Morphological and Immunohistochemical Description of a Splenic Haemangioma in a Captive European Wolf (Canis lupus lupus) and a Review of the Current Literature
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subject
2.2. Pathological Investigations
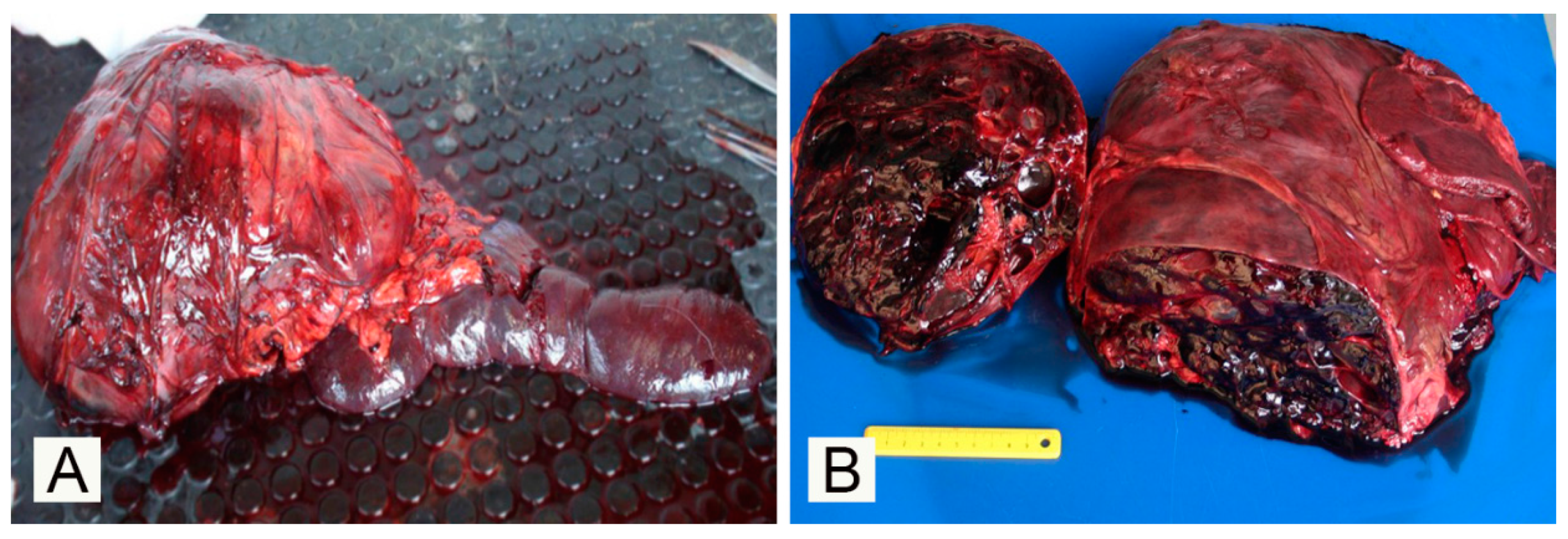
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Robinson, W.F.; Robinson, N.A. Cardiovascular system. In Jubb, Kennedy, and Palmer’s Pathology of Domestic Animals, 6th ed.; Maxie, M.G., Ed.; Elsevier: St. Louis, MO, USA, 2015; pp. 1–101. [Google Scholar]
- Acton, A.E.; Munson, L.; Waddell, W.T. Survey of necropsy results in captive red wolves (Canis rufus), 1992–1996. J. Zoo Wildl. Med. 2000, 31, 2–8. [Google Scholar] [PubMed]
- Sabattini, S.; Bettini, G. An immunohistochemical analysis of canine haemangioma and haemangiosarcoma. J. Comp. Pathol. 2009, 140, 158–168. [Google Scholar] [CrossRef] [PubMed]
- Hendrick, M.J. Mesenchymal tumors of the skin and soft tissues. In Tumors in Domestic Animals, 5th ed.; Meuten, D.J., Ed.; Wiley Blackwell: Hoboken, NJ, USA, 2020; p. 142. [Google Scholar]
- Von Beust, B.R.; Suter, M.M.; Summers, B.A. Factor VIII-related antigen in canine endothelial neoplasms: An immunohistochemical study. Vet. Pathol. 1988, 25, 251–255. [Google Scholar] [CrossRef]
- Rosales Alferez, F.; Tavares Mendoza, F.H.; Pereda Solis, E.M.; Martinez Guerrero, J.H.; Herrera Casio, M.H. Case report of malignant mammary neoplasia in mexican gray wolf (Canis lupus baileyi). J. Anim. Vet. Adv. 2010, 9, 1472–1475. [Google Scholar]
- Bock, P.; Seehusen, F.; Muller, H.; Aupperle, H.; Hewicker-Trautwein, M.; Wohlsein, P. Subcutaneous leiomyosarcoma in a captive European wolf (Canis lupus). Vet. Rec. 2007, 161, 429–430. [Google Scholar] [CrossRef]
- Teifke, J.P.; Lohr, C.V.; Langner, C. Tp53 expressing squamous cell carcinoma of the tonsil in a captive polar wolf (Canis lupus arctos). J. Zoo Wildl. Med. 2005, 36, 538–542. [Google Scholar] [CrossRef]
- Samuel, W.M.; Chalmers, G.A.; Gunson, J.R. Oral papillomatosis in coyotes (Canis latrans) and wolves (Canis lupus) of Alberta. J. Wildl. Dis. 1978, 14, 165–169. [Google Scholar] [CrossRef] [Green Version]
- Seeley, K.E.; Garner, M.M.; Waddell, W.T.; Wolf, K.N. A survey of diseases in captive red wolves (Canis Rufus), 1997–2012. J. Zoo Wildl. Med. 2016, 47, 83–90. [Google Scholar] [CrossRef]
- Shiraki, A.; Yoshida, T.; Kawashima, M.; Murayama, H.; Nagahara, R.; Ito, N.; Shibutani, M. Pulmonary neuroendocrine tumor in a female wolf (Canis lupus lupus). J. Vet. Med. Sci. 2017, 79, 588–592. [Google Scholar] [CrossRef] [Green Version]
- Rothenburger, J.L.; Myers, S.; Lockerbie, B.; Wobeser, B. Novel papillomaviral sequence detected within epidermal plaques in a wolf (Canis lupus). J. Wildl. Dis. 2016, 52, 177–179. [Google Scholar] [CrossRef] [Green Version]
