Effect of Oral Administration of 1,3-1,6 β-Glucans in DWV Naturally Infected Newly Emerged Bees (Apis mellifera L.)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Rearing Conditions
2.2. Feeding Conditions
2.3. Survival Rate Measurement and Food Intake Evaluation
2.4. Sampling
2.5. RNA Extraction and Viral Load Analysis
2.6. Phenoloxidase Activity
2.7. Caspase-3 Activity Assay
2.8. Statistical Analysis
3. Results
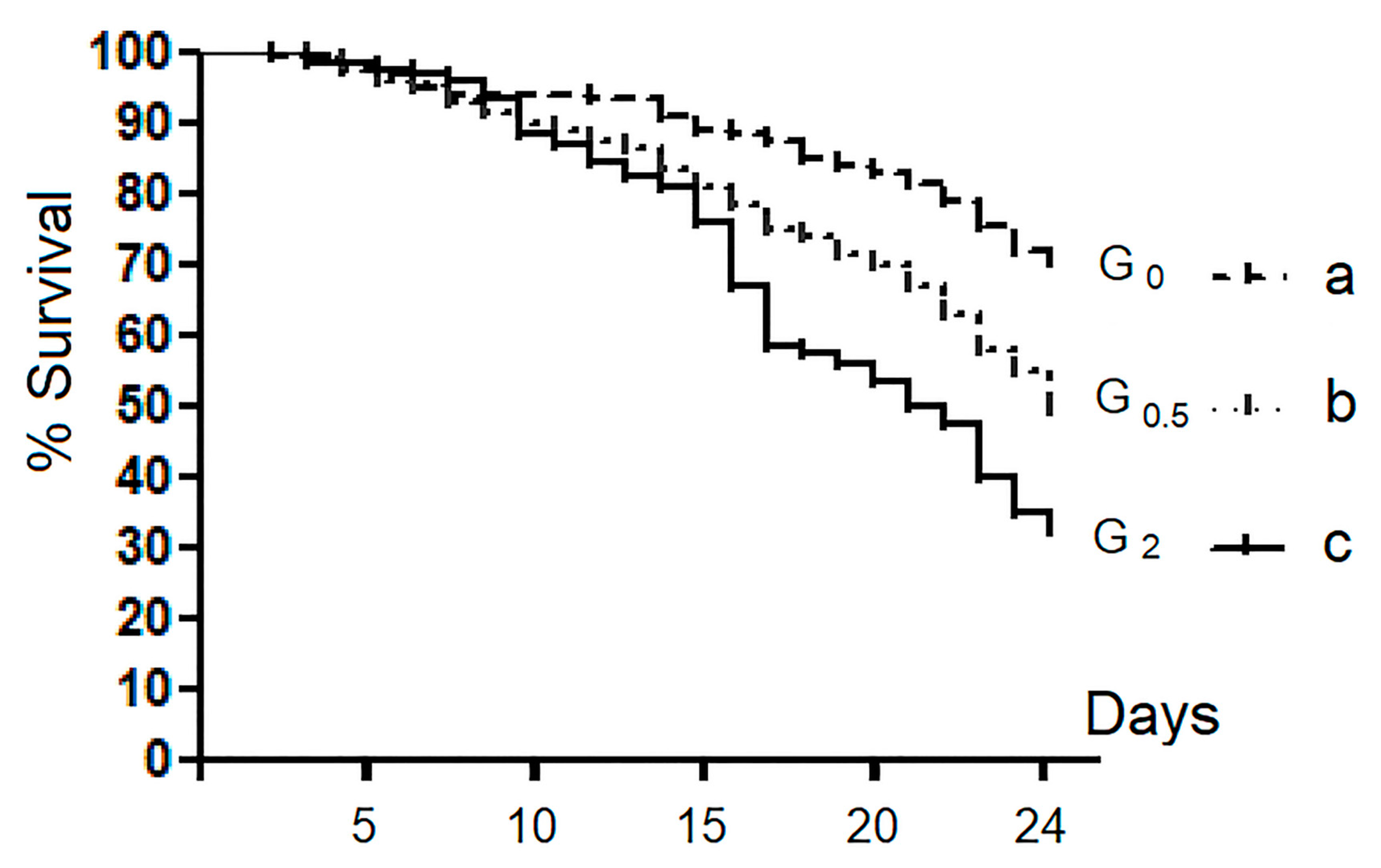
3.1. Survival Rate
3.2. Food Intake
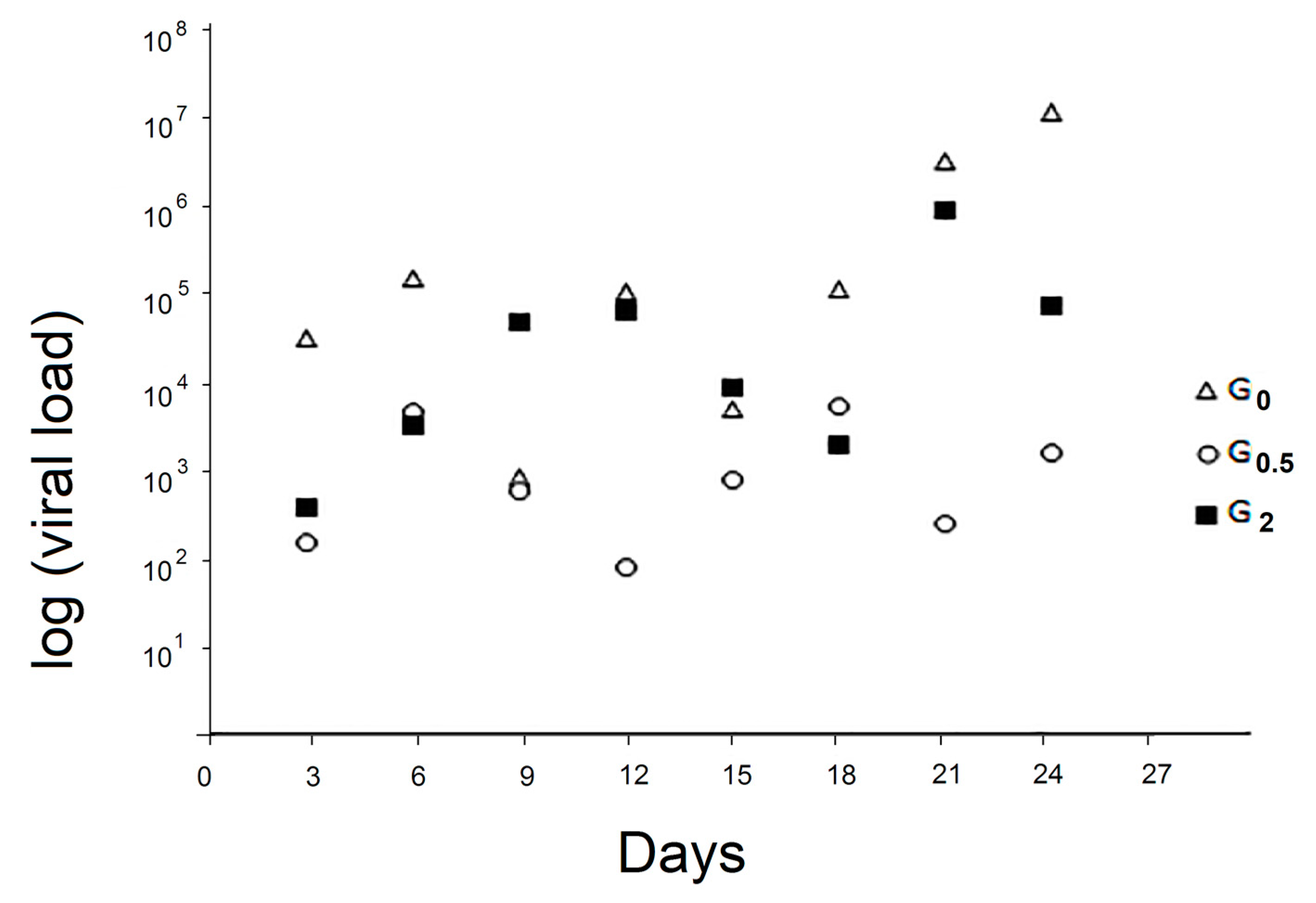
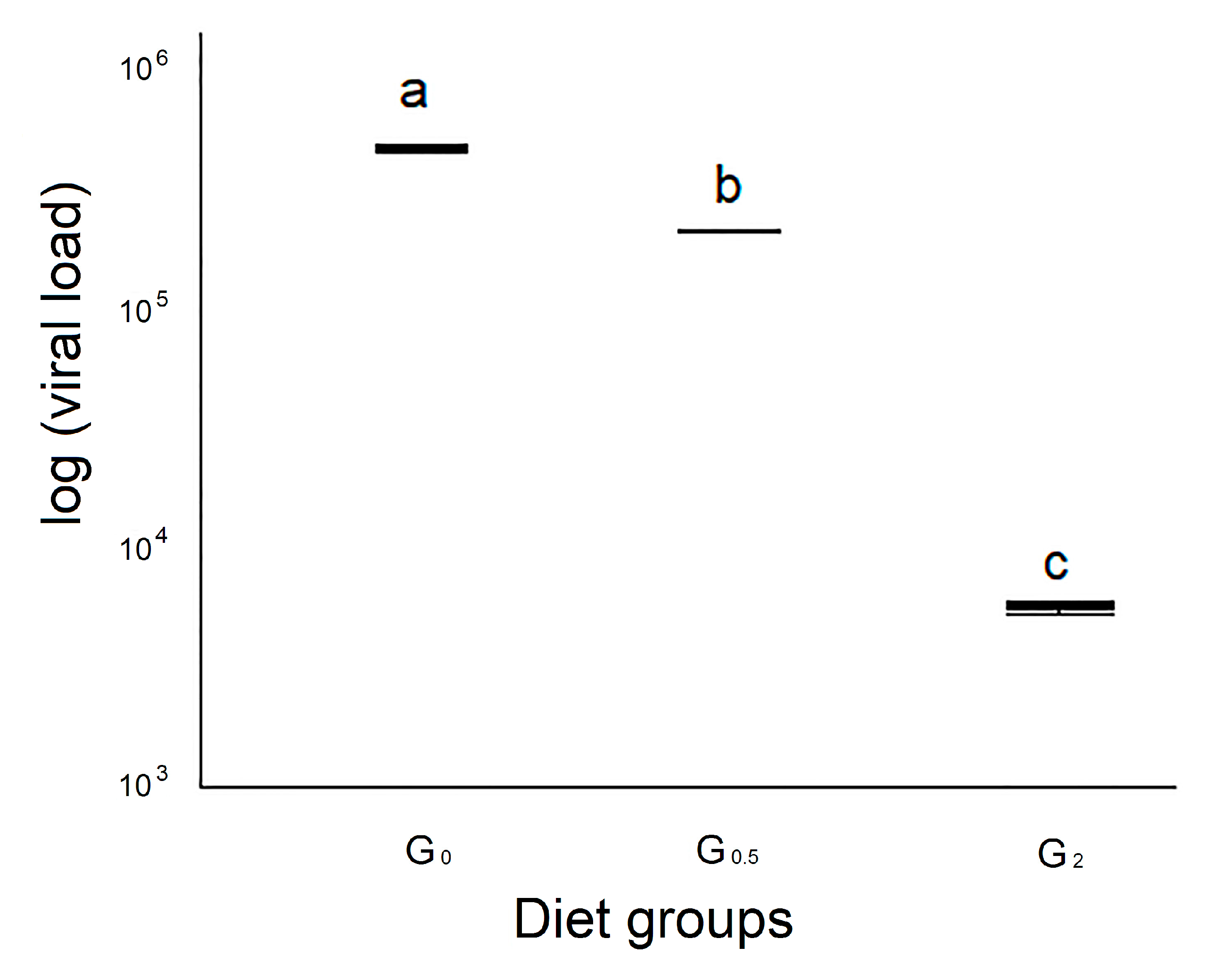
3.3. Viral Loads
3.3.1. Viral Load of the Faecal Samples
3.3.2. Viral Load of Whole T0 Honeybee Samples
3.3.3. Viral Load of the Whole Dead Honeybee Samples
3.3.4. Viral Load of the Whole Survived Bee Samples
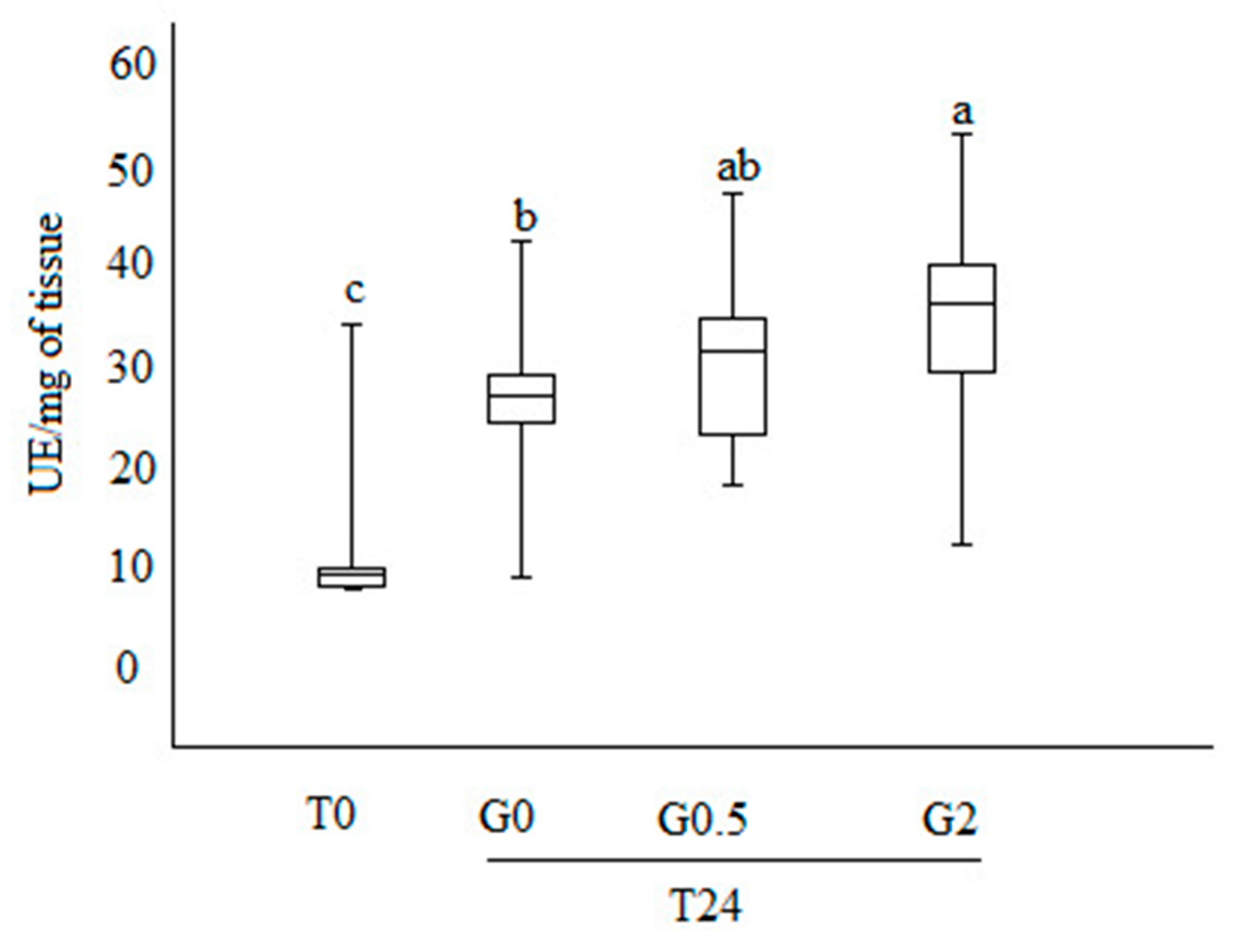
3.4. Phenoloxidase Activity
3.5. Caspase-3 Activity Assay
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Ellis, J.D.; Munn, P.A. The worldwide health status of honey bees. Bee World 2005, 86, 88–101. [Google Scholar] [CrossRef]
- De Miranda, J.R.; Genersch, E. Deformed wing virus. J. Invertebr. Pathol. 2010, 103, S48–S61. [Google Scholar] [CrossRef] [PubMed]
- Francis, R.M.; Nielsen, S.L.; Kryger, P. Varroa-Virus Interaction in Collapsing Honey Bee Colonies. PLoS ONE 2013, 8, e57540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bailey, L.; Ball, B.V. Honey Bee Pathology; Academic Press: London, UK, 1991. [Google Scholar]
