Canine Epithelial Skin Tumours: Expression of the Stem Cell Markers Lgr5, Lgr6 and Sox9 in Light of New Cancer Stem Cell Theories
Abstract
:1. Introduction
2. Material and Methods
2.1. Tumour Samples
2.2. Histological Examination
2.3. Immunohistochemistry (IHC)
2.4. Quantification of Immunolabelling
2.5. RNA Isolation and cDNA Synthesis
2.6. Quantitative Real-Time PCR
2.7. Statistical Analysis
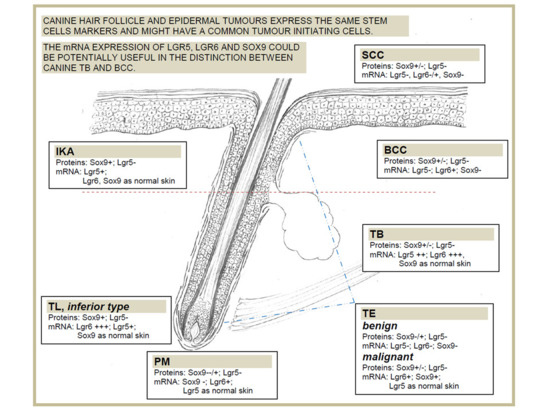
3. Results
3.1. Stem Cell Marker Analyses in Healthy Skin. Sox9 and Lgr5 Protein Expression was Confirmed in Specific Regions of Canine Hair Follicle
3.2. Stem Cell Marker Analyses in Epidermal Tumours. Lgr5 and Sox9 are Downregulated Compared with Normal Skin
3.3. Stem Cell Marker Analyses in Hair Follicle Tumours. TB Samples Show the Highest mRNA Expression of Lgr6, Lgr5 and Sox9, that are Potentially Useful Markers in Differential Diagnosis with BCC
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Qureshi-Baig, K.; Ullmann, P.; Haan, S.; Letellier, E. Tumour-Initiating Cells: A criTICal review of isolation approaches and new challenges in targeting strategies. Mol. Cancer 2017, 16, 40. [Google Scholar] [CrossRef] [PubMed]
- Batlle, E.; Clever, H. Cancer stem cells revisited. Nat. Med. 2017, 23, 1124–1134. [Google Scholar] [CrossRef] [PubMed]
- Curtarelli, R.B.; Gonçalves, J.M.; Dos Santos, L.G.P.; Savi, M.G.; Nör, J.E.; Mezzomo, L.A.M.; Rodríguez Cordeiro, M.M. Expression ofCancer Stem Cell Biomarkers in Human Head and Neck Carcinomas: A Systematic Review. Stem Cell Rev. 2018, 14, 769–784. [Google Scholar] [CrossRef] [PubMed]
- Bongiovanni, L.; Caposano, F.; Romanucci, M.; Grieco, V.; Malatesta, D.; Brachelente, C.; Massimini, M.; Benazzi, C.; Thomas, R.E.; Della Salda, L. Survivin and Sox9: Potential Stem Cell Markers in Canine Normal, Hyperplastic, and Neoplastic Canine Prostate. Vet. Pathol. 2019, 56, 200–207. [Google Scholar] [CrossRef]
- Steinbichler, T.B.; Dudás, J.; Skvortsov, S.; Ganswindt, U.; Riechelmann, H.; Skvortsova, I.-I. Therapy resistance mediated by cancer stem cells. Semin. Cancer Biol. 2018, 53, 156–167. [Google Scholar] [CrossRef]
- Dekoninck, S.; Blanpain, C. Stem cell dynamics, migration and plasticity during wound healing. Nat. Cell Biol. 2019, 21, 18–24. [Google Scholar] [CrossRef] [Green Version]
- Pascucci, L.; Mercati, F.; Gargiulo, A.M.; Pedini, V.; Sorbolini, S.; Ceccarelli, P. CD34 glycoprotein identifies putative stem cells located in the isthmic region of canine hair follicles. Vet. Dermatol. 2006, 17, 244–251. [Google Scholar] [CrossRef]
- Kobayashi, T.; Shimizu, A.; Nishifuji, K.; Amagai, M.; Iwasaki, T.; Ohyama, M. Canine hair-follicle keratinocytes enriched for bulge cells have the highly proliferative characteristic of stem cells. Vet. Dermatol. 2009, 20, 338–346. [Google Scholar] [CrossRef]
- Ohyama, M.; Kobayashi, T. Isolation and characterization of stem cell-enriched human and canine hair follicle keratinocytes. Methods Mol. Biol. 2012, 879, 389–401. [Google Scholar]