- Brown, N.O.; Patnaik, A.K.; MacEwen, E.G. Canine hemangiosarcoma: Retrospective analysis of 104 cases. J. Am. Vet. Med Assoc. 1985, 186, 56–58. [Google Scholar] [PubMed]
- Smith, A.N. Hemangiosarcoma in dogs and cats: Vet. Clin. Nord Am: Small Anim. Pract. 2003, 33, 533–552. [Google Scholar] [CrossRef]
- Pearson, G.R.; Head, K.W. Malignant hemangioendothelioma (angiosarcoma) in the dog. J. Small Anim. Pract. 1976, 17, 737–745. [Google Scholar] [CrossRef] [PubMed]
- Fernades, S.C.; De Nardi, A.B. Hemangiosarcoma. In Oncologia em Cães e Gatos; Daleck, C.R., De Nardi, A.B., Rodaski, S., Eds.; Marca: Roca, Portugal, 2008; pp. 525–537. [Google Scholar]
- Oksanen, A. Hemangiosarcoma in dogs. J. Comp. Pathol. 1978, 88, 585–595. [Google Scholar] [CrossRef]
- Hammer, A.S.; Couto, C.G.; Filppi, J.; Shank, K. Efficacy and toxicity of VAC chemotherapy (vincristine, doxorubicin, and cyclophosphamide) in dogs with hemangiosarcoma. J. Vet. Inter. Med. 1991, 5, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Clifford, C.A.; Mackin, A.J.; Henry, C.J. Treatment of canine hemangiosarcoma: 2000 and beyond. J. Vet. Inter. Med. 2000, 14, 479–485. [Google Scholar] [CrossRef]
- Sorenmo, K.; Duda, L.; Barber, L.; Cronin, K.; Sammarco, C.; Usborne, A.; Goldschmidt, M.; Shofer, F. Canine hemangiosracoma treated with standard chemotherapy and minocycline. J. Vet. Intern. Med. 2000, 14, 395–398. [Google Scholar] [CrossRef]
- Ferrer, L.; Fondevila, D.; Rabanal, R.M.; Vilafranca, M. Immunohistochemical detection of CD31 antigen in normal and neoplastic canine endothelial cells. J. Comp. Pathol. 1995, 112, 319–326. [Google Scholar] [CrossRef]
- Giuffrida, M.A.; Bacon, N.J.; Kamstock, D.A. Use of routine histopathology and factor VIII-related antigen/von Willebrand factor immunohistochemistry to differentiate primary hemangiosarcoma of bone from telangectasic osteosarcoma in 54 dogs. Vet Comp. Oncol. 2017, 15, 1232–1239. [Google Scholar] [CrossRef]
- Laakkonen, P.; Waltari, M.; Holopainen, T.; Takahashi, T.; Pytowski, B.; Steiner, P.; Hicklin, D.; Persaud, K.; Tonra, J.R.; Witte, L.; et al. Vascular endothelial growth factor receptor 3 (VEGFR-3) is involved in tumor angiogenesis and growth. Cancer Res. 2007, 67, 593–599. [Google Scholar] [CrossRef] [Green Version]
- Nóbrega, D.F.; Sehaber, V.F.; Madureira, R.; Bracarense, P.F.R. Canine cutaneous haemangiosarcoma: Biomarkers and survival. J. Comp. Pathol. 2019, 166, 87–96. [Google Scholar]
- Petrova, T.V.; Bono, P.; Holnthoner, W.; Chesnes, J.; Pytowski, B.; Sihto, H.; Laakkonen, P.; Heikkila, P.; Joensuu, H.; Alitalo, K. VEGFR-3 expression is restricted to blood and lymphatic vessels in solid tumors. Cancer Cell 2008, 13, 554–556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Da Silva, L.; Fonseca-Alves, C.E. Pilot assessment of vascular endothelial growth factor receptors and trafficking pathway in recurrent and metastatic canine subcutaneous mast cell tumours. Vet. Med. Sci. 2017, 3, 146–155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neuhauser, T.S.; Derringer, G.A.; Thompson, L.D.; Fanburg-Smith, J.C.; Miettinen, M.; Saaristo, A.; Abbondanzo, S.L. Splenic angiosarcoma: A clinicopathologic and immunophenotypic study of 28 cases. Modern Pathol. 