- Lanzi, G.; de Miranda, J.R.; Boniotti, M.B.; Cameron, C.E.; Lavazza, A.; Capucci, L.; Camazine, S.M.; Rossi, C. Molecular and biological characterization of deformed wing virus of honeybees (Apis mellifera L.). J. Virol. 2006, 80, 4998–5009. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kovac, H.; Crailsheim, K. Lifespan of Apis Mellifera Carnica Pollm. Infested by Varroa Jacobsoni Oud. in Relation to Season and Extent of Infestation. J. Apic. Res. 1988, 27, 230–238. [Google Scholar] [CrossRef]
- Bowen-Walker, P.L.; Martin, S.J.; Gunn, A. The transmission of deformed wing virus between honeybees (Apis mellifera L.) by the ectoparasitic mite Varroa jacobsoni Oud. J. Invertebr. Pathol. 1999, 73, 101–106. [Google Scholar] [CrossRef] [Green Version]
- Yue, C.; Genersch, E. RT-PCR Analysis of Deformed Wing Virus in Honeybees (Apis Mellifera) and Mites (Varroa destructor). J. Gen. Virol. 2005, 86, 3419–3424. [Google Scholar] [CrossRef]
- Mazzei, M.; Carrozza, M.; Luisi, E.; Forzan, M.; Giusti, M.; Sagona, S.; Tolari, F.; Felicioli, A. Infectivity of DWV Associated to Flower Pollen: Experimental Evidence of a Horizontal Transmission Route. PLoS ONE 2014, 9, e113448. [Google Scholar] [CrossRef] [Green Version]
- De Miranda, J.R.; Fries, I. Venereal and Vertical Transmission of Deformed Wing Virus in Honeybees (Apis mellifera L.). J. Invertebr. Pathol. 2008, 98, 184–189. [Google Scholar] [CrossRef]
- Chen, Y.P.; Pettis, J.S.; Collins, A.; Feldlaufer, M.F. Prevalence and Transmission of Honeybee Viruses. Appl. Environ. Microbiol. 2006, 72, 606–611. [Google Scholar] [CrossRef] [Green Version]
- Yue, C.; Schröder, M.; Bienefeld, K.; Genersch, E. Detection of Viral Sequences in Semen of Honeybees (Apis mellifera): Evidence for Vertical Transmission of Viruses through Drones. J. Invertebr. Pathol. 2006, 92, 105–108. [Google Scholar] [CrossRef] [PubMed]
