Longitudinal Volumetric Assessment of Ventricular Enlargement in Pet Dogs Trained for Functional Magnetic Resonance Imaging (fMRI) Studies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Aim of the Study
2.2. Subjects
2.3. Training of the Dogs
2.4. Imaging Technique and Analyses
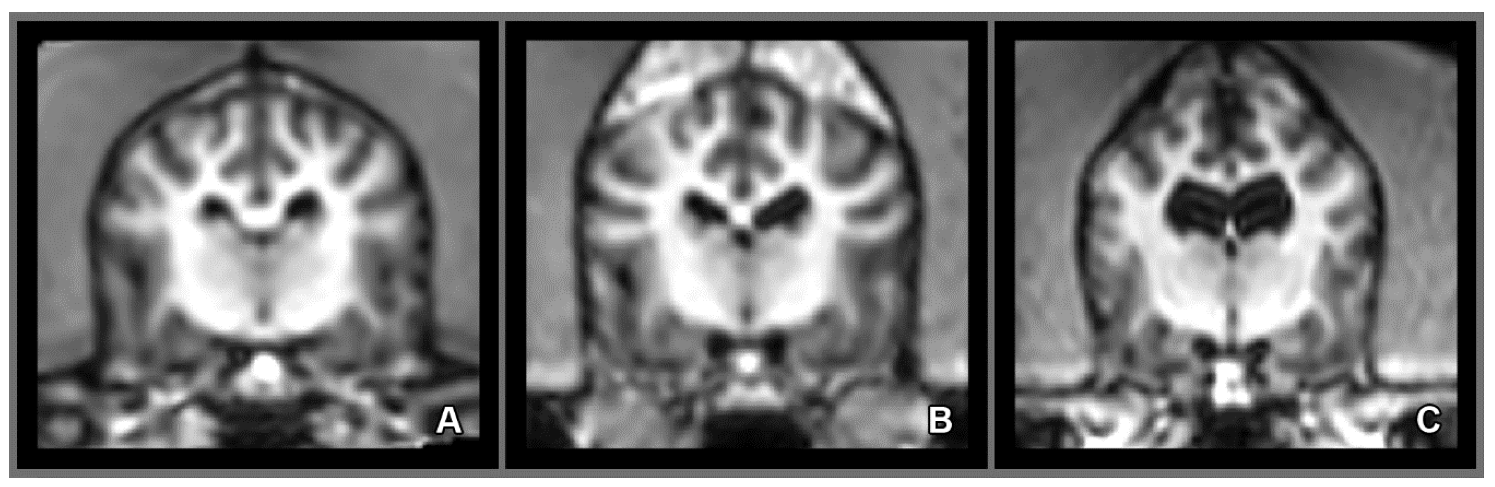
2.5. Morphology and Size of the Measured Brains
2.6. Statistical Analyses
3. Results
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Vullo, T.; Korenman, E.; Manzo, R.P.; Gomez, D.G.; Deck, M.D.F.; Cahill, P.T. Diagnosis of cerebral ventriculomegaly in normal adult beagles using quantitative MRI. Vet. Radiol. Ultrasound 1997, 38, 277–281. [Google Scholar] [CrossRef] [PubMed]
- Thomas, W.B. Nonneoplastic disorders of the brain. Clin. Tech. Small Anim. Pract. 1999, 14, 125–147. [Google Scholar] [CrossRef]
- Ryan, C.T.; Glass, E.N.; Seiler, G.; Zwingenberger, A.L.; Mai, W. Magnetic resonance imaging findings associated with lateral cerebral ventriculomegaly in English bulldogs. Vet. Radiol. Ultrasound 2014, 55, 292–299. [Google Scholar] [CrossRef] [PubMed]
- Laubner, S.; Ondreka, N.; Failing, K.; Kramer, M.; Schmidt, M.J. Magnetic resonance imaging signs of high intraventricular pressure-comparison of findings in dogs with clinically relevant internal hydrocephalus and asymptomatic dogs with ventriculomegaly. BMC Vet. Res. 2015, 11, 181. [Google Scholar] [CrossRef] [Green Version]
- Kii, S.; Uzuka, Y.; Taura, Y.; Nakaichi, M.; Takeuchi, A.; Inokuma, H.; Onishi, T. Magnetic resonance imaging of the lateral ventricles in beagle-type dogs. Vet. Radiol. Ultrasound 1997, 38, 430–433. [Google Scholar] [CrossRef] [PubMed]
- Esteve-Ratsch, B.; Kneissl, S.; Gabler, C. Comparative evaluation of the ventricles in the Yorkshire terrier and the German shepherd dog using low-field MRI. Vet. Radiol. Ultrasound 2001, 42, 410–413. [Google Scholar] [CrossRef] [PubMed]
- Haan, C.E.D.; Kraft, S.L.; Gavin, P.R.; Wendling, L.R.; Griebenow, M.L. Normal variation in size of the lateral ventricles of the labrador retriever dog as assessed by magnetic resonance imaging. Vet. Radiol. Ultrasound 1994, 35, 83–86. [Google Scholar] [CrossRef]
- Thomas, W.B. Hydrocephalus in dogs and cats. Vet. Clin. Small Anim. Pract. 2010, 40, 143–159. [Google Scholar] [CrossRef]
- Vite, C.H.; Insko, E.K.; Schotland, H.M.; Panckeri, K.; Hendricks, J.C. Quantification of cerebral ventricular volume in English bulldogs. Vet. Radiol. Ultrasound 1997, 38, 437–443. [Google Scholar] [CrossRef]
- Driver, C.J.; Chandler, K.; Walmsley, G.; Shihab, N.; Volk, H.A. The association between Chiari-like malformation, ventriculomegaly and seizures in cavalier King Charles spaniels. Vet. J. 2013, 195, 235–237. [Google Scholar] [CrossRef]
- Su, M.; Head, E.; Brooks, W.M.; Wang, Z.; Muggenburg, B.A.; Adam, G.E.; Sutherland, R.; Cotman, C.W.; Nalcioglu, O. Magnetic resonance imaging of anatomic and vascular characteristics in a canine model of human aging. Neurobiol. Aging 1998, 19, 479–485. [Google Scholar] [CrossRef]