- Kobayashi, T.; Iwasaki, T.; Amagai, M.; Ohyama, M. Canine follicle stem cell candidates reside in the bulge and share characteristic features with human bulge cells. J. Investig. Dermatol. 2010, 130, 1988–1995. [Google Scholar] [CrossRef] [Green Version]
- Gerhards, N.M.; Sayar, B.S.; Origgi, F.C.; Galichet, A.; Müller, E.J.; Welle, M.M.; Wiener, D.J. Stem Cell-Associated Marker Expression in Canine Hair Follicles. J. Histochem. Cytochem. 2016, 64, 190–204. [Google Scholar] [CrossRef] [Green Version]
- De Castro, R.V.G.; Tavares, M.R.; Bressan, F.F.; Pieri, N.C.G.; Baracho Trindade Hill, A.; Souza, A.F.; da RN Cruz, N.; Martins, D.S.; Ambrósio, C.E.; Meirelles, F.V.; et al. In vitro identification of a stem cell population from canine hair follicle bulge region. Tissue Cell 2018, 50, 43–50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bongiovanni, L.; Romanucci, M.; Fant, P.; Lagadic, M.; Della Salda, L. Apoptosis and anti-apoptotic heat shock proteins in canine cutaneous infundibular keratinizing acanthomas and squamous cell carcinomas. Vet. Dermatol. 2008, 19, 271–279. [Google Scholar] [CrossRef]
- Bongiovanni, L.; Malatesta, D.; Brachelente, C.; D’Egidio, S.; Della Salda, L. β-catenin in canine skin: Immunohistochemical pattern of expression in normal skin and cutaneous epithelial tumours. J. Comp. Pathol. 2011, 145, 138–147. [Google Scholar] [CrossRef]
- Brachelente, C.; Porcellato, I.; Sforna, M.; Lepri, E.; Mechelli, L.; Bongiovanni, L. The contribution of stem cells to epidermal and hair follicle tumours in the dog. Vet. Dermatol. 2013, 24, 188. [Google Scholar] [CrossRef] [PubMed]
- Bongiovanni, L.; Di Diodoro, F.; Della Salda, L.; Brachelente, C. On the role of survivin as a stem cell biomarker of canine hair follicle and related tumours. Vet. Dermatol. 2014, 25, 138–141. [Google Scholar] [CrossRef] [PubMed]
- Fantinato, E.; Milani, L.; Sironi, G. Sox9 expression in canine epithelial skin tumours. Eur. J. Histochem. 2015, 59, 2514. [Google Scholar] [CrossRef] [Green Version]
- Keong Kok, M.; Chambers, J.K.; Ong, S.M.; Nakayama, H.; Uchida, K. Hierarchical Cluster Analysis of Cytokeratins and Stem Cell Expression Profiles of Canine Cutaneous Epithelial Tumours. Vet. Pathol. 2018, 55, 821–837. [Google Scholar]
- Jang, B.G.; Lee, C.; Kim, H.S.; Shin, M.S.; Cheon, M.S.; Kim, J.W.; Kim, W.H. Distinct expression profile of stem cell markers, LGR5 and LGR6, in basaloid skin tumours. Virchows Arch. 2017, 470, 301–310. [Google Scholar] [CrossRef]
- Hoeck, J.D.; Biehs, B.; Kurtova, A.V.; Kljavin, N.M.; de Sousa E Melo, F.; Alicke, B.; Koeppen, H.; Modrusan, Z.; Piskol, R.; de Sauvage, F.J. Stem cell plasticity enables hair regeneration following Lgr5+ cell loss. Nat. Cell Biol. 2017, 19, 666–676. [Google Scholar] [CrossRef]