2000, 13, 978–987. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Platt, S.R.; Scase, T.J.; Adams, V.; Wieczoreck, L.; Miller, J.; Adamo, F.; Long, S. Vascular endothelial growth factor expression in canine intracranial meningiomas and association with patient survival. J. Vet. Inter. Med. 2006, 20, 663–668. [Google Scholar] [CrossRef]
- Kajita, M.; Itoh, Y.; Chiba, T.; Mori, H.; Okada, A.; Kinoh, H.; Seiki, M. Membrane-type 1 matrix metalloproteinase cleaves CD44 and promotes cell migration. J. Cell Biol. 2001, 153, 893–904. [Google Scholar] [CrossRef]


| Species | Main Findings | Reference |
|---|---|---|
| Mexican gray wolf (Canis lupus baileyi) | Malignant mammary tumor | [6] |
| European wolf (Canis lupus lupus) | Subcutaneous leiomyosarcoma | [7] |
| Polar wolf (Canis lupus arctos) | Oral squamous cell carcinoma | [8] |
| Red wolves (Canis rufus) | Two multicentric lymphomas, one mesenteric round cell tumor, one bronchial carcinoma and two osteosarcomas | [2] |
| American coyotes (Canis latrans) and wolves (Canis lupus). | Oral papillomatosis | [9] |
| Red wolves (Canis rufus) | Forty-three cases in which carcinoma or adenocarcinoma and lymphoma were the most common. There were also reports of pheochromocytomas, osteo-sarcomas, fibrosarcomas, granulosa cell tumors, sarcomas, lymphosarcomas, nerve sheath tumors, sertoli cell tumors, histiocytomas, and adenomas. | [10] |
| Eurasian wolf (Canis lupus lupus) | Pulmonary neuroendocrine tumor | [11] |
| Gray wolf (Canis lupus) | Papillomaviral plaque | [12] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez, J.M.M.; Morandi, F.; Cavicchio, P.; Poli, A.; Verin, R. Morphological and Immunohistochemical Description of a Splenic Haemangioma in a Captive European Wolf (Canis lupus lupus) and a Review of the Current Literature. Vet. Sci. 2020, 7, 102. https://doi.org/10.3390/vetsci7030102
Rodríguez JMM, Morandi F, Cavicchio P, Poli A, Verin R. Morphological and Immunohistochemical Description of a Splenic Haemangioma in a Captive European Wolf (Canis lupus lupus) and a Review of the Current Literature. Veterinary Sciences. 2020; 7(3):102. https://doi.org/10.3390/vetsci7030102
Chicago/Turabian StyleRodríguez, Josep Maria Monné, Federico Morandi, Paolo Cavicchio, Alessandro Poli, and Ranieri Verin. 2020. "Morphological and Immunohistochemical Description of a Splenic Haemangioma in a Captive European Wolf (Canis lupus lupus) and a Review of the Current Literature" Veterinary Sciences 7, no. 3: 102. https://doi.org/10.3390/vetsci7030102





