A Review of the Public Health Challenges of Salmonella and Turtles
Abstract
:1. Introduction
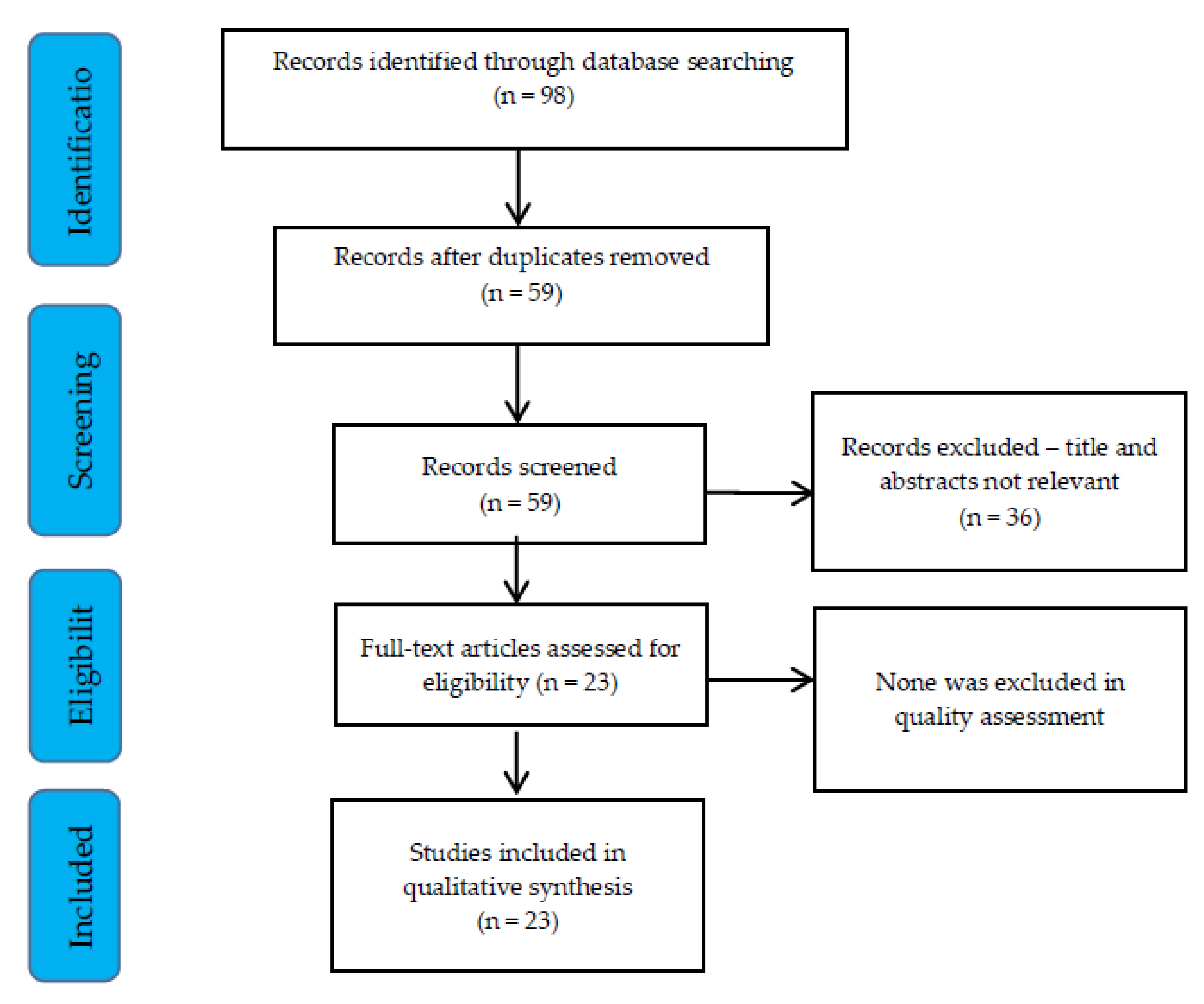
2. Materials and Methods
3. Most Popular Pet Turtles
4. Salmonella Contamination of Turtles and Turtle-Associated Human Salmonellosis around the World
4.1. North America
4.2. South America and Caribbean Island
4.3. Europe
4.4. Asia and Oceania
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lafuente, S.; Bellido, J.B.; Moraga, F.A.; Herrera, S.; Yagüe, A.; Montalvo, T.; de Simó, M.; Simón, P.; Caylà, J.A. Salmonella paratyphi B and Salmonella litchfield outbreaks associated with pet turtle exposure in Spain. Enferm. Infecc. Microbiol. Clin. 2013, 31, 32–35. [Google Scholar] [CrossRef] [PubMed]
- Hohmann, E.L. Nontyphoidal salmonellosis. Clin. Infect. Dis. 2001, 32, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Scallan, E.; Hoekstra, R.M.; Angulo, F.J.; Tauxe, R.V.; Widdowson, M.A.; Roy, S.L.; Jones, J.L.; Griffin, P.M. Foodborne illness acquired in the United States–major pathogens. Emerg. Infect. Dis. 2011, 17, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Musto, J.; Kirk, M.; Lightfoot, D.; Combs, B.G.; Mwanri, L. Multi-drug resistant Salmonella Java infections acquired from tropical fish aquariums, Australia, 2003–2004. Commun. Dis. Intell. 2006, 30, 222–227. [Google Scholar]
- Hale, C.R.; Scallan, E.; Cronquist, A.B.; Dunn, J.; Smith, K.; Robinson, T.; Lathrop, S.; Tobin-D’Angelo, M.; Clogher, P. Estimates of enteric illness attributable to contact with animals and their environments in the United States. Clin. Infect. Dis. 2012, 54, S472–S479. [Google Scholar] [CrossRef] [Green Version]
- Centers for Disease Control and Prevention (CDC). Multistate outbreak of human Salmonella infections associated with exposure to turtles: United States, 2007–2008. MMWR. Morb. Mortal. Wkly. Rep. 2008, 57, 69–72. [Google Scholar]
- Harris, J.R.; Neil, K.P.; Behravesh, C.B.; Sotir, M.J.; Angulo, F.J. Recent multistate outbreaks of human Salmonella infections acquired from turtles: A continuing public health challenge. Clin. Infect. Dis. 2010, 50, 554–559. [Google Scholar] [CrossRef] [Green Version]