- Forzan, M.; Felicioli, A.; Sagona, S.; Bandecchi, P.; Mazzei, M. Complete genome sequence of deformed wing virus isolated from Vespa crabro in Italy. Genome Announc. 2017, 5, e00961-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forzan, M.; Sagona, S.; Mazzei, M.; Felicioli, A. Detection of deformed wing virus in Vespa crabro. Bull. Insectol. 2017, 70, 261–265. [Google Scholar]
- Mazzei, M.; Forzan, M.; Cilia, G.; Sagona, S.; Bortolotti, L.; Felicioli, A. First detection of replicative deformed wing virus (DWV) in Vespa velutina nigrithorax. Bull. Insectol. 2018, 71, 211–216. [Google Scholar]
- Desai, S.D.; Eu, Y.J.; Whyard, S.; Currie, R.W. Reduction in deformed wing virus infection in larval and adult honey bees (Apis mellifera L.) by double-stranded RNA ingestion. Insect. Mol. Biol. 2012, 21, 446–455. [Google Scholar] [CrossRef]
- Soltanian, S.; Stuyven, E.; Cox, E.; Sorgeloos, P.; Bossier, P. Beta-glucans as immunostimulant in vertebrates and invertebrates. Crit. Rev. Microbial. 2009, 35, 109–138. [Google Scholar] [CrossRef]
- Zeković, D.B.; Kwiatkowski, S.; Vrvić, M.M.; Jakovljević, D.; Moran, C.A. Natural and modified (1→3)-β-D-glucans in health promotion and disease alleviation. Crit. Rev. Biotechnol. 2005, 25, 205–230. [Google Scholar] [CrossRef]
- Hedengren, M.; Asling, B.; Dushay, M.S.; Ando, I.; Ekengren, S.; Wihlborg, M.; Hultmark, D. Relish, a central factor in the control of humoral but not cellular immunity in Drosophila. Mol. Cell 1999, 4, 827–837. [Google Scholar] [CrossRef]
- Kingsolver, M.B.; Huang, Z.; Hardy, R.W. Insect antiviral innate immunity: Pathways, effectors, and connections. J. Mol. Biol. 2013, 425, 4921–4936. [Google Scholar] [CrossRef] [Green Version]