- Su, M.-Y.; Tapp, P.D.; Vu, L.; Chen, Y.-F.; Chu, Y.; Muggenburg, B.; Chiou, J.-Y.; Chen, C.; Wang, J.; Bracco, C.; et al. A longitudinal study of brain morphometrics using serial magnetic resonance imaging analysis in a canine model of aging. Prog. Neuropsychopharmacol. Biol. Psychiatry 2005, 29, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Head, E. Neurobiology of the aging dog. AGE 2011, 33, 485–496. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.J.; Laubner, S.; Kolecka, M.; Failing, K.; Moritz, A.; Kramer, M.; Ondreka, N. Comparison of the relationship between cerebral white matter and grey matter in normal dogs and dogs with lateral ventricular enlargement. PLoS ONE 2015, 10, e0124174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmidt, M.J.; Kolecka, M.; Kirberger, R.; Hartmann, A. Dynamic susceptibility contrast perfusion magnetic resonance imaging demonstrates reduced periventricular cerebral blood flow in dogs with ventriculomegaly. Front. Vet. Sci. 2017, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Momjian, S.; Czosnyka, Z.; Czosnyka, M.; Pickard, J.D. Link between vasogenic waves of intracranial pressure and cerebrospinal fluid outflow resistance in normal pressure hydrocephalus. Br. J. Neurosurg. 2004, 18, 56–61. [Google Scholar] [CrossRef]
- Jeppsson, A.; Zetterberg, H.; Blennow, K.; Wikkelsø, C. Idiopathic normal-pressure hydrocephalus: Pathophysiology and diagnosis by CSF biomarkers. Neurology 2013, 80, 1385–1392. [Google Scholar] [CrossRef]
- Iddon, J.L.; Pickard, J.D.; Cross, J.J.L.; Griffiths, P.D.; Czosnyka, M.; Sahakian, B.J. Specific patterns of cognitive impairment in patients with idiopathic normal pressure hydrocephalus and Alzheimer’s disease: A pilot study. J. Neurol. Neurosurg. Psychiatry 1999, 67, 723–732. [Google Scholar] [CrossRef] [Green Version]
- Yamada, H.; Yokota, A.; Haratake, J.; Horie, A. Morphological study of experimental syringomyelia with kaolin-induced hydrocephalus in a canine model. J. Neurosurg. 1996, 84, 999–1005. [Google Scholar] [CrossRef]
- Vullo, T.; Manzo, R.; Gomez, D.G.; Deck, M.D.; Cahill, P.T. A canine model of acute hydrocephalus with MR correlation. Am. J. Neuroradiol. 1998, 19, 1123–1125. [Google Scholar]
- Tapp, P.D.; Siwak, C.T.; Gao, F.Q.; Chiou, J.-Y.; Black, S.E.; Head, E.; Muggenburg, B.A.; Cotman, C.W.; Milgram, N.W.; Su, M.-Y. Frontal lobe volume, function, and β-amyloid pathology in a canine model of aging. J. Neurosci. 2004, 24, 8205–8213. [Google Scholar] [CrossRef] [Green Version]
