Taenia ovis in Small Ruminants in Iran: Prevalence, Pathology, and Economic Loss
Abstract
1. Introduction
2. Materials and Methods
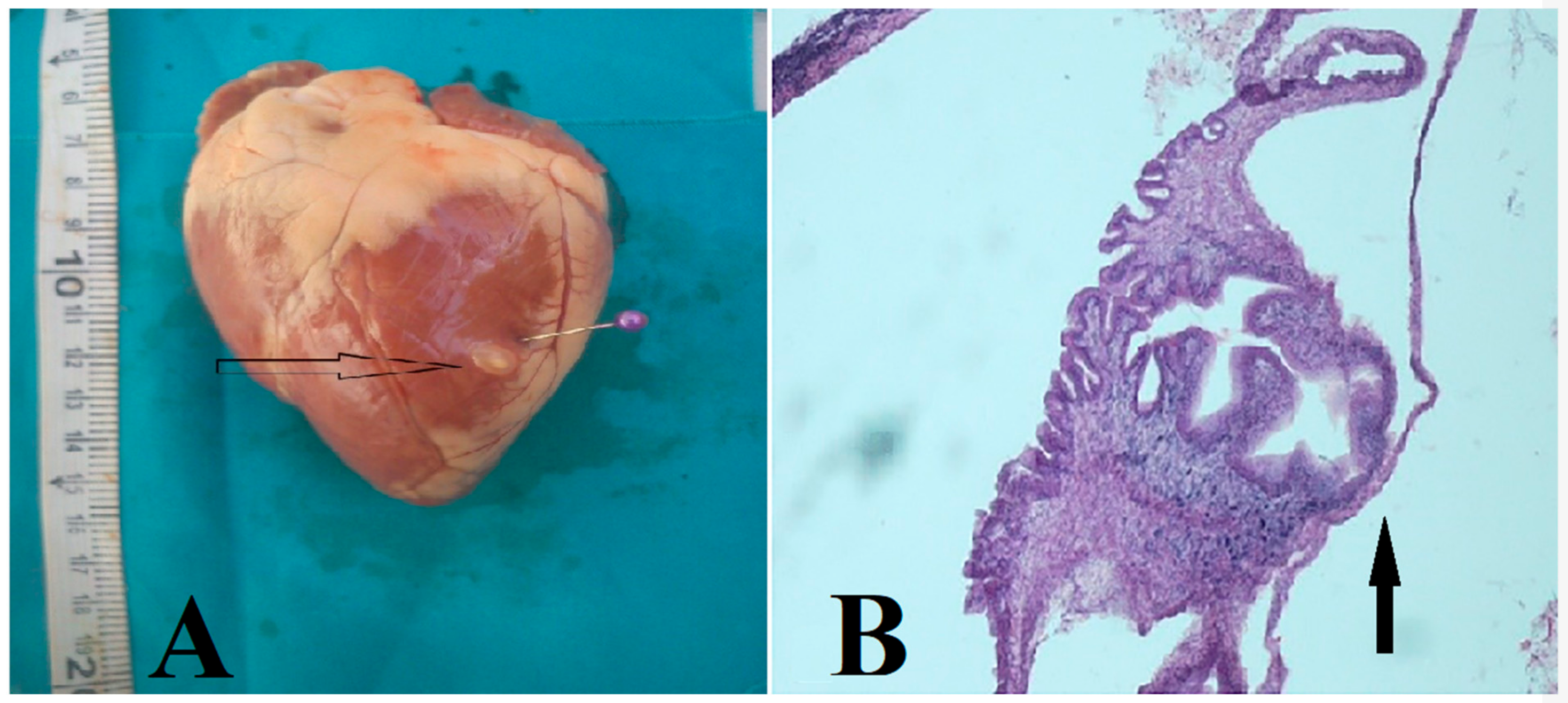
3. Results
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Petersen, H.H.; Al-Sabi, M.N.; Larsen, G.; Jensen, T.K.; Chriél, M. First report of Taenia ovis infection in Danish sheep (Ovis aries). Vet. Parasitol. 2018, 251, 3–6. [Google Scholar] [CrossRef] [PubMed]
- Sissay, M.M.; Uggla, A.; Waller, P.J. Prevalence and seasonal incidence of larval and adult cestode infections of sheep and goats in eastern Ethiopia. Trop. Anim. Health Prod. 2008, 40, 387–394. [Google Scholar] [CrossRef] [PubMed]
- DeWolf, B.D.; Peregrine, A.S.; Jones-Bitton, A.; Jansen, J.T.; MacTavish, J.; Menzies, P.I. Distribution of, and risk factors associated with, sheep carcass condemnations due to Cysticercus ovis infection on Canadian sheep farms. Vet. Parasitol. 2012, 190, 434–441. [Google Scholar] [CrossRef] [PubMed]
- DeWolf, B.D.; Poljak, Z.; Peregrine, A.S.; Jones-Bitton, A.; Jansen, J.T.; Menzies, P.I. Development of a Taenia ovis transmission model and an assessment of control strategies. Vet. Parasitol. 2013, 198, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, D.J.; Urwin, N.A.; Williams, T.M.; Mitchell, K.L.; Lievaart, J.J.; Armua-Fernandez, M.T. Red foxes (Vulpes vulpes) and wild dogs (dingoes (Canis lupus dingo) and dingo/domestic dog hybrids), as sylvatic hosts for Australian Taenia hydatigena and Taenia ovis. Int. J. Parasitol. Parasites Wildl. 2014, 3, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Soulsby, E.J.L. Helminths, Arthropods and Protozoa of Domesticated Animals; Lea and Febiger: Philadelphia, PA, USA, 1982. [Google Scholar]
- Zheng, Y. Taenia ovis: An emerging threat to the Chinese sheep industry? Parasites Vectors 2016, 9, 415. [Google Scholar] [CrossRef] [PubMed]
