Impact of Dehydroepiandrosterone (DHEA) on Bone Mineral Density and Bone Mineral Content in a Rat Model of Male Hypogonadism
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Housing and Diet
2.2. Experimental Groups
2.3. Orchidectomy Rat Model
2.4. Blood Samples Collections
2.5. Measurement of Bone Mineral Density (BMD) and Bone Mineral Content (BMC) of Right Tibia and Femur
2.6. Biochemical Parameters
2.7. Histopathological Examination of Bone
2.8. Statistical Analysis
3. Results
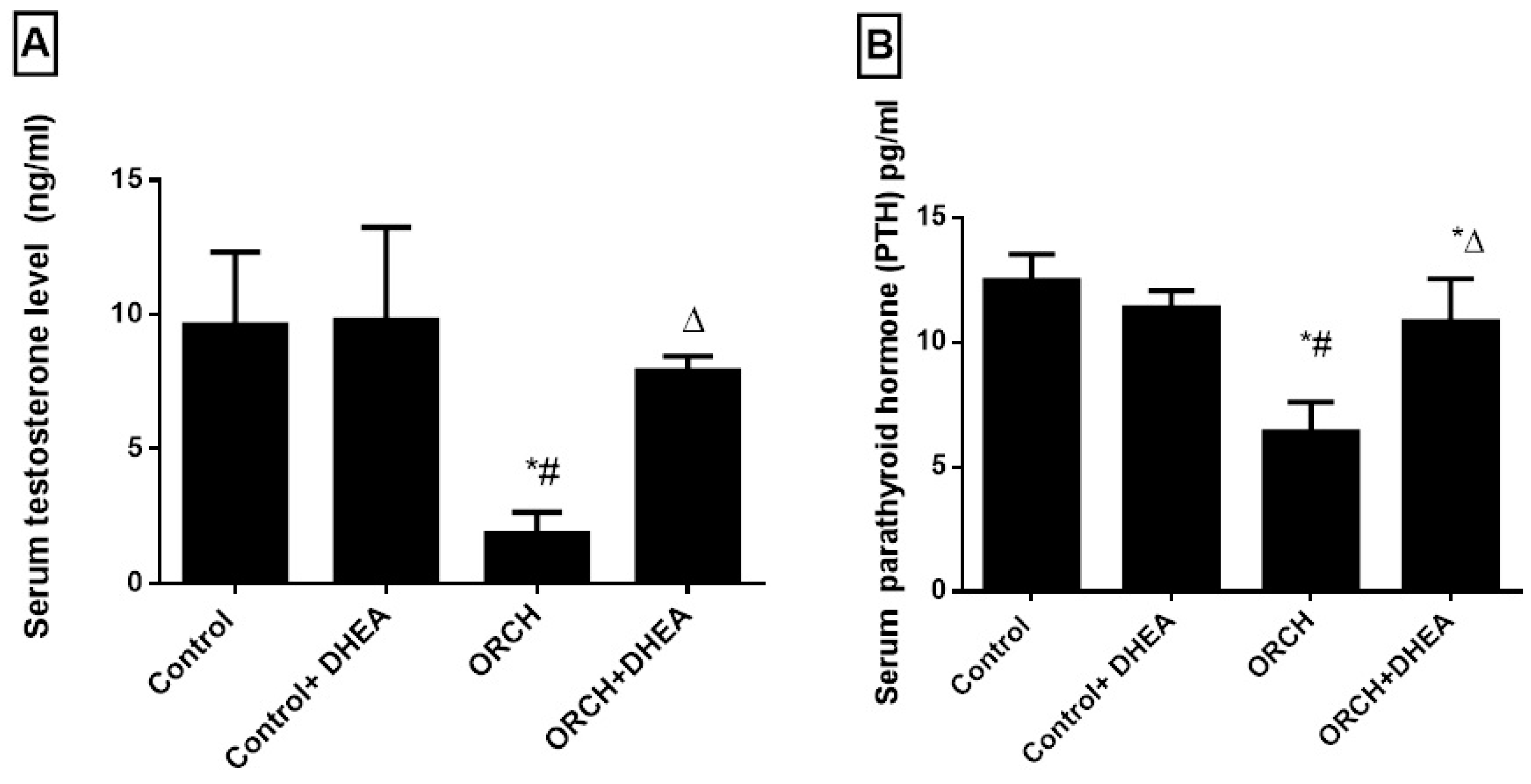
3.1. Effects of Orchidectomy and DHEA Treatment on Serum Levels of Testosterone and Parathyroid Hormones
3.2. Effect of DHEA on BMD and BMC in Orchidectomized Rats
3.3. Effect of DHEA on Osteoprotegerin (OPG) and RANK in Orchidectomized Rats
3.4. Effect of DHEA on Bone Resorption Markers (Deoxypyridinoline (Dpd), Tartrate-Resistant Acid Phosphatase 5b and N-Telopeptide of Type I Collagen (NTXI)) in Orchidectomized Rats
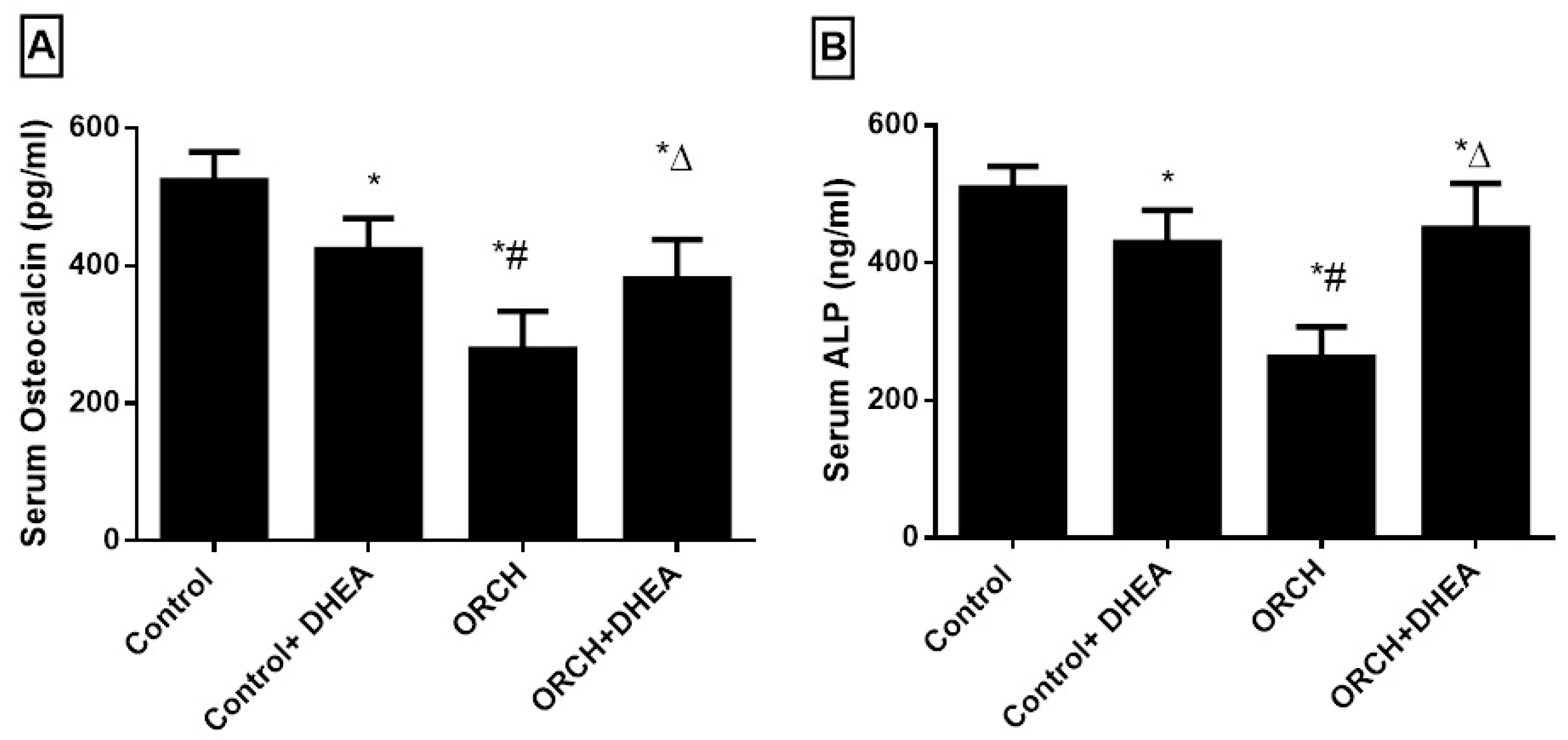
3.5. Effect of DHEA on Bone Formation Markers Osteocalcin (OC) and Alkaline Phosphatase (ALP)
3.6. Effect of DHEA on Bone Morphology in Orchidectomized Rats
3.7. Correlations between Serum PTH and Testosterone and Other Studied Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lerner, U.H. Bone remodeling in post-menopausal osteoporosis. J. Dent. Res. 2006, 85, 584–595. [Google Scholar] [CrossRef] [PubMed]
- Gravallese, E.M.; Goldring, S.R. Cellular mechanisms and the role of cytokines in bone erosions in rheumatoid arthritis. Arthritis Rheum. Off. J. Am. Coll. Rheumatol. 2000, 43, 2143–2151. [Google Scholar] [CrossRef]
- Riggs, B.L.; Khosla, S.; Melton, L.J., III. A unitary model for involutional osteoporosis: Estrogen deficiency causes both type I and type II osteoporosis in postmenopausal women and contributes to bone loss in aging men. J. Bone Miner. Res. 1998, 13, 763–773. [Google Scholar] [CrossRef] [PubMed]
- Burger, H.; De Laet, C.E.; Van Daele, P.L.; Weel, A.E.; Witteman, J.C.; Hofman, A.; Pols, H.A. Risk factors for increased bone loss in an elderly population the rotterdam study. Am. J. Epidemiol. 1998, 147, 871–879. [Google Scholar] [CrossRef] [Green Version]