- Marin, C.; Ingresa-Capaccioni, S.; González-Bodi, S.; Marco-Jiménez, F.; Vega, S. Free-living turtles are a reservoir for Salmonella but not for Campylobacter. PLoS ONE 2013, 8, e72350. [Google Scholar] [CrossRef] [Green Version]
- Bush, E.R.; Baker, S.E.; Macdonald, D.W. Global trade in exotic pets 2006–2012. Conserv. Biol. 2014, 28, 663–676. [Google Scholar] [CrossRef]
- Gambino-Shirley, K.; Stevenson, L.; Concepción-Acevedo, J.; Trees, E.; Wagner, D.; Whitlock, L.; Roberts, J.; Garrett, N.; Van Duyne, S.; McAllister, G.; et al. Flea market finds and global exports: Four multistate outbreaks of human Salmonella infections linked to small turtles, United States-2015. Zoonoses. Public Health 2018, 65, 560–568. [Google Scholar] [CrossRef]
- Mermin, J.; Hutwagner, L.; Vugia, D.; Shallow, S.; Daily, P.; Bender, J.; Koehler, J.; Marcus, R.; Angulo, F.J. Emerging Infections Program FoodNet Working Group. Reptiles, amphibians, and human Salmonella. infection: A population-based, case-control study. Clin. Infect. Dis. 2004, 38, S253–S261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoelzer, K.; Moreno Switt, A.I.; Wiedmann, M. Animal contact as a source of human non-typhoidal salmonellosis. Vet. Res. 2011, 42, 34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wells, E.V.; Boulton, M.; Hall, W.; Bidol, S.A. Reptile-associated salmonellosis in preschool-aged children in Michigan, January 2001–June 2003. Clin. Infect. Dis. 2004, 39, 687–691. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagano, N.; Oana, S.; Nagano, Y.; Arakawa, Y. A severe Salmonella enterica serotype Paratyphi infection in a child related to a pet turtle, Trachemys scripta elegans. Jpn. J. Infect. Dis. 2006, 59, 132–134. [Google Scholar] [PubMed]
- Henkel, J. A Trail of Tiny Turtles; US Food and Drug Administration Investigators’ Reports; US Food and Drug Administration: Washington, DC, USA, 1997. Available online: http://permanent.access.gpo.gov/lps1609/www.fda.gov/fdac/departs/1997/597_irs.html (accessed on 11 January 2010).