- Tapp, P.D.; Chu, Y.; Araujo, J.A.; Chiou, J.-Y.; Head, E.; Milgram, N.W.; Su, M.-Y. Effects of scopolamine challenge on regional cerebral blood volume. A pharmacological model to validate the use of contrast enhanced magnetic resonance imaging to assess cerebral blood volume in a canine model of aging. Prog. Neuropsychopharmacol. Biol. Psychiatry 2005, 29, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Head, E. A canine model of human aging and Alzheimer’s disease. Biochim. Biophys. Acta 2013, 1832, 1384–1389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katz, M.L.; Coates, J.R.; Sibigtroth, C.M.; Taylor, J.D.; Carpentier, M.; Young, W.M.; Wininger, F.A.; Kennedy, D.; Vuillemenot, B.R.; O’Neill, C.A. Enzyme replacement therapy attenuates disease progression in a canine model of late-infantile neuronal ceroid lipofuscinosis (CLN2 disease). J. Neurosci. Res. 2014, 92, 1591–1598. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schütt, T.; Helboe, L.; Pedersen, L.Ø.; Waldemar, G.; Berendt, M.; Pedersen, J.T. Dogs with cognitive dysfunction as a spontaneous model for early Alzheimer’s disease: A translational study of neuropathological and inflammatory markers. J. Alzheimers Dis. 2016, 52, 433–449. [Google Scholar] [CrossRef]
- Mazzatenta, A.; Carluccio, A.; Robbe, D.; Giulio, C.D.; Cellerino, A. The companion dog as a unique translational model for aging. Semin. Cell Dev. Biol. 2017, 70, 141–153. [Google Scholar] [CrossRef]
- Sándor, S.; Kubinyi, E. Genetic pathways of aging and their relevance in the dog as a natural model of human aging. Front. Genet. 2019, 10. [Google Scholar] [CrossRef]
- Hines, C.D.G.; Song, X.; Kuruvilla, S.; Farris, G.; Markgraf, C.G. Magnetic resonance imaging assessment of the ventricular system in the brains of adult and juvenile beagle dogs treated with posaconazole IV Solution. J. Pharmacol. Toxicol. Methods 2015, 76, 55–64. [Google Scholar] [CrossRef]
- Andics, A.; Gácsi, M.; Faragó, T.; Kis, A.; Miklósi, A. Voice-sensitive regions in the dog and human brain are revealed by comparative fMRI. Curr. Biol. CB 2014, 24, 574–578. [Google Scholar] [CrossRef] [Green Version]
- Andics, A.; Gábor, A.; Gácsi, M.; Faragó, T.; Szabó, D.; Miklósi, Á. Neural mechanisms for lexical processing in dogs. Science 2016, 353, 1030–1032. [Google Scholar] [CrossRef]
- Smith, S.M.; Jenkinson, M.; Woolrich, M.W.; Beckmann, C.F.; Behrens, T.E.J.; Johansen-Berg, H.; Bannister, P.R.; De Luca, M.; Drobnjak, I.; Flitney, D.E.; et al. Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage 2004, 23 (Suppl. 1), S208–S219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jenkinson, M.; Beckmann, C.F.; Behrens, T.E.J.; Woolrich, M.W.; Smith, S.M. FSL. NeuroImage 2012, 62, 782–790. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Brady, M.; Smith, S. Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Trans. Med. Imaging 2001, 20, 45–57. [Google Scholar] [CrossRef] [PubMed]
- Czakó, L. Egy új hazai kezdeményezés: Kutya Agy- és Szövetbank Létesítése és Működtetése [A New Hungarian Initiative: Creating the Canine Brain and Tissue Bank]. Master’s thesis, ELTE Eötvös Loránd University (Institute of Biology, Department of Ethology) and the University of Veterinary Medicine (Department of Animal Hygiene, Herd Health and Mobile Clinic), Budapest, Hungary, 2018. [Google Scholar]
