Food Safety Considerations Related to the Consumption and Handling of Game Meat in North America
Abstract
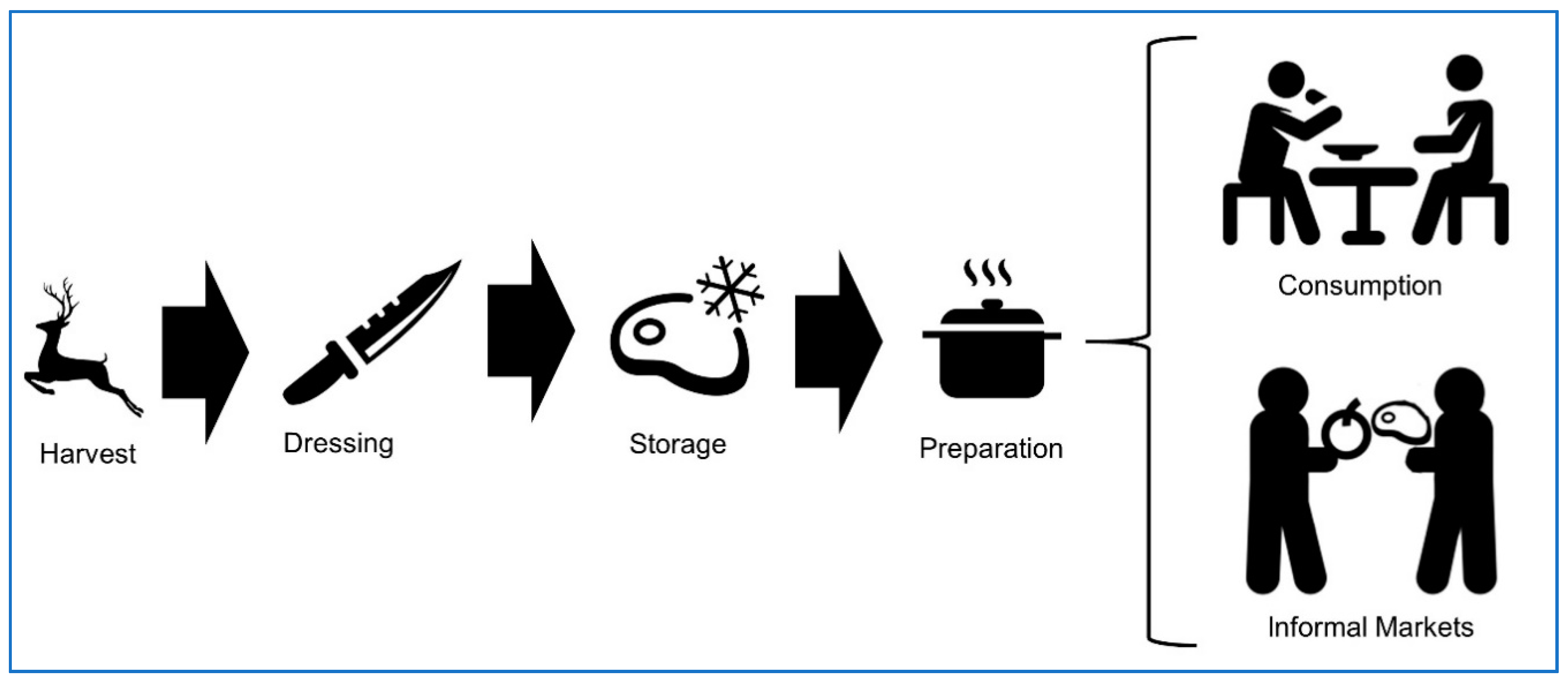
:1. Introduction
2. Infectious Disease Transmission Risk from Wildlife Baiting and Shared Environment
3. Game Meat Hygiene
4. Overview of Foodborne Diseases
4.1. Bacterial Pathogens
4.2. Parasites
4.3. Viruses
4.4. Lead Exposure
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jones, K.E.; Patel, N.G.; Levy, M.A.; Storeygard, A.; Balk, D.; Gittleman, J.L.; Daszak, P. Global trends in emerging infectious diseases. Nature 2008, 451, 990–993. [Google Scholar] [CrossRef]
- Watsa, M. Rigorous wildlife disease surveillance. Science 2020, 369, 145–147. [Google Scholar] [CrossRef]
- Su, C.; Stover, D.T.; Buss, B.F.; Carlson, A.V. LS Occupational animal exposure among persons with campylobacteriosis and cryptosporidiosis—Nebraska, 2005–2015. MMWR Morb. Mortal. Wkly. Rep. 2017, 66, 955–958. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, C.; de Perio, M.A.; Fagan, K.; Smith, M.L.; Salehi, E.; Levine, S.; Gruszynski, K.; Luckhaupt, S.E. Occupational distribution of campylobacteriosis and salmonellosis cases—Maryland, Ohio, and Virginia, 2014. MMWR. Morb. Mortal. Wkly. Rep. 2017, 66, 850–853. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Conrad, C.C.; Stanford, K.; Narvaez-Bravo, C.; Callaway, T.; McAllister, T. Farm fairs and petting zoos: A review of animal contact as a source of zoonotic enteric disease. Foodborne Pathog. Dis. 2017, 14, 59–73. [Google Scholar] [CrossRef] [PubMed]
- Varga, C.; Middleton, D.; Walton, R.; Savage, R.; Tighe, M.-K.; Allen, V.; Ahmed, R.; Rosella, L. Evaluating risk factors for endemic human Salmonella Enteritidis infections with different phage types in Ontario, Canada using multinomial logistic regression and a case-case study approach. BMC Public Health 2012, 12, 866. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, M.; Havelaar, A.H.; Hoffmann, S.; Hald, T.; Kirk, M.D.; Torgerson, P.R.; Devleesschauwer, B. Global disease burden of pathogens in animal source foods, 2010. PLoS ONE 2019, 14, e0216545. [Google Scholar] [CrossRef] [PubMed]
- Bennett, S.D.; Sodha, S.V.; Ayers, T.L.; Lynch, M.F.; Gould, L.H.; Tauxe, R.V. Produce-associated foodborne disease outbreaks, USA, 1998–2013. Epidemiol. Infect. 2018, 146, 1397–1406. [Google Scholar] [CrossRef] [Green Version]