- de Jong, B.; Andersson, Y.; Ekdahl, K. Effect of regulation and education on reptile-associated salmonellosis. Emerg. Infect. Dis. 2005, 11, 398–403. [Google Scholar] [CrossRef]
- Hernández, E.; Rodriguez, J.L.; Herrera-León, S.; García, I.; de Castro, V.; Muniozguren, N. Salmonella Paratyphi B var Java infections associated with exposure to turtles in Bizkaia, Spain, September 2010 to October 2011. Euro Surveill. 2012, 17, 20201. [Google Scholar]
- Walters, M.S.; Simmons, L.; Anderson, T.C.; DeMent, J.; Van Zile, K.; Matthias, L.P.; Etheridge, S.; Baker, R.; Healan, C.; Bagby, R.; et al. Outbreaks of salmonellosis from small turtles. Pediatrics 2016, 137, e20151735. [Google Scholar] [CrossRef] [Green Version]
- Hersey, E.; Mason, D.V. Salmonella Surveillance Report No. 10; CDC: Atlanta, GA, USA, 1963. [Google Scholar]
- Basler, C.; Bottichio, L.; Higa, J.; Prado, B.; Wong, M.; Bosch, S. Multistate outbreak of human Salmonella Poona infections associated with pet turtle exposure—United States, 2014. MMWR. Morb. Mortal. Wkly. Rep. 2015, 64, 804. [Google Scholar] [CrossRef] [Green Version]
- Kraus, F. Alien Reptiles and Amphibians: A Scientific Compendium and Analysis, 1st ed.; Springer: Dordrecht, The Netherlands, 2009. [Google Scholar]
- Available online: http://www.reptilesmagazine.com/Most-Popular-Pet-Turtles/ (accessed on 12 February 2010).
- Bosch, S.; Tauxe, R.V.; Behravesh, C.B. Turtle-Associated Salmonellosis, United States, 2006–2014. Emerg. Infect. Dis. 2016, 22, 1149–1155. [Google Scholar] [CrossRef]
- Code of Federal Regulations. Turtles intrastate and interstate requirements, 21 C.F.R. § 1240.62 2014. Available online: http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/cfrsearch.cfm?fr=1240.62 (accessed on 1 April 2019).
- Gambino-Shirley, K.; Stevenson, L.; Wargo, K.; Burnworth, L.; Roberts, J.; Garrett, N.; Van Duyne, S.; McAllister, G.; Nichols, M. Notes from the Field: Four Multistate Outbreaks of Human Salmonella Infections Linked to Small Turtle Exposure—United States, 2015. MMWR. Morb. Mortal. Wkly. Rep. 2016, 65, 655–656. [Google Scholar] [CrossRef] [Green Version]
- Multistate Outbreak of Salmonella Agbeni Infections Linked to Pet Turtles, 2017 (Final Update). Available online: https://www.cdc.gov/salmonella/agbeni-08-17/ (accessed on 29 August 2017).
- Centers for Disease Control and Prevention (CDC). Surveillance for Foodborne Disease Outbreaks; United States, 2016: Annual report; US Department of Health and Human Services, CDC: Atlanta, GA, USA, 2018. Available online: https://www.cdc.gov/fdoss/pdf/2016_FoodBorneOutbreaks_508.pdf (accessed on 29 August 2017).