- Füllgrabe, A.; Joost, S.; Are, A.; Jacob, T.; Sivan, U.; Haegebarth, A.; Linnarsson, S.; Simons, B.D.; Clevers, H.; Toftgård, R.; et al. Dynamics of Lgr6⁺ Progenitor Cells in the Hair Follicle, Sebaceous Gland, and Interfollicular Epidermis. Stem Cell Rep. 2015, 5, 843–855. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nowak, J.A.; Polak, L.; Pasolli, H.A.; Fuchs, E. Hair follicle stem cells are specified and function in early skin morphogenesis. Cell Stem Cell 2008, 3, 33–43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goldschmidt, M.H.; Dunstan, R.W.; Stannard, A.A.; von Tscharner, C.; Walder, E.J.; Yager, J.A. Histological Classification of Epithelial and Melanocytic Tumours of the Skin of Domestic Animals. In International Histological Classification of Tumours of Domestic Animals; World Health Organization, Armed Forces Institute of Pathology, American Registry of Pathology: Washington, DC, USA, 1998; pp. 20–22. [Google Scholar]
- Gross, T.L.; Ihrke, P.J.; Walder, E.J.; Affolter, V.K. Epidermal tumours. In Skin Diseases of the Dog and Cat; Blackwell Publishing: Oxford, UK, 2005; pp. 562–640. [Google Scholar]
- Rao, X.; Huang, X.; Zhou, Z.; Lin, X. An improvement of the 2ˆ(-delta delta CT) method for quantitative real-time polymerase chain reaction data analysis. Biostat. Bioinform. Biomath. 2013, 3, 71–85. [Google Scholar]
- Huang, P.Y.; Kandyba, E.; Jabouille, A.; Sjolund, J.; Kumar, A.; Halliwill, K.; McCreery, M.; DelRosario, R.; Kang, H.C.; Wong, C.E.; et al. Lgr6 is a stem cell marker in mouse skin squamous cell carcinoma. Nat. Genet. 2017, 49, 1624–1632. [Google Scholar] [CrossRef] [Green Version]
- Youssef, M.; Cuddihy, A.; Darido, C. Long-Lived Epidermal Cancer-Initiating Cells. Int. J. Mol. Sci. 2017, 18, 1369. [Google Scholar] [CrossRef] [Green Version]
- Cammareri, P.; Rose, A.M.; Vincent, D.F.; Wang, J.; Nagano, A.; Libertini, S.; Ridgway, R.A.; Athineos, D.; Coates, P.J.; McHugh, A.; et al. Inactivation of TGFβ receptors in stem cells drives cutaneous squamous cell carcinoma. Nat. Commun. 2016, 7, 12493. [Google Scholar] [CrossRef]
- Vidal, V.P.; Chaboissier, M.C.; Lützkendorf, S.; Cotsarelis, G.; Mill, P.; Hui, C.C.; Ortonne, N.; Ortonne, J.P.; Schedl, A. Sox9 is essential for outer root sheath differentiation and the formation of the hair stem cell compartment. Curr. Biol. 2005, 15, 1340–1351. [Google Scholar] [CrossRef] [Green Version]
- Vidal, V.P.I.; Ortonne, N.; Schedl, A. Sox9 expression is a general marker of basal cell carcinoma and adnexal-related neoplasms. J. Cutan. Pathol. 2008, 35, 373–379. [Google Scholar] [CrossRef]
- Malanchi, I.; Peinado, H.; Kassen, D.; Hussenet, T.; Metzger, D.; Chambon, P.; Huber, M.; Hohl, D.; Cano, A.; Birchmeier, W.; et al. Cutaneous cancer stem cell maintenance is dependent on beta-catenin signalling. Nature 2008, 452, 650–653. [Google Scholar] [CrossRef]