- Zahedi, A.; Paparini, A.; Jian, F.; Robertson, I.; Ryan, U. Public health significance of zoonotic Cryptosporidium species in wildlife: Critical insights into better drinking water management. Int. J. Parasitol. Parasites Wildl. 2016, 5, 88–109. [Google Scholar] [CrossRef] [Green Version]
- Paulsen, P.; Bauer, A.; Vodnansky, M.; Winkelmayer, R.; Smulders, F.J.M.; Paulsen, P.; Bauer, A. Game Meat Hygiene in Focus Microbiology, Epidemiology, Risk Analysis and Quality Assurance; Wageningen Academic Publishers: Wageningen, The Netherlands, 2011; ISBN 978-90-8686-165-1. [Google Scholar]
- Hoffman, L.C.; Wiklund, E. Game and venison—meat for the modern consumer. Meat Sci. 2006, 74, 197–208. [Google Scholar] [CrossRef]
- Valencak, T.G.; Gamsjäger, L.; Ohrnberger, S.; Culbert, N.J.; Ruf, T. Healthy n-6/n-3 fatty acid composition from five European game meat species remains after cooking. BMC Res. Notes 2015, 8, 273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lillehaug, A.; Bergsjø, B.; Schau, J.; Bruheim, T.; Vikøren, T.; Handeland, K. Campylobacter spp., Salmonella spp., verocytotoxic Escherichia coli, and antibiotic resistance in indicator organisms in wild cervids. Acta Vet. Scand. 2005, 46, 23. [Google Scholar] [CrossRef] [PubMed]
- Vieira-Pinto, M.; Morais, L.; Caleja, C.; Themudo, P.; Torres, C.; Igrejas, G.; Poeta, P.; Martins, C. Salmonella sp. in game (Sus scrofa and Oryctolagus cuniculus). Foodborne Pathog. Dis. 2011, 8, 739–740. [Google Scholar] [CrossRef] [PubMed]
- Hilbert, F.; Smulders, F.J.M.; Chopra-Dewasthaly, R.; Paulsen, P. Salmonella in the wildlife-human interface. Food Res. Int. 2012, 45, 603–608. [Google Scholar] [CrossRef]
- Miko, A.; Pries, K.; Haby, S.; Steege, K.; Albrecht, N.; Krause, G.; Beutin, L. Assessment of Shiga toxin-producing Escherichia coli isolates from wildlife meat as potential pathogens for humans. Appl. Environ. Microbiol. 2009, 75, 6462–6470. [Google Scholar] [CrossRef] [Green Version]
- Keene, W.E. An outbreak of Escherichia coli 0157:H7 infections traced to jerky made from deer meat. JAMA J. Am. Med. Assoc. 1997, 277, 1229. [Google Scholar] [CrossRef]
- Hove, T.; Mukaratirwa, S. Seroprevalence of Toxoplasma gondii in farm-reared ostriches and wild game species from Zimbabwe. Acta Trop. 2005, 94, 49–53. [Google Scholar] [CrossRef]
- Malmsten, J.; Jakubek, E.-B.; Björkman, C. Prevalence of antibodies against Toxoplasma gondii and Neospora caninum in moose (Alces alces) and roe deer (Capreolus capreolus) in Sweden. Vet. Parasitol. 2011, 177, 275–280. [Google Scholar] [CrossRef]