- Madari, A.; Farbakova, J.; Katina, S.; Smolek, T.; Novak, P.; Weissova, T.; Novak, M.; Zilka, N. Assessment of severity and progression of canine cognitive dysfunction syndrome using the CAnine DEmentia Scale (CADES). Appl. Anim. Behav. Sci. 2015, 171, 138–145. [Google Scholar] [CrossRef]
- Yamada, S.; Ducker, T.B.; Perot, P.L. Dynamic changes of cerebrospinal fluid in upright and recumbent shunted experimental animals. Pediatr. Neurosurg. 1975, 1, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Ravussin, P.; Guinard, J.P.; Ralley, F.; Thorin, D. Effect of propofol on cerebrospinal fluid pressure and cerebral perfusion pressure in patients undergoing craniotomy. Anaesthesia 1988, 43, 37–41. [Google Scholar] [CrossRef]
- Harrington, M.L.; Bagley, R.S.; Moore, M.P.; Tyler, J.W. Effect of craniectomy, durotomy, and wound closure on intracranial pressure in healthy cats. Am. J. Vet. Res. 1996, 57, 1659–1661. [Google Scholar]




| Ventricular Enlargement between 1st and 2nd Measurement of the 7 Dogs | ||||
|---|---|---|---|---|
| Age in months at the time of the scan | Enlargement in % | |||
| ID | 1st | 2nd | left | Right |
| 1 | 39 | 82 | 61.96 | 24.04 |
| 2 | 27 | 78 | 48.32 | 86.10 |
| 3 | 60 | 115 | 44.21 | 46.60 |
| 4 | 99 | 144 | 6.45 | 11.33 |
| 5 | 44 | 95 | 70.77 | 0.34 |
| 6 | 23 | 76 | 41.52 | 56.15 |
| 7 | 32 | 80 | 58.98 | 97.92 |
| Average | 47.46 | 46.07 | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gunde, E.; Czeibert, K.; Gábor, A.; Szabó, D.; Kis, A.; Arany-Tóth, A.; Andics, A.; Gácsi, M.; Kubinyi, E. Longitudinal Volumetric Assessment of Ventricular Enlargement in Pet Dogs Trained for Functional Magnetic Resonance Imaging (fMRI) Studies. Vet. Sci. 2020, 7, 127. https://doi.org/10.3390/vetsci7030127
Gunde E, Czeibert K, Gábor A, Szabó D, Kis A, Arany-Tóth A, Andics A, Gácsi M, Kubinyi E. Longitudinal Volumetric Assessment of Ventricular Enlargement in Pet Dogs Trained for Functional Magnetic Resonance Imaging (fMRI) Studies. Veterinary Sciences. 2020; 7(3):127. https://doi.org/10.3390/vetsci7030127
Chicago/Turabian StyleGunde, Eva, Kálmán Czeibert, Anna Gábor, Dóra Szabó, Anna Kis, Attila Arany-Tóth, Attila Andics, Márta Gácsi, and Enikő Kubinyi. 2020. "Longitudinal Volumetric Assessment of Ventricular Enlargement in Pet Dogs Trained for Functional Magnetic Resonance Imaging (fMRI) Studies" Veterinary Sciences 7, no. 3: 127. https://doi.org/10.3390/vetsci7030127
APA StyleGunde, E., Czeibert, K., Gábor, A., Szabó, D., Kis, A., Arany-Tóth, A., Andics, A., Gácsi, M., & Kubinyi, E. (2020). Longitudinal Volumetric Assessment of Ventricular Enlargement in Pet Dogs Trained for Functional Magnetic Resonance Imaging (fMRI) Studies. Veterinary Sciences, 7(3), 127. https://doi.org/10.3390/vetsci7030127