- Merkling, S.H.; van Rij, R.P. Beyond RNAi: Antiviral defense strategies in Drosophila and mosquito. J. Insect. Physiol. 2013, 59, 159–170. [Google Scholar] [CrossRef]
- Guselle, N.J.; Markham, R.J.F.; Speare, D.J. Timing of intraperitoneal administration of β-1,3/1,6 glucan to rainbow trout, Oncorhynchus mykiss (Walbaum), affects protection against the microsporidian Loma salmonae. J. Fish Dis. 2007, 30, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Kepka, M.; Verburg-van Kemenade, B.M.; Homa, J.; Chadzinska, M. Mechanisms involved in apoptosis of carp leukocytes upon in vitro an in vivo immunostimulation. Fish Shellfish Immunol. 2014, 39, 386–395. [Google Scholar] [CrossRef] [PubMed]
- Miest, J.J.; Hoole, D. Time and concentration dependency of MacroGard® induced apoptosis. Fish Shellfish Immunol. 2015, 42, 363–366. [Google Scholar] [CrossRef] [PubMed]
- Cohen, G.M. Caspases: The executioners of apoptosis. Biochem. J. 1997, 326, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Cooper, D.M.; Granville, D.J.; Lowenberger, C. The insect caspases. Apoptosis 2009, 14, 247–256. [Google Scholar] [CrossRef]
- Rossi, C.A.; Roat, T.C.; Tavares, D.A.; Cintra-Socolowski, P.; Malaspina, O. Brain morphophysiology of Africanized bee Apis mellifera exposed to sublethal doses of imidacloprid. Arch. Environ. Con. Tox. 2013, 65, 234–243. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.Y.; Zhou, T.; Wang, Q.; Dai, P.L.; Xu, S.F.; Jia, H.R.; Wang, X. Programmed cell death in the honey bee (Apis mellifera) (Hymenoptera: Apidae) worker brain induced by imidacloprid. J. Econ. Entomol. 2015, 108, 1486–1494. [Google Scholar] [CrossRef]