- Gaulin, C.; Ramsay, D.; Thivierge, K.; Tataryn, J.; Courville, A.; Martin, C.; Cunningham, P.; Désilets, J.; Morin, D.; Dion, R. Acute toxoplasmosis among Canadian deer hunters associated with consumption of undercooked deer meat hunted in the United States. Emerg. Infect. Dis. 2020, 26, 199–205. [Google Scholar] [CrossRef] [Green Version]
- Murrell, K.D. The dynamics of Trichinella spiralis epidemiology: Out to pasture? Vet. Parasitol. 2016, 231, 92–96. [Google Scholar] [CrossRef]
- Holzbauer, S.M.; Agger, W.A.; Hall, R.L.; Johnson, G.M.; Schmitt, D.; Garvey, A.; Bishop, H.S.; Rivera, H.; de Almeida, M.E.; Hill, D.; et al. Outbreak of Trichinella spiralis infections associated with a wild boar hunted at a game farm in Iowa. Clin. Infect. Dis. 2014, 59, 1750–1756. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Warenik-Bany, M.; Maszewski, S.; Mikolajczyk, S.; Piskorska-Pliszczynska, J. Impact of environmental pollution on PCDD/F and PCB bioaccumulation in game animals. Environ. Pollut. 2019, 255. [Google Scholar] [CrossRef] [PubMed]
- American Veterinary Medical Association (AVMA). Disease Precautions for Hunters. Available online: https://www.avma.org/resources/public-health/disease-precautions-hunters (accessed on 3 November 2020).
- United States Department of Agriculture (USDA). Roasting Those “Other” Holiday Meats. Available online: https://www.fsis.usda.gov/wps/portal/fsis/topics/food-safety-education/get-answers/food-safety-fact-sheets/seasonal-food-safety/roasting-those-other-holiday-meats/ct_index (accessed on 3 November 2020).
- United States Department of Agriculture (USDA). Harvesting Wild Game. Available online: https://www.fsis.usda.gov/wps/wcm/connect/fsis-content/internet/main/newsroom/meetings/newsletters/small-plant-news/small-plant-news-archive/volume-5/spn-vol5-no4 (accessed on 3 November 2020).
- Schantz, P.M.; Moorhead, A.; Grunenwald, P.E.; Dietz, V.J. Trichinellosis in the United States, 1991–1996: Declining but not gone. Am. J. Trop. Med. Hyg. 1999, 60, 66–69. [Google Scholar] [CrossRef] [Green Version]
- U.S. Department of the Interior; U.S. Fish and Wildlife Service; U.S. Department of Commerce; U.S. Census Bureau. 2011 National Survey of Fishing, Hunting, and Wildlife-Associated Recreation. Available online: https://www.census.gov/content/dam/Census/library/publications/2014/demo/fhw11-nat.pdf (accessed on 3 November 2020).