- Office for State, Tribal, Local and Territorial Support. Centers for Disease Control and Prevention. Menu of State Turtle-Associated Salmonellosis Laws. Available online: https://www.cdc.gov/phlp/docs/turtle-menu.pdf (accessed on 30 April 2015).
- Braun, S.; Spalloni, W.; Ferreccio, F.; Postigo, J.; Fernández, A.; Porte, L.; Saldivia, A.; Wigant, W.; Triantafilo, V. Salmonella spp. gastroenteritis associated to pet turtles in three infants. Rev. Chilena Infectol. 2015, 32, 334–338. (In Spanish) [Google Scholar] [CrossRef] [Green Version]
- Ives, A.K.; Antaki, E.; Stewart, K.; Francis, S.; Jay-Russell, M.T.; Sithole, F.; Kearney, M.T.; Griffin, M.J.; Soto, E. Detection of Salmonella enterica Serovar Montevideo and Newport in Free-ranging Sea Turtles and Beach Sand in the Caribbean and Persistence in Sand and Seawater Microcosms. Zoonoses Public Health 2017, 64, 450–459. [Google Scholar] [CrossRef]
- Santoro, M.; Hernandez, G.; Caballero, M. Aerobic bacterial flora of nesting green turtles (Chelonia mydas) from Tortuguero National Park, Costa Rica. J. Zoo Wildl. Med. 2006, 37, 549–552. [Google Scholar] [CrossRef] [PubMed]
- Santoro, M.; Hernandez, G.; Caballero, M.; Garcıa, F. Potential bacterial pathogens carried by nesting leatherback turtles (Dermochelys coriacea) in Costa Rica. Chelonian Conserv. Biol. 2007, 7, 104–108. [Google Scholar] [CrossRef]
- Dutton, C.S.; Revan, F.; Wang, C.; Xu, C.; Norton, T.M.; Stewart, K.M.; Kaltenboeck, B.; Soto, E. Salmonella enterica prevalence in leatherback sea turtles (Dermochelys coriacea) in St. Kitts, West Indies. J. Zoo. Wildl. Med. 2013, 44, 765–768. [Google Scholar] [CrossRef] [PubMed]
- Bradley, T.; Angulo, F.J.; Raiti, P. Association of Reptilian and Amphibian Veterinarians guidelines for reducing risk of transmission of Salmonella spp from reptiles to humans. J. Am. Vet. Med. Assoc. 1998, 213, 51–52. [Google Scholar]
- Ricard, C.; Mellentin, J.; Ben Abdallah Chabchoub, R.; Kingbede, P.; Heuclin, T.; Ramdame, A.; Bouquet, A.; Couttenier, F.; Hendricx, S. Salmonella meningitis in an infant due to a pet turtle. Arch. Pediatr. 2015, 22, 605–607. (In French) [Google Scholar] [CrossRef]
- Marin, C.; Vega, S.; Marco-Jiménez, F. Tiny Turtles Purchased at Pet Stores are a Potential High Risk for Salmonella Human Infection in the Valencian Region, Eastern Spain. Vector Borne. Zoonotic Dis. 2016, 16, 455–460. [Google Scholar] [CrossRef]
- Angot, M.; Labbe, F.; Duquenoy, A.; Le Roux, P. Rotavirus-Salmonella coinfection due to turtles: Two cases with exotic pets. Arch. Pediatr. 2017, 24, 747–748. (In French) [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Kuang, D.; Wang, F.; Meng, J.; Jin, H.; Yang, X.; Liao, M.; Klena, J.D.; Wu, S.; Zhang, Y.; et al. Turtles as a possible reservoir of nontyphoidal Salmonella in Shanghai, China. Foodborne Pathog. Dis. 2016, 13, 428–433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Silva, B.C.J.; Hossain, S.; Wimalasena, S.H.M.P.; Pathirana, H.N.K.S.; Wendt, M.; Heo, G.J. Quinolone susceptibility and genetic characterization of Salmonella enterica subsp. enterica isolated from pet turtles. Lab. Anim. Res. 2017, 33, 49–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Back, D.S.; Shin, G.W.; Wendt, M.; Heo, G.J. Prevalence of Salmonella spp. in pet turtles and their environment. Lab. Anim. Res. 2016, 32, 166–170. [Google Scholar] [CrossRef] [Green Version]