- Stanley, H.L.; Schmitt, B.P.; Poses, R.M.; Deiss, W.P. Does hypogonadism contribute to the occurrence of a minimal trauma hip fracture in elderly men? J. Am. Geriatr. Soc. 1991, 39, 766–771. [Google Scholar] [CrossRef]
- Boonen, S.; Vanderschueren, D.; Cheng, X.G.; Verbeke, G.; Dequeker, J.; Geusens, P.; Broos, P.; Bouillon, R. Age-Related (Type II) Femoral Neck Osteoporosis in Men: Biochemical Evidence for Both Hypovitaminosis D–and Androgen Deficiency–Induced Bone Resorption. J. Bone Miner. Res. 1997, 12, 2119–2126. [Google Scholar] [CrossRef] [PubMed]
- Kenny, A.M.; Prestwood, K.M.; Marcello, K.M.; Raisz, L.G. Determinants of bone density in healthy older men with low testosterone levels. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2000, 55, M492–M497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scopacasa, F.; Horowitz, M.; Wishart, J.M.; Morris, H.A.; Chatterton, B.E.; Need, A.G. The relation between bone density, free androgen index, and estradiol in men 60 to 70 years old. Bone 2000, 27, 145–149. [Google Scholar] [CrossRef]
- Steeve, K.T.; Marc, P.; Sandrine, T.; Dominique, H.; Yannick, F. IL-6, RANKL, TNF-alpha/IL-1: Interrelations in bone resorption pathophysiology. Cytokine Growth Factor Rev. 2004, 15, 49–60. [Google Scholar] [CrossRef]
- Genazzani, A.D.; Lanzoni, C.; Genazzani, A.R. Might DHEA be considered a beneficial replacement therapy in the elderly? Drugs Aging 2007, 24, 173–185. [Google Scholar] [CrossRef]
- Nawata, H.; Tanaka, S.; Tanaka, S.; Takayanagi, R.; Sakai, Y.; Yanase, T.; Ikuyama, S.; Haji, M. Aromatase in bone cell: Association with osteoporosis in postmenopausal women. J. Steroid Biochem. Mol. Biol. 1995, 53, 165–174. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Y.D.; Wang, W.J.; Zhu, Y.; Li, D.J. Dehydroepiandrosterone improves murine osteoblast growth and bone tissue morphometry via mitogen-activated protein kinase signaling pathway independent of either androgen receptor or estrogen receptor. J. Mol. Endocrinol. 2007, 38, 467–479. [Google Scholar] [CrossRef] [PubMed]
- Clarke, B.L.; Ebeling, P.R.; Jones, J.D.; Wahner, H.W.; O’fallon, W.M.; Riggs, B.L.; Fitzpatrick, L.A. Predictors of bone mineral density in aging healthy men varies by skeletal site. Calcif. Tissue Int. 2002, 70, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.D.; Tao, M.F.; Cheng, W.W.; Liu, X.H.; Wan, X.P.; KeMi, C. Dehydroepiandrosterone indirectly inhibits human osteoclastic resorption via activating osteoblastic viability by the MAPK pathway. Chin. Med. J. 2012, 125, 1230–1235. [Google Scholar]