- Higes, M.; Juarranz, Á.; Dias-Almeida, J.; Lucena, S.; Botías, C.; Meana, A.; García-Palencia, P.; Martín-Hernández, R. Apoptosis in the pathogenesis of Nosema ceranae (Microsporidia: Nosematidae) in honey bees (Apis mellifera). Environ. Microbiol. Rep. 2013, 5, 530–536. [Google Scholar] [CrossRef]
- Mazzei, M.; Fronte, B.; Sagona, S.; Carrozza, M.; Forzan, M.; Pizzurro, F.; Bibbiani, C.; Miragliotta, V.; Abramo, F.; Millanta, F.; et al. Effect of 1,3-1,6 β Glucan on Natural and Experimental Deformed Wing Virus Infection in Newly Emerged Honeybees (Apis mellifera ligustica). PLoS ONE 2016, 11, e0166297. [Google Scholar] [CrossRef]
- González-Santoyo, I.; Córdoba-Aguilar, A. Phenoloxidase: A key component of the insect immune system. Entomol. Exp. Appl. 2012, 142, 1–16. [Google Scholar] [CrossRef]
- Cerenius, L.; Lee, B.L.; Söderhäll, K. The proPO-system: Pros and cons for its role in invertebrate immunity. Trends Immunol. 2008, 29, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Schmid, M.R.; Brockmann, A.; Pirk, C.W.; Stanley, D.W.; Tautz, J. Adult honeybees (Apis mellifera L.) abandon hemocytic, but not phenoloxidase-based immunity. J. Insect. Physiol. 2008, 54, 439–444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Millanta, F.; Sagona, S.; Mazzei, M.; Forzan, M.; Poli, A.; Felicioli, A. Phenoloxidase activity and haemolymph cytology in honeybees challenged with a virus suspension (deformed wings virus DWV) or phosphate buffered suspension (PBS). Cienc. Rural. 2019, 49. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez-Andres, J.; Rani, S.; Varjak, M.; Chase-Topping, M.E.; Beck, M.H.; Ferguson, M.C.; Schnettler, E.; Fragkoudis, R.; Barry, G.; Merits, A.; et al. Phenoloxidase activity acts as a mosquito innate immune response against infection with Semliki Forest virus. PLoS Pathog. 2012, 8, e1002977. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Büyükgüzel, E. Eicosanoids mediate cellular immune response and phenoloxidase reaction to viral infection in adult Pimpla turionellae. Arch. Insect Biochem. Physiol. 2012, 81, 20–33. [Google Scholar] [CrossRef] [PubMed]
- Alaux, C.; Ducloz, F.; Crauser, D.; Le Conte, Y. Diet effects on honeybee immunocompetence. Biol. Lett. 2010, 6, 562–565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Pasquale, G.; Salignon, M.; Le Conte, Y.; Belzunces, L.P.; Decourtye, A.; Kretzschmar, A.; Suchail, S.; Brunet, J.L.; Alaux, C. Influence of pollen nutrition on honey bee health: Do pollen quality and diversity matter? PLoS ONE 2013, 8, e72016. [Google Scholar] [CrossRef] [Green Version]
- Frias, B.E.D.; Barbosa, C.D.; Lourenço, A.P. Pollen nutrition in honey bees (Apis mellifera): Impact on adult health. Apidologie 2016, 47, 15–25. [Google Scholar] [CrossRef] [Green Version]
- Söderhäll, K.; Cerenius, L. Role of the prophenoloxidase-activating system in invertebrate immunity. Curr. Opin. Immunol. 1998, 10, 23–28. [Google Scholar] [CrossRef]
- Vetvicka, V.; Sima, P. β-Glucan in invertebrates. Invertebr. Surviv. J. 2004, 1, 60–65. [Google Scholar]