- Smith, K.F.; Yabsely, M.J.; Sanchez, S.; Casey, C.L.; Behrens, M.D.; Hernandez, S.M. Salmonella isolates from wild-caught Tokay geckos (Gekko gecko) imported to the U.S. from Indonesia. Vector Borne Zoonotic. Dis. 2012, 12, 575–582. [Google Scholar] [CrossRef] [Green Version]
- Hossain, S.; De Silva, B.C.J.; Dahanayake, P.S.; Shin, G.W.; Heo, J.G. Molecular characterization of virulence, antimicrobial resistance genes and class 1 integron gene casettes in Salmonella enterica subsp. entrica isolated from pet turtles in Seoul, Korea. J. Exot. Pet. Med. 2019, 28, 209217. [Google Scholar] [CrossRef]
- Bertrand, S.; Rimhanen-Finne, R.; Weill, F.X.; Rabsch, W.; Thornton, L.; Perevoscikovs, J.; van Pelt, W.; Heck, M. (Eds.) Salmonella infections associated with reptiles: The current situation in Europe. Euro Surveill. 2008, 13, 18902. [Google Scholar]
- Bruce, H.L.; Barrow, P.A.; Rycroft, A.N. Zoonotic potential of Salmonella enterica carried by pet tortoises. Vet Rec. 2018, 182, 141. [Google Scholar] [CrossRef] [Green Version]
- Corrente, M.; Sangiorgio, G.; Grandolfo, E.; Bodnar, L.; Catella, C.; Trotta, A.; Martella, V.; Buonavoglia, D. Risk for zoonotic Salmonella transmission from pet reptiles: A survey on knowledge, attitudes and practices of reptile-owners related to reptile husbandry. Prev. Vet. Med. 2017, 146, 73–78. [Google Scholar] [CrossRef]
- Saelinger, C.A.; Lewbart, G.A.; Christian, L.S.; Lemons, C.L. Prevalence of Salmonella spp in cloacal, fecal, and gastrointestinal mucosal samples from wild North American turtles. J. Am. Vet. Med. Assoc. 2006, 229, 266–268. [Google Scholar] [CrossRef]
- Sánchez-Jiménez, M.M.; Rincón-Ruiz, P.A.; Duque, S.; Giraldo, M.A.; Ramírez-Monroy, D.M.; Jaramillo, G.; Cardona-Castro, N. Salmonella enterica in semi-aquatic turtles in Colombia. J. Infect. Dev. Ctries. 2011, 5, 361–364. [Google Scholar]
- Gavrilovici, C.; Pânzaru, C.V.; Cozma, S.; Mârţu, C.; Lupu, V.V.; Ignat, A.; Miron, I.; Stârcea, M. “Message from a turtle”: Otitis with Salmonella arizonae in children: Case report. Medicine (Baltimore) 2017, 96, e8455. [Google Scholar] [CrossRef] [PubMed]
- Nakadai, A.; Kuroki, T.; Kato, Y.; Suzuki, R.; Yamai, S.; Yaginuma, C.; Shiotani, R.; Yamanouchi, A.; Hayashidani, H. Prevalence of Salmonella spp. in pet reptiles in Japan. J. Vet. Med. Sci. 2005, 67, 97–101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hidalgo-Vila, J.; Díaz-Paniagua, C.; Pérez-Santigosa, N.; de Frutos- Escobar, C.; Herrero-Herrero, A. Salmonella in free-living exotic and native turtles and in pet exotic turtles from SW Spain. Res. Vet. Sci. 2008, 85, 449–452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geue, L.; Löschner, U. Salmonella enterica in reptiles of German and Austrian origin. Vet. Microbiol. 2002, 84, 79–91. [Google Scholar] [CrossRef]
- Mitchell, J.C.; McAvoy, B.V. Enteric bacteria in natural populations of freshwater turtles in Virginia. Va. J. Sci. 1990, 41, 233–242. [Google Scholar]