- Panjari, M.; Davis, S.R. DHEA therapy for women: Effect on sexual function and wellbeing. Hum. Reprod. Update 2007, 13, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Erben, R.G.; Eberle, J.; Stahr, K.; Goldberg, M. Androgen deficiency induces high turnover osteopenia in aged male rats: A sequential histomorphometric study. J. Bone Miner. Res. 2000, 15, 1085–1098. [Google Scholar] [CrossRef]
- Prakasam, G.; Yeh, J.K.; Chen, M.M.; Castro-Magana, M.; Liang, C.T.; Aloia, J.F. Effects of growth hormone and testosterone on cortical bone formation and bone density in aged orchidectomized rats. Bone 1999, 24, 491–497. [Google Scholar] [CrossRef]
- Iwamoto, J.; Yeh, J.K.; Takeda, T. Effect of vitamin K2 on cortical and cancellous bones in orchidectomized and/or sciatic neurectomized rats. J. Bone Miner. Res. 2003, 18, 776–783. [Google Scholar] [CrossRef]
- Saki, F.; Kasaee, S.R.; Sadeghian, F.; Talezadeh, P.; Omrani, G.H.R. The effect of testosterone itself and in combination with letrozole on bone mineral density in male rats. J. Bone Miner. Metab. 2019, 37, 668–675. [Google Scholar] [CrossRef]
- Ryu, S.J.; Ryu, D.S.; Kim, J.Y.; Park, J.Y.; Kim, K.H.; Chin, D.K.; Kim, K.S.; Cho, Y.E.; Kuh, S.U. Changes in Bone Metabolism in Young Castrated Male Rats. Yonsei Med. J. 2016, 57, 1386–1394. [Google Scholar] [CrossRef] [Green Version]
- Ryu, S.J.; Ryu, D.S.; Kim, J.Y.; Park, J.Y.; Kim, K.H.; Chin, D.K.; Kim, K.S.; Cho, Y.E.; Kuh, S.U. Bone mineral density changes after orchiectomy using a scrotal approach in rats. Korean J. Spine 2015, 12, 55–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Broulik, P.D.; Rosenkrancová, J.; Ruůzicka, P.; Sedlácek, R. Effect of alendronate administration on bone mineral density and bone strength in castrated rats. Horm. Metab. Res. 2005, 37, 414–418. [Google Scholar] [CrossRef] [PubMed]