- Rickard, M.D.; Bell, K.J. Successful vaccination of lambs against infection with Taenia ovis using antigens produced during in vitro cultivation of the larval stages. Res. Vet. Sci. 1971, 12, 401–402. [Google Scholar] [CrossRef]
- Herenda, D.C.; Chambers, P.; Ettriqui, A.; Seneviratna, P.; da Silva, T.J.P. Manual on Meat Inspection for Developing Countries; FAO Animal Production and Health Paper, 119; Food and Agriculture Organization: Rome, Italy, 1994. [Google Scholar]
- Whitten, L. The effect of freezing on the viability of Taenia ovis cysts. N. Z. Vet. J. 1971, 19, 223. [Google Scholar] [CrossRef] [PubMed]
- McNab, J.; Robertson, T. Cysticercus ovis survey: Summary of three years’ results. N. Z. Vet. J. 1972, 20, 66–68. [Google Scholar] [CrossRef] [PubMed]
- Hashemnia, M.; Shahbazi, Y.; Frajani, G.K. Prevalence and pathological lesions of ovine cysticercosis in slaughtered sheep in western Iran. J. Parasit. Dis. 2016, 40, 1575–1578. [Google Scholar] [CrossRef] [PubMed]
- Statistical Centre of Iran. Selected Results of Livestock Slaughter in the National Slaughterhouses – The Year 1396 (March 2017- March 2018). Available online: https://www.amar.org.ir/Portals/1/releases/agriculture/Livestock_survey_1396.pdf (accessed on 26 October 2019).
- Heath, D.; Lawrence, S.; Twaalfhoven, H. Taenia ovis cysts in lamb meat: The relationship between the number of cysts observed at meat inspection and the number of cysts found by fine slicing of tissue. N. Z. Vet. J. 1985, 33, 152–154. [Google Scholar] [CrossRef] [PubMed]
- DeWolf, B.; Peregrine, A.; Jones-Bitton, A.; Jansen, J.; Menzies, P. Taenia ovis infection and its control: A Canadian perspective. N. Z. Vet J. 2014, 62, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Sergeant, E.S.G. Epitools Epidemiological Calculators. Ausvet. Available online: http://epitools.ausvet.com.au (accessed on 25 January 2020).