- Brenner, D.; Lewbart, G.; Stebbins, M.; Herman, D.W. Health survey of wild and captive bog turtles (Clemmys muhlenbergii) in North Carolina and Virginia. J. Zoo. Wildl. Med. 2002, 33, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Richards, J.M.; Brown, J.D.; Kelly, T.R.; Fountain, A.; Sleeman, J.M. Absence of detectable Salmonella cloacal shedding in free-living reptiles on admission to the wildlife center of Virginia. J. Zoo. Wildl. Med. 2004, 35, 562–563. [Google Scholar] [CrossRef]
- Hidalgo-Vila, J.; Díaz-Paniagua, C.; de Frutos-Escobar, C.; Jiménez-Martínez, C.; Pérez-Santigosa, N. Salmonella in free living terrestrial and aquatic turtles. Vet. Microbiol. 2007, 119, 311–315. [Google Scholar] [CrossRef] [Green Version]
- Readel, A.M.; Phillips, C.A.; Goldberg, T.L. Absence of cloacal shedding of Salmonella in wild red-eared sliders (Trachemys scripta elegans). Herpetol. Rev. 2008, 39, 427–430. [Google Scholar]
- Scheelings, T.F.; Lightfoot, D.; Holz, P. Prevalence of Salmonella in Australian reptiles. J. Wildl. Dis. 2011, 47, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Kuroki, T.; Ito, K.; Ishihara, T.; Furukawa, I.; Kaneko, A.; Suzuki, Y.; Seto, J.; Kamiyama, T. Turtle-Associated Salmonella Infections in Kanagawa, Japan. Jpn. J. Infect Dis. 2015, 68, 333–337. [Google Scholar] [CrossRef] [Green Version]
- Feeley, J.C.; Treger, M.D. Penetration of turtle eggs by Salmonella braen-derup. Public Health Rep. 1969, 84, 156–158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koski, L.; Stevenson, L.; Huffman, J.; Robbins, A.; Latash, J.; Omoregie, E.; Kline, K.; Nichols, M. Notes from the Field: An Outbreak of Salmonella Agbeni Infections Linked to Turtle Exposure—United States, 2017. MMWR. Morb. Mortal. Wkly. Rep. 2018, 67, 1350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Scientific Name | Common Name | Adult Size (Inches) | Origin |
|---|---|---|---|
| Trachemys scripta elegans | Red-Eared Slider | 8 to 10 | The United States, Asia and Europe |
| Terrapene carolina carolina | Eastern Box Turtle | 4 to 8 | The United States, Mexico |
| Chrysemys picta bellii | Western Painted Turtle | 7 to 8 | The United States and Canada |
| Graptemys geographica | Map Turtle | 6 to 10 | The United States and Canada |
| Glyptemys (Clemmys) insculpta | Wood Turtle | 5 to 9 | The United States and Canada |
| Country | Year | Outbreak/ Case Report | Age Range or Median Patient’s Age | No. of Infected Cases | No. of Hospitalization | No. of Death | Source | Salmonella Serovar(s) | Reference |
|---|---|---|---|---|---|---|---|---|---|
| USA | 2017 | A multistate outbreak | 21 years | 76 | 30 | - | Turtles were from street or roadside vendor, a retail store, and festivals. | S. enterica subsp. enterica serovar Abgeni | [35] |
| USA | 2015 | Four multistate outbreaks | Children aged <5 years | 143 | 39 | - | Small turtles purchased from flea markets or street vendors. | S. enterica subsp. enterica serovars Sandiego, Poona, Pomona | [10] |
| USA | 2011–14 | Ten multistate outbreaks | 6 years | 645 | 99 | - | S. enterica subsp. enterica serovars Paratyphi B var. Java, Sandiego, Newport, Pomona, Poona, Typhimurium, I 4,[5],12:i- | [23] | |
