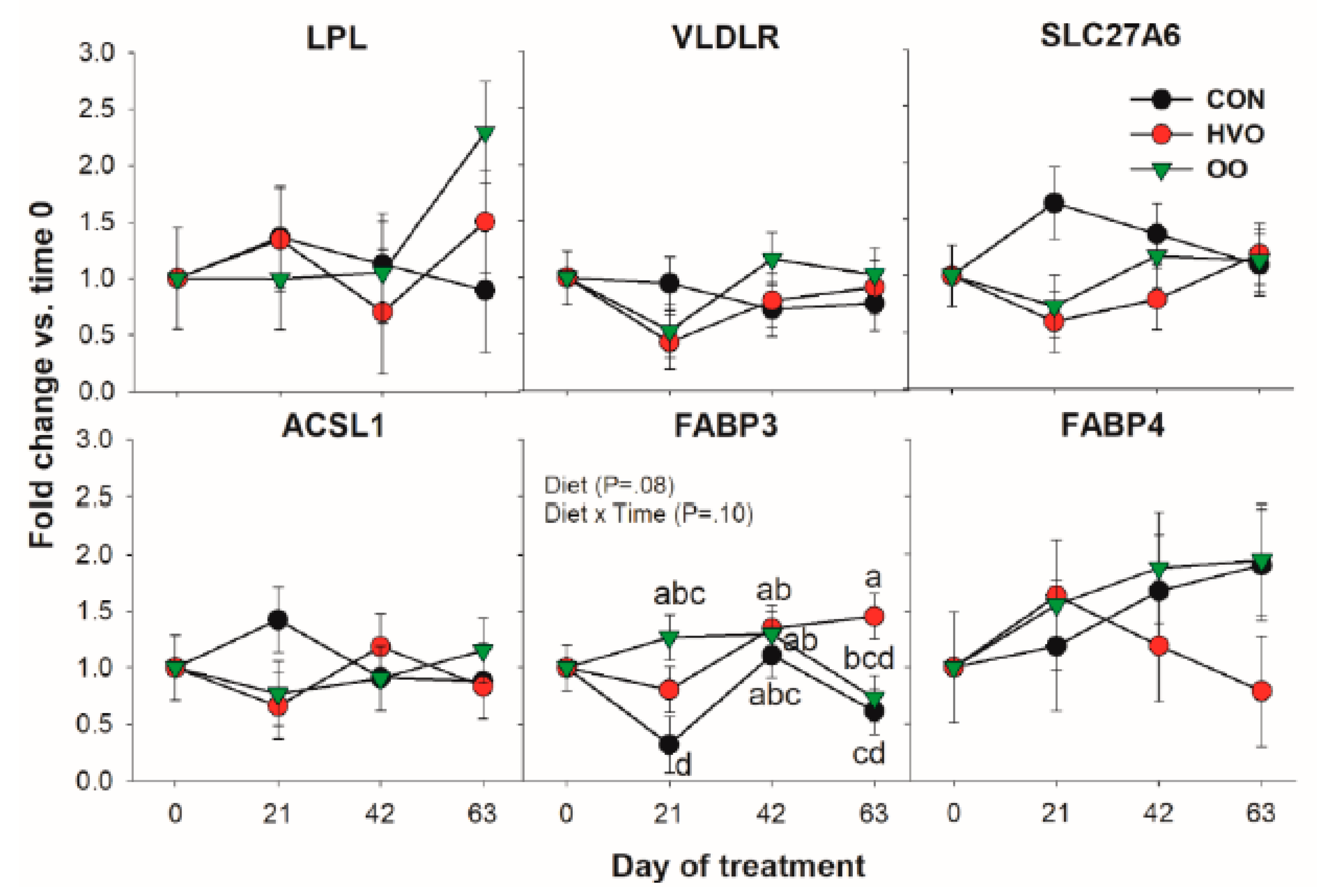
3.1.1. LCFA Transport and Activation
3.1.2. De Novo Fatty Acid Synthesis
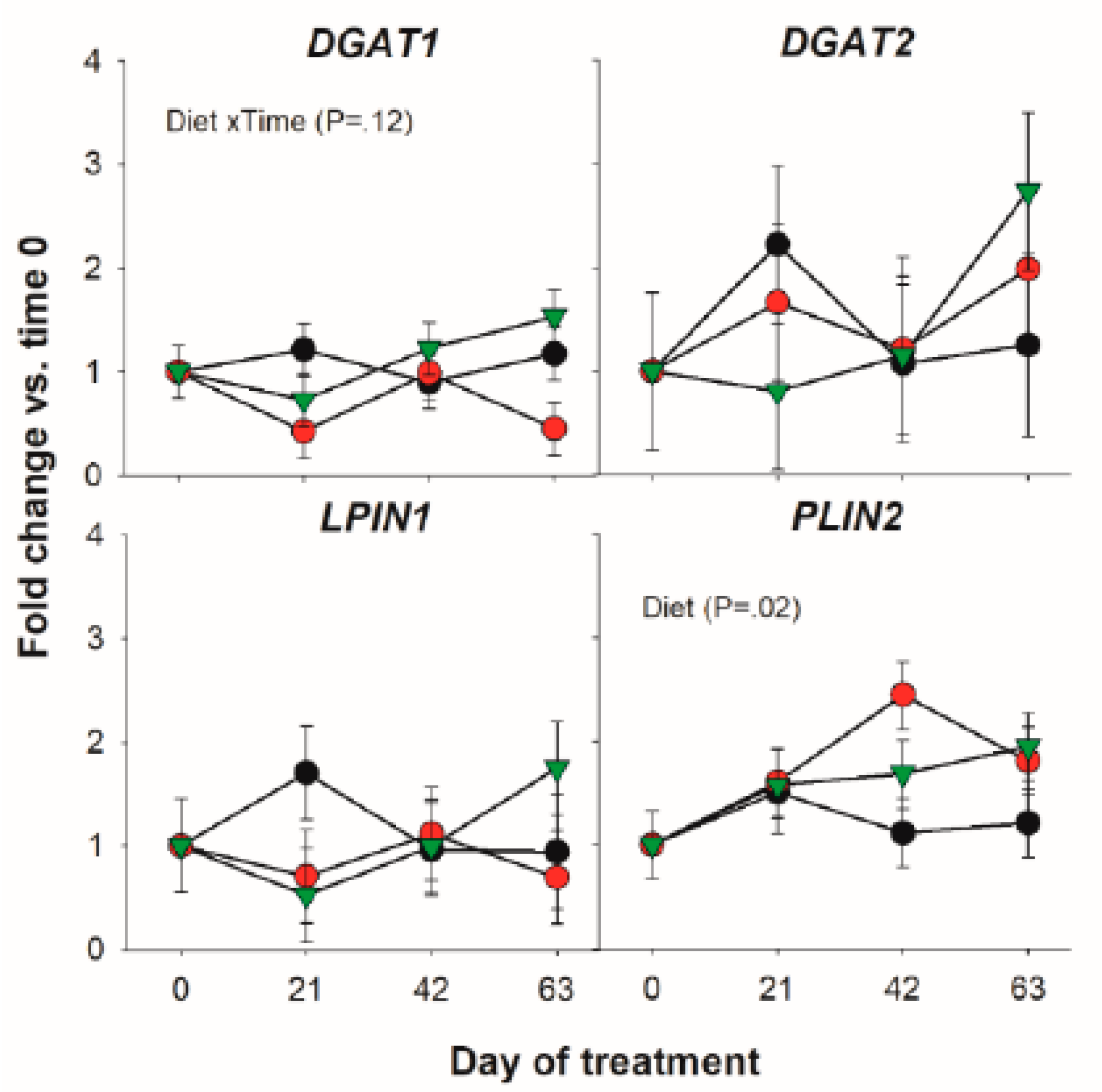
3.1.3. Triacylglycerol Synthesis and Lipid Droplet Formation
3.1.4. Transcription Regulation
3.1.5. Correlation Between Transcripts
4. Discussion
4.1. Cow’s Performance
4.2. Lipid Supplements Might Have Anti-Lipolitic and Anti-Adipogenic Effect
4.3. Long-Term Supplementation of Lipids Does Not Improve the Nutrigenomic Effect of Lcfa in Mid-Lactation Cows
4.4. Limitations of The Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Bionaz, M.; Chen, S.; Khan, M.J.; Loor, J.J. Functional role of PPARs in ruminants: Potential targets for fine-tuning metabolism during growth and lactation. PPAR Res. 2013, 2013, 684159. [Google Scholar] [CrossRef] [PubMed]
- Bionaz, M.; Osorio, J.; Loor, J.J. TRIENNIAL LACTATION SYMPOSIUM: Nutrigenomics in dairy cows: Nutrients, transcription factors, and techniques. J. Anim. Sci. 2015, 93, 5531–5553. [Google Scholar] [CrossRef] [PubMed]
- Harvatine, K.J.; Bauman, D.E. SREBP1 and thyroid hormone responsive spot 14 (s14) are involved in the regulation of bovine mammary lipid synthesis during diet-induced milk fat depression and treatment with CLA. J. Nutr. 2006, 136, 2468–2474. [Google Scholar] [CrossRef] [PubMed]
- Invernizzi, G.; Thering, B.J.; McGuire, M.A.; Savoini, G.; Loor, J.J. Sustained upregulation of stearoyl-CoA desaturase in bovine mammary tissue with contrasting changes in milk fat synthesis and lipogenic gene networks caused by lipid supplements. Funct. Integr. Genom. 2010, 10, 561–575. [Google Scholar] [CrossRef] [PubMed]
- Thering, B.J.; Graugnard, D.E.; Piantoni, P.; Loor, J.J. Adipose tissue lipogenic gene networks due to lipid feeding and milk fat depression in lactating cows. J. Dairy Sci. 2009, 92, 4290–4300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vargas-Bello-Pérez, E.; Zhao, W.; Bionaz, M.; Luo, J.; Loor, J.J. Nutrigenomic effect of saturated and unsaturated long chain fatty acids on lipid-related genes in goat mammary epithelial cells: What is the role of PPARγ? Vet. Sci. 2019, 6, 54. [Google Scholar] [CrossRef]