- Wink, C.S.; Felts, W.J. Effects of castration on the bone structure of male rats: A model of osteoporosis. Calcif. Tissue Int. 1980, 32, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Papierska, L.; Rabijewski, M.; Kasperlik-Załuska, A.; Zgliczyński, W. Effect of DHEA supplementation on serum IGF-1, osteocalcin, and bone mineral density in postmenopausal, glucocorticoid-treated women. Adv. Med. Sci. 2012, 57, 51–57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, J.; Omi, N. DHEA administration has limited effect onintestinal Ca absorption in ovariectomized rats. J. Exerc. Nutr. Biochem. 2014, 18, 333–337. [Google Scholar] [CrossRef] [Green Version]
- Jankowski, C.M.; Gozansky, W.S.; Schwartz, R.S.; Dahl, D.J.; Kittelson, J.M.; Scott, S.M.; Van Pelt, R.E.; Kohrt, W.M. Effects of dehydroepiandrosterone replacement therapy on bone mineral density in older adults: A randomized, controlled trial. J. Clin. Endocrinol. Metab. 2006, 91, 2986–2993. [Google Scholar] [CrossRef] [Green Version]
- Hussein, F.; Hatem, B.M. The Possible Effect of DHEA on Hepatic and Metabolic Dysfunction in a Rat Model of Male Hypogonadism. Med. J. Cairo Univ. 2019, 87, 4769–4776. [Google Scholar] [CrossRef]
- Arlt, W.; Callies, F.; van Vlijmen, J.C.; Koehler, I.; Reincke, M.; Bidlingmaier, M.; Huebler, D.; Oettel, M.; Ernst, M.; Schulte, H.M.; et al. Dehydroepiandrosterone replacement in women with adrenal insufficiency. N. Engl. J. Med. 1999, 341, 1013–1020. [Google Scholar] [CrossRef]
- Villareal, D.T.; Holloszy, J.O.; Kohrt, W.M. Effects of DHEA replacement on bone mineral density and body composition in elderly women and men. Clin. Endocrinol. 2000, 53, 561–568. [Google Scholar] [CrossRef] [Green Version]
- Corona, G.; Rastrelli, G.; Giagulli, V.A.; Sila, A.; Sforza, A.; Forti, G.; Mannucci, E.; Maggi, M. Dehydroepiandrosterone supplementation in elderly men: A meta-analysis study of placebo-controlled trials. J. Clin. Endocrinol. Metab. 2013, 98, 3615–3626. [Google Scholar] [CrossRef] [Green Version]
- Hock, J.M.; Gera, I.; Fonseca, J.; Raisz, L.G. Human parathyroid hormone-(1-34) increases bone mass in ovariectomized and orchidectomized rats. Endocrinology 1988, 122, 2899–2904. [Google Scholar] [CrossRef] [PubMed]