- Gamborg, C.; Sandøe, P.; Palmer, C. Ethical management of wildlife. Lethal versus nonlethal control of white-tailed deer. Conserv. Sci. Pract. 2020, 2. [Google Scholar] [CrossRef] [Green Version]
- Blossey, B.; Curtis, P.; Boulanger, J.; Dávalos, A. Red oak seedlings as indicators of deer browse pressure: Gauging the outcome of different white-tailed deer management approaches. Ecol. Evol. 2019, 9, 13085–13103. [Google Scholar] [CrossRef] [PubMed]
- Mateus-Pinilla, N.; Weng, H.-Y.; Ruiz, M.O.; Shelton, P.; Novakofski, J. Evaluation of a wild white-tailed deer population management program for controlling chronic wasting disease in Illinois, 2003–2008. Prev. Vet. Med. 2013, 110, 541–548. [Google Scholar] [CrossRef] [Green Version]
- Manjerovic, M.B.; Green, M.L.; Mateus-Pinilla, N.; Novakofski, J. The importance of localized culling in stabilizing chronic wasting disease prevalence in white-tailed deer populations. Prev. Vet. Med. 2014, 113, 139–145. [Google Scholar] [CrossRef] [Green Version]
- VerCauteren, K.C.; Lavelle, M.J.; Campa, H. Persistent spillback of bovine tuberculosis from white-tailed deer to cattle in Michigan, USA: Status, strategies, and needs. Front. Vet. Sci. 2018, 5. [Google Scholar] [CrossRef]
- Cunningham, A.A.; Daszak, P.; Wood, J.L.N. One Health, emerging infectious diseases and wildlife: Two decades of progress? Philos. Trans. R. Soc. B 2017. [Google Scholar] [CrossRef] [Green Version]
- Jenkins, E.J.; Simon, A.; Bachand, N.; Stephen, C. Wildlife parasites in a One Health world. Trends Parasitol. 2015, 31, 174–180. [Google Scholar] [CrossRef]
- Godfroid, J. Brucellosis in livestock and wildlife: Zoonotic diseases without pandemic potential in need of innovative one health approaches. Arch. Public Health 2017, 75, 34. [Google Scholar] [CrossRef] [PubMed]
- Sorensen, A.; van Beest, F.M.; Brook, R.K. Impacts of wildlife baiting and supplemental feeding on infectious disease transmission risk: A synthesis of knowledge. Prev. Vet. Med. 2014, 113, 356–363. [Google Scholar] [CrossRef] [PubMed]
- Weidman, T.; Litvaitis, J.A. Can supplemental food increase winter survival of a threatened cottontail rabbit? Biol. Conserv. 2011, 144, 2054–2058. [Google Scholar] [CrossRef]
- Mathisen, K.M.; Milner, J.M.; van Beest, F.M.; Skarpe, C. Long-term effects of supplementary feeding of moose on browsing impact at a landscape scale. For. Ecol. Manag. 2014, 314, 104–111. [Google Scholar] [CrossRef] [Green Version]
- Andreassen, H.P.; Gundersen, H.; Storaas, T. The effect of scent-marking, forest clearing, and supplemental feeding on moose-train collisions. J. Wildl. Manag. 2005, 69, 1125–1132. [Google Scholar] [CrossRef]
- Sahlsten, J.; Bunnefeld, N.; Månsson, J.; Ericsson, G.; Bergström, R.; Dettki, H. Can supplementary feeding be used to redistribute moose Alces alces? Wildl. Biol. 2010, 16, 85–92. [Google Scholar] [CrossRef] [Green Version]
- Geisser, H.; Reyner, H.U. Efficacy of hunting, feeding, and fencing to reduce crop damage by wild boars. J. Wildl. Dis. 2004, 68, 939–946. [Google Scholar] [CrossRef]
- Steyaert, S.M.J.G.; Kindberg, J.; Jerina, K.; Krofel, M.; Stergar, M.; Swenson, J.E.; Zedrosser, A. Behavioral correlates of supplementary feeding of wildlife: Can general conclusions be drawn? Basic Appl. Ecol. 2014, 15, 669–676. [Google Scholar] [CrossRef]
- Mysterud, A.; Viljugrein, H.; Solberg, E.J.; Rolandsen, C.M. Legal regulation of supplementary cervid feeding facing chronic wasting disease. J. Wildl. Manag. 2019, 83, 1667–1675. [Google Scholar] [CrossRef]
- Thompson, A.K.; Samuel, M.D.; Van Deelen, T.R. Alternative feeding strategies and potential disease transmission in Wisconsin white-tailed deer. J. Wildl. Manag. 2008, 72, 416–421. [Google Scholar] [CrossRef]