| USA | 2014 | A multistate outbreak | 5 years | 40 | 8 | - | Small turtles (<4 inches). | S. enterica subsp. enterica serovar Poona | [20] |
| Chile | - | Case report in three infants | - | 3 | - | - | Pet turtles | S. enterica subsp. enterica serovars Montevideo, Newport, Pomona | [29] |
| Spain | 2009 | Outbreak | 11 months and 4 years | 2 | - | - | Freshwater turtles (Trachemys scripta troosti) purchased from the same pet-shop. | S. enterica subsp. enterica serovars paratyphi B var. Java. | [1] |
| 11 month | 1 | - | - | ||||||
| Spain | 2010–11 | Outbreak | Mostly three months to ten years | 11 | - | - | Turtles | S. enterica subsp. enterica serovars Paratyphi B var Java, Paratyphi B var Java monophasic variant 4,5,12:b:- dT+. and Paratyphi B sensu stricto | [17] |
| France | - | Case report | - | 2 | - | - | Turtles kept at home | Salmonella–rotavirus co-infection | [36] |
| France | - | Case report | 1-month-old infant | 1 | - | - | Pet turtle | S. enterica subsp. enterica serovar Vitkin | [37] |
| Romania | - | Case report | 16-year-old boy | 1 | - | - | Turtles in the lake | S. enterica subsp. arizonae | [38] |
| Japan | 2007–8 | Case report | 5-year-old boy | 1 | - | - | Turtle kept at the patient’s home | S. enterica subsp. enterica serovar Poona | [39] |
| 4-year-old boy | 1 | - | - | Tortoise kept at the patient’s home | S. enterica subsp. enterica serovar Abony |
| Country | Turtle Species | Turtle’s Source | Sample | Sample Size | No. of Positive (%) | Salmonella Serovar(s) | Reference |
|---|---|---|---|---|---|---|---|
| Korea | Six commercially popular species: Chinese stripe-necked turtles (Ocadia sinensis), River cooters (Pseudemys concinna concinna), Yellow-bellied sliders (Trachemys scripta scripta), Common musk turtles (Sternotherus odoratus),Western painted turtles (Chrysemys picta belli), Northern Chinese softshell turtles (Pelodiscus maackii) | Nine pet shops and eight online markets | Fecal samples | 59 | 35 (59.3) | S. enterica subsp. enterica serovars Pomona, Paratyphi, Typhimurium, Thompson, Stanley, Braenderup, Kentucky, Singapore, and Potsdam | [40] |
| Korea | Six commercially popular species:Chinese stripe-necked turtles (Ocadia sinensis), yellow-bellied sliders (Trachemys scripta scripta), River cooters (Pseudemys concinna concinna), Northern Chinese softshell turtles (Pelodiscus maackii), Western painted turtles (Chrysemys picta belli) and common musk turtles (Sternotherus odoratus) | Different pet shops and online markets | Fecal samples | 35 | 21 (60.0%) | S. enterica subsp. enterica | [41] |
| Korea | Six commercially popular species: Chinese stripe-necked turtles (Ocadia sinensis), yellow belly sliders (Trachemysscripta scripta), river cooters (Pseudemys concinna concinna), northern Chinese softshell turtles (Pelodiscusmaackii), western painted turtles (Chrysemys picta belli) and common musk turtles (Sternotherus odoratus) | Nine pet shops and eight online markets | Fecal samples | 34 | 17 (50.0%) | S. enterica subsp. enterica | [42] |
| China | Soft-shelled terrapins | Supermarkets and farmer’s markets | Fecal samples | 172 | 51 (29.7%) | S. enterica subsp. enterica. belonged to twenty-two serovars including Thompson, Hvittingfoss, Typhimurium, Wandsworth, Virchow, Stanley, Saintpaul, Singapore, Kedougou and other subtypes | [43] |