- Kadegowda, A.K.; Bionaz, M.; Piperova, L.S.; Erdman, R.A.; Loor, J.J. Peroxisome proliferator-activated receptor-γ activation and long-chain fatty acids alter lipogenic gene networks in bovine mammary epithelial cells to various extents. J. Dairy Sci. 2009, 92, 4276–4289. [Google Scholar] [CrossRef]
- Litherland, N.B.; Bionaz, M.; Wallace, R.L.; Loor, J.J.; Drackley, J.K. Effects of the peroxisome proliferator-activated receptor-α agonists clofibrate and fish oil on hepatic fatty acid metabolism in weaned dairy calves. J. Dairy Sci. 2010, 93, 2404–2418. [Google Scholar] [CrossRef]
- Palmquist, D.L. Milk fat: origin of fatty acids and influence of nutritional factors thereon. In Advanced Dairy Chemistry, 3rd ed.; Fox, P.F., McSweeney, P.L., Eds.; Springer Science: New York, NY, USA, 2016; pp. 43–92. [Google Scholar] [CrossRef]
- Bionaz, M.; Loor, J.J. Gene networks driving bovine milk fat synthesis during the lactation cycle. BMC Genom. 2008, 9, 366. [Google Scholar] [CrossRef]
- Vargas-Bello-Pérez, E.; Geldsetzer-Mendoza, C.; Morales, M.S.; Toro-Mujica, P.; Fellenberg, M.A.; Ibañez, R.A.; Gómez-Cortés, P.; Garnsworthy, P.C. Effect of olive oil in dairy cow diets on the fatty acid profile and sensory characteristics of cheese. Int. Dairy J. 2018, 85, 8–15. [Google Scholar] [CrossRef]
- Harvatine, K.J.; Perfield II, J.W.; Bauman, D.E. Expression of enzymes and key regulators of lipid synthesis is upregulated in adipose tissue during CLA-induced milk fat depression in dairy cows. J. Nutr. 2009, 139, 849–854. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, E.; Ballou, M.A.; Correa, M.N.; DePeters, E.J.; Drackley, J.K.; Loor, J.J. Dietary lipid during the transition period to manipulate subcutaneous adipose tissue peroxisome proliferator-activated receptor-γ co-regulator and target gene expression. J. Dairy Sci. 2011, 94, 5913–5925. [Google Scholar] [CrossRef] [PubMed]
- Ruijter, J.M.; Ramakers, C.; Hoogaars, W.M.; Karlen, Y.; Bakker, O.; van den Hoff, M.J.; Moorman, A.F. Amplification efficiency: linking baseline and bias in the analysis of quantitative PCR data. Nucleic Acids Res. 2009, 37, e45. [Google Scholar] [CrossRef] [PubMed]