- Zanella, G.; Duvauchelle, A.; Hars, J.; Moutou, F.; Boschiroli, M.L.; Durand, B. Patterns of lesions of bovine tuberculosis in wild red deer and wild boar. Vet. Rec. 2008, 163, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Putman, R.J.; Staines, B.W. Supplementary winter feeding of wild red deer Cervus elaphus in Europe and North America: Justifications, feeding practice and effectiveness. Mamm. Rev. 2004, 34, 285–306. [Google Scholar] [CrossRef]
- Robb, G.N.; McDonald, R.A.; Chamberlain, D.E.; Bearhop, S. Food for thought: Supplementary feeding as a driver of ecological change in avian populations. Front. Ecol. Environ. 2008, 6, 476–484. [Google Scholar] [CrossRef]
- Murray, M.H.; Becker, D.J.; Hall, R.J.; Hernandez, S.M. Wildlife health and supplemental feeding: A review and management recommendations. Biol. Conserv. 2016, 204, 163–174. [Google Scholar] [CrossRef]
- Lavelle, M.J.; Phillips, G.E.; Fischer, J.W.; Burke, P.W.; Seward, N.W.; Stahl, R.S.; Nichols, T.A.; Wunder, B.A.; VerCauteren, K.C. Mineral licks: Motivational factors for visitation and accompanying disease risk at communal use sites of elk and deer. Environ. Geochem. Health 2014, 36, 1049–1061. [Google Scholar] [CrossRef] [Green Version]
- Craft, M.E. Infectious disease transmission and contact networks in wildlife and livestock. Philos. Trans. R. Soc. B Biol. Sci. 2015, 370, 20140107. [Google Scholar] [CrossRef]
- Daniels, M.J.; Hutchings, M.R.; Greig, A. The risk of disease transmission to livestock posed by contamination of farm stored feed by wildlife excreta. Epidemiol. Infect. 2003, 130, 561–568. [Google Scholar] [CrossRef]
- Hedman, H.D.; Zhang, L.; Trueba, G.; Vinueza Rivera, D.L.; Zurita Herrera, R.A.; Villacis Barrazueta, J.J.; Gavilanes Rodriguez, G.I.; Butt, B.; Foufopoulos, J.; Berrocal, V.J.; et al. Spatial exposure of agricultural antimicrobial resistance in relation to free-ranging domestic chicken movement patterns among agricultural communities in Ecuador. Am. J. Trop. Med. Hyg. 2020. [Google Scholar] [CrossRef]
- Brochu, N.M.; Guerin, M.T.; Varga, C.; Lillie, B.N.; Brash, M.L.; Susta, L. A two-year prospective study of small poultry flocks in Ontario, Canada, part 1: Prevalence of viral and bacterial pathogens. J. Vet. Diagn. Investig. 2019, 31, 327–335. [Google Scholar] [CrossRef] [Green Version]
- Thomas, L.F.; de Glanville, W.A.; Cook, E.A.; Fevre, E.M. The spatial ecology of free-ranging domestic pigs (Sus scrofa) in western Kenya. BMC Vet. Res. 2013, 9, 46. [Google Scholar] [CrossRef] [Green Version]
- Lund, B.M.; O’Brien, S.J. The occurrence and prevention of foodborne disease in vulnerable people. Foodborne Pathog. Dis. 2011, 8, 961–973. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giuggioli, G.; Olivastri, A.; Pennisi, L.; Paludi, D.; Ianieri, A.; Vergara, A. The hygiene-sanitary control in the wild game meats. Ital. J. Food Saf. 2018, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mesinger, D.; Ocieczek, A. Consumer education as an important condition for increasing wild animal meat consumption in the context of promoting the idea of sustainable development in Poland. Pol. J. Environ. Stud. 2020, 29, 3485–3492. [Google Scholar] [CrossRef]
- European Parliament and of the Council Regulation (EC) No 178/2002 of the European Parliament and of the Council of 28 January 2002 Laying Down the General Principles and Requirements of Food Law, Establishing the European Food Safety Authority and Laying Down Procedures in Matters of Food Safty. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=celex%3A32002R0178 (accessed on 3 November 2020).
- Illinois Department of Natural Resources (IDNR). Summary of 2013–2014 Illinois Deer Seasons. Available online: https://www2.illinois.gov/dnr/hunting/deer/Documents/2019-2020 Illinois Deer Harvest Report.pdf (accessed on 3 November 2020).