| pet turtles | 164 | 31 (18.9%) | |||||
| UK | Tortoises | Veterinary practice | Cloacal swabs | 89 | 5 (5.6) | S. enterica Group D | [44] |
| Italy | Testudinidae, Trachemys scripta | Reptile owners | Cloacal swabs | 10 | 3 (30) | Salmonella spp. | [45] |
| Spain | Thirty five turtle species | Pet stores and | Water samples | 120 | 24 (20) | Eighteen different serovars belonged to S. enterica subsp. enterica including Typhimurium and Pomona | [46] |
| Private owners | 120 | 96 (80) | |||||
| Spain | Free-living native (Emys orbicularis) and exotic (Trachemys scripta elegans) turtles | Captured turtles | Water samples from exotic and native turtle containers | 200 | 8.0 ± 2.5 | Eight different serovars belonged to S. enterica subsp. enterica serovars Typhimurium and Thompson S. enterica subsp. salamaeS. enterica subsp. diarizonaeS. enterica subsp. houtenae | [8] |
| Cloacal swabs from exotic and native turtles | 200 | 3.0 ± 1.5 | |||||
| Intestinal content samples from only exotic turtles | 117 | 12.0 ± 3.0 | |||||
| Saint Kitts | Leatherback sea turtles | Sea | Cloacal swabs | 9 | 3 (33.3) | S. enterica subsp. enterica serovars Montevideo and Newport. | [30] |
| Hawksbill sea turtles | 14 | 1 (7.1) | |||||
| Green sea turtles | 9 | 0 (0) | |||||
| Saint Kitts, West Indies | Leatherback sea turtles (Dermochelys coriacea) | Sea | Cloacal swabs | 21 | 3 (14.2) | S. enterica subsp. enterica | [33] |
| Colombia | Semi-aquatic turtles | - | Fecal samples | 110 | 30 (27%) | S. enterica subsp. enterica serovars Enteritidis and Typhimurium | [34] |
| Australia | Common long-neck tortoise (Chelodina longicollis) | Captive/Wild | Cloacal swabs | 19 | 2 (10.5) | S. enterica subsp. enterica serovar Typhimurium | [47] |
| Murray River turtle (Emydura macquarii) | 12 | 0 (0) | |||||
| Mary River turtle (Elusor macrurus) | 2 | 0 (0) | |||||
| Sawshell turtle (Elseya latisternum) | 1 | 0 (0) | |||||
| Broadshell turtle (Macrochelodina expansa) | 1 | 0 (0) | |||||
| Krefft’s turtle (Emydura krefftii) | 1 | 0 (0) | |||||
| Irwin’s turtle (Elseya irwini) | 2 | 0 (0) | |||||
| Painted turtle (Emydura subglobosa) | 2 | 0 (0) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sodagari, H.R.; Habib, I.; Shahabi, M.P.; Dybing, N.A.; Wang, P.; Bruce, M. A Review of the Public Health Challenges of Salmonella and Turtles. Vet. Sci. 2020, 7, 56. https://doi.org/10.3390/vetsci7020056
Sodagari HR, Habib I, Shahabi MP, Dybing NA, Wang P, Bruce M. A Review of the Public Health Challenges of Salmonella and Turtles. Veterinary Sciences. 2020; 7(2):56. https://doi.org/10.3390/vetsci7020056
Chicago/Turabian StyleSodagari, Hamid Reza, Ihab Habib, Majedeh Pakzad Shahabi, Narelle A. Dybing, Penghao Wang, and Mieghan Bruce. 2020. "A Review of the Public Health Challenges of Salmonella and Turtles" Veterinary Sciences 7, no. 2: 56. https://doi.org/10.3390/vetsci7020056
APA StyleSodagari, H. R., Habib, I., Shahabi, M. P., Dybing, N. A., Wang, P., & Bruce, M. (2020). A Review of the Public Health Challenges of Salmonella and Turtles. Veterinary Sciences, 7(2), 56. https://doi.org/10.3390/vetsci7020056