- Vandesompele, J.; De Preter, K.; Pattyn, F.; Poppe, B.; Van Roy, N.; De Paepe, A.; Speleman, F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002, 3, RESEARCH0034. [Google Scholar] [CrossRef] [PubMed]
- Rosa, F.; Osorio, J.S.; Trevisi, E.; Yanqui-Rivera, F.; Estill, C.T.; Bionaz, M. 2,4-Thiazolidinedione Treatment Improves the Innate Immune Response in Dairy Goats with Induced Subclinical Mastitis. PPAR Res. 2017, 2017, 7097450. [Google Scholar] [CrossRef] [PubMed]
- Palmquist, D.L.; Jenkins, T.C. Fat in lactation rations: Review. J. Dairy Sci. 1980, 63, 1–14. [Google Scholar] [CrossRef]
- Bionaz, M.; Monaco, E.; Wheeler, M.B. Transcription adaptation during in vitro adipogenesis and osteogenesis of porcine mesenchymal stem cells: Dynamics of pathways, biological processes, up-stream regulators, and gene networks. PLoS ONE 2015, 10, e01377644. [Google Scholar] [CrossRef]
- Ladeira, M.M.; Schoonmaker, J.P.; Gionbelli, M.; Dias, J.C.O.; Gionbelli, T.R.S.; Carvalho, J.R.R.; Teixeira, P.D. Nutrigenomics and beef quality: A review about lipogenesis. Int. J. Mol. Sci. 2016, 10, 918. [Google Scholar] [CrossRef]
- Mizoguchi, Y.; Moriya, M.; Taniguchi, D.; Hasegawa, A. Effect of retinoic acid on gene expression profiles of bovine intramuscular preadipocytes during adipogenesis. Anim. Sci. J. 2014, 85, 101–111. [Google Scholar] [CrossRef]
- Fougère, H.; Bernard, L. Effect of diets supplemented with starch and corn oil, marine algae, or hydrogenated palm oil on mammary lipogenic gene expression in cows and goats: A comparative study. J. Dairy Sci. 2018, 102, 768–779. [Google Scholar] [CrossRef]
- Yanting, C.; Yang, Q.Y.; Ma, G.L.; Du, M.; Harrison, J.H.; Block, E. Dose- and type-dependent effects of long-chain fatty acids on adipogenesis and lipogenesis of bovine adipocytes. J. Dairy Sci. 2017, 101, 1601–1615. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.H.; Silvey, D.T.; Johnson, B.J.; Doumit, M.E.; Chung, K.Y.; Sawyer, J.E.; Go, G.W.; Smith, S.B. Conjugated linoleic acid (t-10, c-12) reduces fatty acid synthesis de novo, but not expression of genes for lipid metabolism in bovine adipose tissue ex vivo. Lipids 2014, 49, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Lohakare, J.; Osorio, J.S.; Bionaz, M. Peroxisome proliferator-activated receptor β/δ does not regulate glucose uptake and lactose synthesis in bovine mammary epithelial cells cultivated in vitro. J. Dairy Res. 2018, 85, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Castro-Carrera, T.; Frutos, P.; Leroux, C.; Chilliard, Y.; Hervás, G.; Belenguer, A.; Bernard, L.; Toral, P.G. Dietary sunflower oil modulates milk fatty acid composition without major changes in adipose and mammary tissue fatty acid profile or related gene mRNA abundance in sheep. Animal 2015, 9, 582–591. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Symbol | Name | Function |
|---|---|---|
| ACACA | Acetyl-CoA carboxylase alfa | Catalyzes the rate-limiting reaction in the de novo synthesis of long-chain fatty acids (LCFA) |
| ACSL1 | Acyl-CoA Synthetase Long Chain Family Member 1 | Convert LCFA into acyl-CoA esters, transport of exogenous fatty acid (FA) |
| ACSS2 | Acyl-CoA Synthetase Short Chain Family Member 2 | The chemical reactions and pathways resulting in the formation of acetyl-CoA from acetate |
| PLIN2 | Adipose Differentiation-Related Protein | Involved in formation and maintenance of lipid droplets |
| DGAT1 and 2 | Diacylglycerol O-acyltransferase Homolog 1 and 2 | Acyltransferase that catalyzes the terminal and only committed step in triacylglycerol synthesis |
| FABP3 and 4 | Fatty Acid Binding Protein 3 and 4 | Intracellular transport of acyl-CoA; regulation of gene expression by providing LCFA to PPARγ |
| FADS2 | Fatty acid desaturase 2 | Desaturase introducing a cis double bond at carbon 6 of the fatty acyl chain |
| FASN | Fatty acid synthase | Fatty acid synthetase catalyzes the formation of long-chain fatty acids from acetyl-CoA, malonyl-CoA and NADPH |
| SLC27A6 | Soluble Carrier Protein 27A | LCFA translocation (high uptake); Convert LCFA into acyl-CoA esters |