- Hunt, W.G.; Watson, R.T.; Oaks, J.L.; Parish, C.N.; Burnham, K.K.; Tucker, R.L.; Belthoff, J.R.; Hart, G. Lead bullet fragments in venison from rifle-killed deer: Potential for human dietary exposure. PLoS ONE 2009, 4, e5330. [Google Scholar] [CrossRef] [Green Version]
- Buenz, E.J.; Parry, G.J. Chronic lead intoxication from eating wild-harvested game. Am. J. Med. 2018, 131, e181–e184. [Google Scholar] [CrossRef]
- Prüss-Ustün, A.; Bartram, J.; Clasen, T.; Colford, J.M.; Cumming, O.; Curtis, V.; Bonjour, S.; Dangour, A.D.; De France, J.; Fewtrell, L.; et al. Burden of disease from inadequate water, sanitation and hygiene in low- and middle-income settings: A retrospective analysis of data from 145 countries. Trop. Med. Int. Health 2014, 19, 894–905. [Google Scholar] [CrossRef] [Green Version]
- Arnold, B.F.; Null, C.; Luby, S.P.; Unicomb, L.; Stewart, C.P.; Dewey, K.G.; Ahmed, T.; Ashraf, S.; Christensen, G.; Clasen, T.; et al. Cluster-randomised controlled trials of individual and combined water, sanitation, hygiene and nutritional interventions in rural Bangladesh and Kenya: The WASH Benefits study design and rationale. BMJ Open 2013, 3. [Google Scholar] [CrossRef] [Green Version]
- Gill, C.O. Microbiological conditions of meats from large game animals and birds. Meat Sci. 2007, 77, 149–160. [Google Scholar] [CrossRef]
- Illinois Department of Agriculture (IDOA). Handling Carcasses and Venison. Available online: https://www2.illinois.gov/sites/agr/Animals/AnimalHealth/AnimalDiseases/Pages/CWD-Processing.aspx (accessed on 3 November 2020).
- Paulsen, P.; Smulders, F.J.M.; Hilbert, F. Salmonella in meat from hunted game: A central European perspective. Food Res. Int. 2012, 45, 609–616. [Google Scholar] [CrossRef]
- Carrasco-Garcia, R.; Barroso, P.; Perez-Olivares, J.; Montoro, V.; Vicente, J. Consumption of big game remains by scavengers: A potential risk as regards disease transmission in central Spain. Front. Vet. Sci. 2018, 5. [Google Scholar] [CrossRef] [Green Version]
- United States Department of Agriculture (USDA). FSIS Compliance Guide: Modernization of Poultry Slaughter. Available online: https://www.fsis.usda.gov/wps/wcm/connect/7a0a728e-3b29-49e9-9c1b-ec55f2f04887/Chilling-Requirements-1014.pdf?MOD=AJPERES (accessed on 3 November 2020).
- United States Department of Agriculture (USDA). Keep Food Safe! Food Safety Basics. Available online: https://www.fsis.usda.gov/wps/portal/fsis/topics/food-safety-education/get-answers/food-safety-fact-sheets/safe-food-handling/keep-food-safe-food-safety-basics/CT_Index/!ut/p/a1/jZHRToMwFIafhsvSInNh3hESM9CBy9R13CwFDqURWkKrqE9vtyXGmU3XXvWc78tp_-IcU5xL9iY4M (accessed on 3 November 2020).
- University of Minnesota Extension Cooking Venison for Flavor and Safety. Available online: https://extension.umn.edu/preserving-and-preparing/cooking-venison-flavor-and-safety (accessed on 3 November 2020).
- Clemson Cooperative Extension Safe handling of Wild Game Meats. Factsheet.HGIC 3516. Available online: https://hgic.clemson.edu/factsheet/safe-handling-of-wild-game-meats (accessed on 3 November 2020).
- United States Department of Agriculture (USDA). Foodborne Illness Peaks in Summer—What Can You Do to Prevent It? Available online: https://www.fsis.usda.gov/wps/portal/fsis/topics/food-safety-education/get-answers/food-safety-fact-sheets/foodborne-illness-and-disease/foodborne-illness-peaks-in-summer/ct_index#:~:text=Cook meat and poultry completely,°F %2F74 °C (accessed on 3 November 2020).