- Leblebici, C.; Bambul Sığırcı, B.; Kelten Talu, C.; Koca, S.B.; Huq, G.E. CD10, TDAG51, CK20, AR, INSM1, and Nestin Expression in the Differential Diagnosis of Trichoblastoma and Basal Cell Carcinoma. Int. J. Surg. Pathol. 2019, 27, 19–27. [Google Scholar] [CrossRef]
- Stanoszek, L.M.; Wang, G.Y.; Harms, P.W. Histologic Mimics of Basal Cell Carcinoma. Arch. Pathol. Lab. Med. 2017, 141, 1490–1502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krahl, D.; Sellheyer, K. Sox9, more than a marker of the outer root sheath: Spatiotemporal expression pattern during human cutaneous embryogenesis. J. Cutan. Pathol. 2010, 37, 350–356. [Google Scholar] [CrossRef] [PubMed]
- Asada, M.; Schaart, F.M.; de Almeida, H.L., Jr.; Korge, B.; Kurokawa, I.; Asada, Y.; Orfanos, C.E. Solid basal cell epithelioma (BCE) possibly originates from the outer root sheath of the hair follicle. Acta Derm Venereol 1993, 73, 286–292. [Google Scholar] [PubMed]
- Youssef, K.K.; Lapouge, G.; Bouvrée, K.; Rorive, S.; Brohée, S.; Appelstein, O.; Larsimont, J.C.; Sukumaran, V.; Van de Sande, B.; Pucci, D.; et al. Adult interfollicular tumour-initiating cells are reprogrammed into an embryonic hair follicle progenitor-like fate during basal cell carcinoma initiation. Nat. Cell Biol. 2012, 14, 1282–1294. [Google Scholar] [CrossRef] [PubMed]
- Bongiovanni, L.; Suter, M.M.; Inverso, A.; Malatesta, D.; Romanucci, M.; Della Salda, L.; Brachelente, C. SOX9 and CK15 stem cell markers in canine sebaceous lesions. J. Comp. Pathol. 2015, 152, 73. [Google Scholar] [CrossRef]













| Tumour Type | Total Number of Cases | Absent | Low 0%–10% | Moderate 10%–25% | High 25%–50% | Very High >50% | Overall Expression |
|---|---|---|---|---|---|---|---|
| SCC | 9 | 5 | 0 | 3 | 1 | 0 | 5/9 absent |
| BCC | 6 | 3 | 0 | 2 | 0 | 1 | 3/6 (50% of cases) absent |
| TL | 3 | 1 | 1 | 1 | 0 | 0 | variable (from absent to moderate) |
| IKA | 9 | 2 | 2 | 3 | 1 | 1 | 5/9 moderate/low |
| TB | 8 | 3 | 1 | 2 | 1 | 1 | 4/8 (50% of cases) absent/low |
| TE | 11 | 5 | 4 | 1 | 1 | 0 | 9/11 absent/low |
| PM | 6 | 1 | 3 | 1 | 0 | 1 | 4/6 absent/low |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bongiovanni, L.; Brachelente, C.; Moreno, E.; Welle, M.M. Canine Epithelial Skin Tumours: Expression of the Stem Cell Markers Lgr5, Lgr6 and Sox9 in Light of New Cancer Stem Cell Theories. Vet. Sci. 2020, 7, 62. https://doi.org/10.3390/vetsci7020062
Bongiovanni L, Brachelente C, Moreno E, Welle MM. Canine Epithelial Skin Tumours: Expression of the Stem Cell Markers Lgr5, Lgr6 and Sox9 in Light of New Cancer Stem Cell Theories. Veterinary Sciences. 2020; 7(2):62. https://doi.org/10.3390/vetsci7020062
Chicago/Turabian StyleBongiovanni, Laura, Chiara Brachelente, Eva Moreno, and Monika M. Welle. 2020. "Canine Epithelial Skin Tumours: Expression of the Stem Cell Markers Lgr5, Lgr6 and Sox9 in Light of New Cancer Stem Cell Theories" Veterinary Sciences 7, no. 2: 62. https://doi.org/10.3390/vetsci7020062