- Cherdthong, A.; Seankamsorn, A.; Suriyapha, C.; Chanjula, P.; Wanapat, M. Effect of beta-glucan supplementation on feed intake, digestibility of nutrients and ruminal fermentation in Thai native beef cattle. J. Anim. Physiol. Anim. Nutr. 2018, 102, 1509–1514. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, C.A.; Vetvicka, V.; Zanuzzo, F.S. β-Glucan successfully stimulated the immune system in different jawed vertebrate species. Comp. Immunol. Microbiol. Infect. Dis. 2019, 62, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Vetvicka, V.; Vannucci, L.; Sima, P. The effects of β–glucan on fish immunity. N. Am. J. Med. Sci. 2013, 5, 580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Felicioli, A.; Forzan, M.; Sagona, S.; D’Agostino, P.; Baido, D.; Fronte, B.; Mazzei, M. Effect of Oral Administration of 1,3-1,6 β-Glucans in DWV Naturally Infected Newly Emerged Bees (Apis mellifera L.). Vet. Sci. 2020, 7, 52. https://doi.org/10.3390/vetsci7020052
Felicioli A, Forzan M, Sagona S, D’Agostino P, Baido D, Fronte B, Mazzei M. Effect of Oral Administration of 1,3-1,6 β-Glucans in DWV Naturally Infected Newly Emerged Bees (Apis mellifera L.). Veterinary Sciences. 2020; 7(2):52. https://doi.org/10.3390/vetsci7020052
Chicago/Turabian StyleFelicioli, Antonio, Mario Forzan, Simona Sagona, Paola D’Agostino, Diego Baido, Baldassare Fronte, and Maurizio Mazzei. 2020. "Effect of Oral Administration of 1,3-1,6 β-Glucans in DWV Naturally Infected Newly Emerged Bees (Apis mellifera L.)" Veterinary Sciences 7, no. 2: 52. https://doi.org/10.3390/vetsci7020052
APA StyleFelicioli, A., Forzan, M., Sagona, S., D’Agostino, P., Baido, D., Fronte, B., & Mazzei, M. (2020). Effect of Oral Administration of 1,3-1,6 β-Glucans in DWV Naturally Infected Newly Emerged Bees (Apis mellifera L.). Veterinary Sciences, 7(2), 52. https://doi.org/10.3390/vetsci7020052