| INSIG1 | Insulin Induced Gene 1 | Mediates feedback control of cholesterol synthesis by controlling SCAP and HMGCR |
| LPIN1 | Lipin 1 | Dephosphorylation of phosphatidate yielding diacylglycerol; Gene expression (PPARα co- factor) |
| LPL | Lipoprotein Lipase | Catalyzes the hydrolysis of triglycerides from circulating chylomicrons and very low-density lipoproteins |
| PPARG | Peroxisome Proliferator Activated Receptor Gamma | Regulate transcription of lipogenic and adipogenic genes. |
| SCAP | SREBP Chaperone | Protein required for cholesterol as well as lipid homeostasis. Chaperone for activation of SREBP1 |
| SCD1 | Stearoyl-CoA desaturase 1 | Desaturase introducing introduce the first double bond into saturated fatty acyl-CoA substrates |
| SREBF1 | Sterol Regulatory Element Binding Transcription Factor | Transcriptional regulation of cholesterol synthesis and lipogenesis genes |
| THRSP | Thyroid Hormone Responsive | Nuclear protein which is important in the regulation of lipid metabolism |
| VLDLR | Very Low-Density Lipoprotein Receptor | Binds very low-density lipoproteins assisting LPL |
| Gene | CON | HVO | OO | SEM | Diet (D) | Time (T) | D × T |
|---|---|---|---|---|---|---|---|
| Fatty acid transport and activation | |||||||
| LPL | 1.10 | 1.14 | 1.34 | 0.20 | 0.64 | 0.47 | 0.62 |
| VLDLR | 0.86 | 0.78 | 0.93 | 0.12 | 0.72 | 0.27 | 0.60 |
| SLC27A6 | 1.28 | 0.89 | 1.01 | 0.17 | 0.34 | 0.89 | 0.27 |
| FABP3 | 0.76 b | 1.15 a | 1.07 ab | 0.11 | 0.08 | 0.06 | 0.10 |
| FABP4 | 1.44 | 1.15 | 1.59 | 0.33 | 0.65 | 0.43 | 0.77 |
| ACSL1 | 1.05 | 0.92 | 0.93 | 0.21 | 0.90 | 0.97 | 0.13 |
| De-novo synthesis and desaturation | |||||||
| ACACA | 1.11 | 1.55 | 1.41 | 0.52 | 0.83 | 0.50 | 0.19 |
| FASN | 28.0 | 39.5 | 52.4 | 13.0 | 0.41 | 0.15 | 0.22 |
| ACSS2 | 1.25 | 1.75 | 1.58 | 0.77 | 0.90 | 0.59 | 0.28 |
| FADS2 | 1.03 ab | 0.73 b | 1.06 a | 0.11 | 0.08 | 0.34 | 0.92 |
| SCD1 | 1.40 | 1.88 | 2.25 | 0.83 | 0.77 | 0.16 | 0.34 |
| Triacylglycerol synthesis and lipid droplet formation | |||||||
| LPIN1 | 1.15 | 0.88 | 1.07 | 0.28 | 0.78 | 0.98 | 0.32 |