- Tezval, M.; Serferaz, G.; Rack, T.; Kolios, L.; Sehmisch, S.; Schmelz, U.; Tezval, H.; Stuermer, K.M.; Stuermer, E.K. Effect of parathyroid hormone on hypogonadism induced bone loss of proximal femur of orchiectomized rat. World J. Urol. 2011, 29, 529–534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Said, M.A.A.; Abdel-Kareem, H.M.; Abdallah, H.A. Leptin exerts a bone protective effect in ovariectomized rats by inhibiting osteoclastogenesis. Bull. Egypt. Soc. Physiol. Sci. 2020, 40, 166–179. [Google Scholar] [CrossRef]
- He, X.F.; Zhang, L.; Zhang, C.H.; Zhao, C.R.; Li, H.; Zhang, L.F.; Tian, G.F.; Guo, M.F.; Dai, Z.; Sui, F.G. Berberine alleviates oxidative stress in rats with osteoporosis through receptor activator of NF-kB/receptor activator of NF-kB ligand/osteoprotegerin (RANK/RANKL/OPG) pathway. Bosn. J. Basic Med. Sci. 2017, 17, 295–301. [Google Scholar] [CrossRef] [Green Version]
- Li, C.W.; Liang, B.; Shi, X.L.; Wang, H. Opg/Rankl mRNA dynamic expression in the bone tissue of ovariectomized rats with osteoporosis. Genet. Mol. Res. 2015, 14, 9215–9224. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Wang, W. Effects of tanshinol on markers of bone turnover in ovariectomized rats and osteoblast cultures. PLoS ONE 2017, 12, e0181175. [Google Scholar] [CrossRef] [Green Version]
- Janckila, A.J.; Yam, L.T. Biology and clinical significance of tartrate-resistant acid phosphatases: New perspectives on an old enzyme. Calcif. Tissue Int. 2009, 85, 465–483. [Google Scholar] [CrossRef]
- Chao, T.Y.; Yu, J.C.; Ku, C.H.; Chen, M.M.; Lee, S.H.; Janckila, A.J.; Yam, L.T. Tartrate-resistant acid phosphatase 5b is a useful serum marker for extensive bone metastasis in breast cancer patients. Clin. Cancer Res. 2005, 11, 544–550. [Google Scholar]
- García-Pérez, M.A.; Moreno-Mercer, J.; Tarín, J.J.; Cano, A. Similar efficacy of low and standard doses of transdermal estradiol in controlling bone turnover in postmenopausal women. Gynecol. Endocrinol. 2006, 22, 179–184. [Google Scholar] [CrossRef]
- Hanson, D.A.; Weis, M.A.; Bollen, A.M.; Maslan, S.L.; Singer, F.R.; Eyre, D.R. A specific immunoassay for monitoring human bone resorption: Quantitation of type I collagen cross-linked N-telopeptides in urine. J. Bone Miner. Res. 1992, 7, 1251–1258. [Google Scholar] [CrossRef]