- Oryan, A.; Goorgipour, S.; Moazeni, M.; Shirian, S. Abattoir prevalence, organ distribution, public health and economic importance of major metacestodes in sheep, goats and cattle in Fars, southern Iran. Trop. Biomed. 2012, 29, 349–359. [Google Scholar] [PubMed]
- Phythian, C.; Jackson, B.; Bell, R.; Citer, L.; Barwell, R.; Windsor, P. Abattoir surveillance of Sarcocystis spp., Cysticercosis ovis and Echinococcus granulosus in Tasmanian slaughter sheep, 2007–2013. Aust. Vet. J. 2018, 96, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Bakhraibah, A.O.; Alsulami, M.N. Prevalence of Cysticercus ovis among slaughtered goats in Makkah, Saudi Arabia. Biosci. Biotechnol. Res. Asia 2018, 15, 909–914. [Google Scholar] [CrossRef]
- Lawson, J.; Gemmell, M. The potential role of blowflies in the transmission of taeniid tapeworm eggs. Parasitology 1985, 91, 129–143. [Google Scholar] [CrossRef] [PubMed]
- Rickard, M.; Arundel, J. Passive protection of lambs against infection with Taenia ovis via colostrum. Aust. Vet. J. 1974, 50, 22–24. [Google Scholar] [CrossRef] [PubMed]
- Oryan, A.; Moghaddar, N.; Gaur, S. Metacestodes of sheep with special reference to their epidemiological status, pathogenesis and economic implications in Fars Province, Iran. Vet. Parasitol. 1994, 51, 231–240. [Google Scholar] [CrossRef]

| Age | Sex | Season | Total | ||||||
|---|---|---|---|---|---|---|---|---|---|
| <1 y | ≥1 y | Female | Male | Spring | Summer | Autumn | Winter | ||
| Sheep | |||||||||
| Sampled | 10,960 | 5220 | 7600 | 8580 | 4045 | 4045 | 4045 | 4045 | 16,180 |
| Positive | 399 | 78 | 440 | 37 | 330 | 37 | 45 | 74 | 477 |
| % | 3.6 1 | 1.5 | 5.8 1 | 0.4 | 8.2 1 | 0.9 | 1.1 | 1.8 | 2.9 |
| 95% CI% | 3.3–4.0 | 1.2–1.9 | 5.3–6.3 | 0.3–0.6 | 7.3–9.0 | 0.6–1.3 | 0.8–1.5 | 1.4–2.3 | 2.7–3.2 |
| Goats | |||||||||
| Sampled | 2520 | 5040 | 5320 | 2240 | 1890 | 1890 | 1890 | 1890 | 7560 |
| Positive | 50 | 40 | 70 | 20 | 41 | 14 | 18 | 17 | 90 |
| % | 2.0 1 | 0.8 | 1.3 | 0.9 | 2.2 1 | 0.7 | 1.0 | 0.9 | 1.2 |
| 95% CI% | 1.5–2.6 | 0.6–1.1 | 1.0–1.7 | 0.5–1.4 | 1.6–2.9 | 0.4–1.2 | 0.6–1.5 | 0.5–1.4 | 1.0–1.5 |
| Masseter Muscle | Heart Muscle | Diaphragm | Triceps | Intercostal Muscle | Thigh Muscle | Liver | Spleen | Intestinal Mucosa | |
|---|---|---|---|---|---|---|---|---|---|
| Sheep | |||||||||
| Positive | 368 | 450 | 380 | 350 | 289 | 234 | 143 | 98 | 199 |
| % | 2.3 | 2.8 | 2.3 | 2.2 | 1.8 | 1.4 | 0.9 | 0.6 | 1.2 |
| 95% CI% | 2.1–2.5 | 2.5–3.0 | 2.1–2.6 | 1.9–2.4 | 1.6–2.0 | 1.3–1.6 | 0.7–1.0 | 0.5–0.7 | 1.1–1.4 |
| Goats | |||||||||
| Positive | 80 | 83 | 78 | 71 | 47 | 0 | 0 | 0 | 0 |
| % | 1.1 | 1.1 | 1.0 | 0.9 | 0.6 | ||||
| 95% CI% | 0.8–1.3 | 0.9–1.4 | 0.8–1.3 | 0.7–1.2 | 0.5–0.8 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hajipour, N.; Allah Rashidzadeh, H.; Ketzis, J.; Esmaeili seraji, R.; Azizi, H.; Karimi, I.; Bagherniaee, H.; Montazeri, R. Taenia ovis in Small Ruminants in Iran: Prevalence, Pathology, and Economic Loss. Vet. Sci. 2020, 7, 34. https://doi.org/10.3390/vetsci7010034
Hajipour N, Allah Rashidzadeh H, Ketzis J, Esmaeili seraji R, Azizi H, Karimi I, Bagherniaee H, Montazeri R. Taenia ovis in Small Ruminants in Iran: Prevalence, Pathology, and Economic Loss. Veterinary Sciences. 2020; 7(1):34. https://doi.org/10.3390/vetsci7010034
Chicago/Turabian StyleHajipour, Nasser, Habib Allah Rashidzadeh, Jennifer Ketzis, Rouhollah Esmaeili seraji, Hamidreza Azizi, Iraj Karimi, Hossein Bagherniaee, and Rohollah Montazeri. 2020. "Taenia ovis in Small Ruminants in Iran: Prevalence, Pathology, and Economic Loss" Veterinary Sciences 7, no. 1: 34. https://doi.org/10.3390/vetsci7010034
APA StyleHajipour, N., Allah Rashidzadeh, H., Ketzis, J., Esmaeili seraji, R., Azizi, H., Karimi, I., Bagherniaee, H., & Montazeri, R. (2020). Taenia ovis in Small Ruminants in Iran: Prevalence, Pathology, and Economic Loss. Veterinary Sciences, 7(1), 34. https://doi.org/10.3390/vetsci7010034