- Bryan, F.L. Factors that contribute to outbreaks of foodborne disease. J. Food Prot. 1978, 41, 816–827. [Google Scholar] [CrossRef] [PubMed]
- Goguen, A.D.; Riley, S.J. Consumption of wild-harvested meat in society. Wildl. Soc. Bull. 2020, 44, 553–563. [Google Scholar] [CrossRef]
- Goguen, A.D.; Riley, S.J.; Organ, J.F.; Rudolph, B.A. Wild-harvested venison yields and sharing by Michigan deer hunters. Hum. Dimens. Wildl. 2018, 23, 197–212. [Google Scholar] [CrossRef]
- Ljung, P.E.; Riley, S.J.; Heberlein, T.A.; Ericsson, G. Eat prey and love: Game-meat consumption and attitudes toward hunting. Wildl. Soc. Bull. 2012, 36, 669–675. [Google Scholar] [CrossRef]
- Illinois Department of Natural Resources (IDNR). Chronic Wasting Disease Management. Available online: https://www2.illinois.gov/dnr/programs/CWD/Pages/default.aspx (accessed on 3 November 2020).
- Dorn, C.R. Review of foodborne outbreak of Escherichia coli O157:H7 infection in the western United States. J. Am. Vet. Med. Assoc. 1993, 203, 1583–1587. [Google Scholar]
- Bell, B.P. A Multistate outbreak of Escherichia coli O157:H7—Associated bloody diarrhea and hemolytic uremic syndrome from hamburgers. JAMA J. Am. Med. Assoc. 1994, 272, 1349–1353. [Google Scholar] [CrossRef]
- Meng, X.J.; Lindsay, D.S.; Sriranganathan, N. Wild boars as sources for infectious diseases in livestock and humans. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 2697–2707. [Google Scholar] [CrossRef] [Green Version]
- Chlebicz, A.; Śliżewska, K. Campylobacteriosis, salmonellosis, yersiniosis, and listeriosis as zoonotic foodborne diseases: A review. Int. J. Environ. Res. Public Health 2018, 15, 863. [Google Scholar] [CrossRef] [Green Version]
- Membré, J.-M.; Laroche, M.; Magras, C. Assessment of levels of bacterial contamination of large wild game meat in Europe. Food Microbiol. 2011, 28, 1072–1079. [Google Scholar] [CrossRef]
- Rabatsky-Ehr, T.; Dingman, D.; Marcus, R.; Howard, R.; Kinney, A.; Mshar, P. Deer meat as the source for a sporadic case of Escherichia coli O157:H7 infection, Connecticut. Emerg. Infect. Dis. 2002, 8, 525–527. [Google Scholar] [CrossRef]
- Laidler, M.R.; Tourdjman, M.; Buser, G.L.; Hostetler, T.; Repp, K.K.; Leman, R.; Samadpour, M.; Keene, W.E. Escherichia coli O157:H7 infections associated with consumption of locally grown strawberries contaminated by deer. Clin. Infect. Dis. 2013, 57, 1129–1134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Madar, C.S.; Cardile, A.P.; Cunningham, S.; Magpantay, G.; Finger, D. A case of Salmonella gastroenteritis following ingestion of raw venison sashimi. Hawaii J. Med. Public Health 2012, 71, 49–50. [Google Scholar] [PubMed]
- Atanassova, V.; Apelt, J.; Reich, F.; Klein, G. Microbiological quality of freshly shot game in Germany. Meat Sci. 2008, 78, 414–419. [Google Scholar] [CrossRef] [PubMed]
- Avagnina, A.; Nucera, D.; Grassi, M.A.; Ferroglio, E.; Dalmasso, A.; Civera, T. The microbiological conditions of carcasses from large game animals in Italy. Meat Sci. 2012, 91, 266–271. [Google Scholar] [CrossRef]
- Obwegeser, T.; Stephan, R.; Hofer, E.; Zweifel, C. Shedding of foodborne pathogens and microbial carcass contamination of hunted wild ruminants. Vet. Microbiol. 2012, 159, 149–154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, S.; Sun, M.; Fitzgerald, E.; Hwang, S.-A. Did summer weather factors affect gastrointestinal infection hospitalizations in New York State? Sci. Total Environ. 2016, 550, 38–44. [Google Scholar] [CrossRef]