- DeLaurier, A.; Jackson, B.; Ingham, K.; Pfeiffer, D.; Horton, M.A.; Price, J.S. Biochemical markers of bone turnover in the domestic cat: Relationships with age and feline osteoclastic resorptive lesions. J. Nutr. 2002, 132 (Suppl. S2), 1742S–1744S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vanderschueren, D.; Jans, I.; Van Herck, E.; Moermans, K.; Verhaeghe, J.; Bouillon, R. Time-related increase of biochemical markers of bone turnover in androgen-deficient male rats. Bone Miner. 1994, 26, 123–131. [Google Scholar] [CrossRef]
- Kobayashi, T.; Koie, H.; Watanabe, A.; Ino, A.; Watabe, K.; Kim, M.; Kanayama, K.; Otsuji, K. Effects of food enriched with egg yolk hydrolysate (bone peptide) on bone metabolism in orchidectomized dogs. J. Vet. Med. Sci. 2015, 77, 503–506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhardwaj, P.; Rai, D.V.; Garg, M.L. Zinc as a nutritional approach to bone loss prevention in an ovariectomized rat model. Menopause 2013, 20, 1184–1193. [Google Scholar] [CrossRef] [PubMed]




| BMD | BMC | Serum OPG | Serum RANK | Serum DPD | Serum TRAP-5b | Serum NTX | Serum OC | Serum ALP | Serum Testost. | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Serum PTH | r | −0.1055 | 0.08628 | −0.04852 | −0.2230 | −0.4931 | −0.4002 | −0.09896 | 0.4616 | 0.6523 | 0.4950 |
| p | 0.6079 | 0.6752 | 0.8260 | 0.3063 | 0.0022 | 0.0803 | 0.5658 | 0.0078 | <0.0001 | 0.0139 | |
| Serum Testost. | r | 0.4503 | 0.6955 | 0.3570 | −0.5527 | 0.1078 | −0.6808 | 0.05743 | 0.6500 | 0.6155 | |
| p | 0.0272 | 0.0002 | 0.0945 | 0.0062 | 0.6160 | 0.0010 | 0.7898 | 0.0019 | 0.0014 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sakr, H.F.; Hussein, A.M.; Eid, E.A.; Boudaka, A.; Lashin, L.S. Impact of Dehydroepiandrosterone (DHEA) on Bone Mineral Density and Bone Mineral Content in a Rat Model of Male Hypogonadism. Vet. Sci. 2020, 7, 185. https://doi.org/10.3390/vetsci7040185
Sakr HF, Hussein AM, Eid EA, Boudaka A, Lashin LS. Impact of Dehydroepiandrosterone (DHEA) on Bone Mineral Density and Bone Mineral Content in a Rat Model of Male Hypogonadism. Veterinary Sciences. 2020; 7(4):185. https://doi.org/10.3390/vetsci7040185
Chicago/Turabian StyleSakr, Hussein F., Abdelaziz M. Hussein, Elsayed A. Eid, Ammar Boudaka, and Lashin S. Lashin. 2020. "Impact of Dehydroepiandrosterone (DHEA) on Bone Mineral Density and Bone Mineral Content in a Rat Model of Male Hypogonadism" Veterinary Sciences 7, no. 4: 185. https://doi.org/10.3390/vetsci7040185