| DGAT1 | 1.07 | 0.72 | 1.12 | 0.17 | 0.26 | 0.44 | 0.12 |
| DGAT2 | 1.39 | 1.46 | 1.42 | 0.58 | 0.99 | 0.23 | 0.50 |
| PLIN2 | 1.21 b | 1.71 a | 1.55 ab | 0.13 | 0.02 | 0.03 | 0.43 |
| Transcription regulation | |||||||
| PPARG | 1.07 a | 0.70 b | 0.62 b | 0.09 | 0.05 | 0.40 | 0.83 |
| INSIG1 | 1.19 | 1.38 | 1.62 | 0.38 | 0.73 | 0.07 | 0.78 |
| SCAP | 1.24 | 0.89 | 0.86 | 0.14 | 0.18 | 0.46 | 0.10 |
| SREBF1 | 0.82 | 0.72 | 0.72 | 0.14 | 0.86 | 0.04 | <0.01 |
| THRSP | 1.97 | 3.21 | 2.68 | 1.35 | 0.81 | 0.13 | 0.47 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vargas-Bello-Pérez, E.; Bionaz, M.; Sciarresi-Arechabala, P.; Cancino-Padilla, N.; Morales, M.S.; Romero, J.; Leskinen, H.; Garnsworthy, P.C.; Loor, J.J. Long-Term Effects of Dietary Olive Oil and Hydrogenated Vegetable Oil on Expression of Lipogenic Genes in Subcutaneous Adipose Tissue of Dairy Cows. Vet. Sci. 2019, 6, 74. https://doi.org/10.3390/vetsci6030074
Vargas-Bello-Pérez E, Bionaz M, Sciarresi-Arechabala P, Cancino-Padilla N, Morales MS, Romero J, Leskinen H, Garnsworthy PC, Loor JJ. Long-Term Effects of Dietary Olive Oil and Hydrogenated Vegetable Oil on Expression of Lipogenic Genes in Subcutaneous Adipose Tissue of Dairy Cows. Veterinary Sciences. 2019; 6(3):74. https://doi.org/10.3390/vetsci6030074
Chicago/Turabian StyleVargas-Bello-Pérez, Einar, Massimo Bionaz, Pietro Sciarresi-Arechabala, Nathaly Cancino-Padilla, María Sol Morales, Jaime Romero, Heidi Leskinen, Philip C. Garnsworthy, and Juan J. Loor. 2019. "Long-Term Effects of Dietary Olive Oil and Hydrogenated Vegetable Oil on Expression of Lipogenic Genes in Subcutaneous Adipose Tissue of Dairy Cows" Veterinary Sciences 6, no. 3: 74. https://doi.org/10.3390/vetsci6030074
APA StyleVargas-Bello-Pérez, E., Bionaz, M., Sciarresi-Arechabala, P., Cancino-Padilla, N., Morales, M. S., Romero, J., Leskinen, H., Garnsworthy, P. C., & Loor, J. J. (2019). Long-Term Effects of Dietary Olive Oil and Hydrogenated Vegetable Oil on Expression of Lipogenic Genes in Subcutaneous Adipose Tissue of Dairy Cows. Veterinary Sciences, 6(3), 74. https://doi.org/10.3390/vetsci6030074