- Wallace, D.J.; Van Gilder, T.; Shallow, S.; Fiorentino, T.; Segler, S.D.; Smith, K.E.; Shiferaw, B.; Etzel, R.; Garthright, W.E.; Angulo, F.J. Incidence of foodborne illnesses reported by the Foodborne Diseases Active Surveillance Network (FoodNet)—1997. J. Food Prot. 2000, 63, 807–809. [Google Scholar] [CrossRef]
- Varga, C.; John, P.; Cooke, M.; Majowicz, S.E. Spatial and space-time clustering and demographic characteristics of human nontyphoidal Salmonella infections with major serotypes in Toronto, Canada. PLoS ONE 2020, 15, e0235291. [Google Scholar] [CrossRef]
- Paulsen, P.; Winkelmayer, R. Seasonal variation in the microbial contamination of game carcasses in an Austrian hunting area. Eur. J. Wildl. Res. 2004. [Google Scholar] [CrossRef]
- Sauvala, M.; Laaksonen, S.; Laukkanen-Ninios, R.; Jalava, K.; Stephan, R.; Fredriksson-Ahomaa, M. Microbial contamination of moose (Alces alces) and white-tailed deer (Odocoileus virginianus) carcasses harvested by hunters. Food Microbiol. 2019, 78, 82–88. [Google Scholar] [CrossRef]
- Sunstrum, J.; Shoyinka, A.; Power, L.E.; Maxwell, D.; Stobierski, M.G.; Signs, K.; Sidge, J.L.; O’Brien, D.J.; Robbe-Austerman, S.; Davidson, P. Notes from the Field: Zoonotic Mycobacterium bovis Disease in Deer Hunters—Michigan, 2002–2017. MMWR Morb. Mortal. Wkly. Rep. 2019, 68, 807–808. [Google Scholar] [CrossRef] [PubMed]
- Wilkins, M.J.; Meyerson, J.; Bartlett, P.C.; Spieldenner, S.L.; Berry, D.E.; Mosher, L.B.; Kaneene, J.B.; Robinson-Dunn, B.; Stobierski, M.G.; Boulton, M.L. Human Mycobacterium bovis infection and bovine tuberculosis outbreak, Michigan, 1994–2007. Emerg. Infect. Dis. 2008, 14, 657–660. [Google Scholar] [CrossRef] [PubMed]
- Brown, V.R.; Bowen, R.A.; Bosco-Lauth, A.M. Zoonotic pathogens from feral swine that pose a significant threat to public health. Transbound. Emerg. Dis. 2018, 65, 649–659. [Google Scholar] [CrossRef]
- Olsen, S.C. Brucellosis in the United States: Role and significance of wildlife reservoirs. Vaccine 2010, 28 (Suppl. 5), 73–76. [Google Scholar] [CrossRef] [PubMed]
- Dorny, P.; Praet, N.; Deckers, N.; Gabriel, S. Emerging food-borne parasites. Vet. Parasitol. 2009, 163, 196–206. [Google Scholar] [CrossRef] [PubMed]
- Appelbee, A.J.; Thompson, R.C.A.; Olson, M.E. Giardia and Cryptosporidium in mammalian wildlife—Current status and future needs. Trends Parasitol. 2005, 21, 370–376. [Google Scholar] [CrossRef]
- Sandfoss, M.; DePerno, C.; Patton, S.; Flowers, J.; Kennedy-Stoskopf, S. Prevalence of antibody to toxoplasma gondii and trichinella spp. In feral pigs (Sus scrofa) of eastern North Carolina. J. Wildl. Dis. 2011, 47, 338–343. [Google Scholar] [CrossRef] [Green Version]
- Fredebaugh, S.L.; Mateus-Pinilla, N.E.; McAllister, M.; Warner, R.E.; Weng, H.-Y. Prevalence of antibody to toxoplasma gondii in terrestrial wildlife in a natural area. J. Wildl. Dis. 2011, 47, 381–392. [Google Scholar] [CrossRef] [Green Version]
- Diaz, J.H.; Warren, R.J.; Oster, M.J. The disease ecology, epidemiology, clinical manifestations, and management of trichinellosis linked to consumption of wild animal meat. Wilderness Environ. Med. 2020, 31, 235–244. [Google Scholar] [CrossRef] [Green Version]
- Springer, Y.P.; Casillas, S.; Helfrich, K.; Mocan, D.; Smith, M.; Arriaga, G.; Mixson, L.; Castrodale, L.; McLaughlin, J. Two outbreaks of trichinellosis linked to consumption of walrus meat—Alaska, 2016–2017. MMWR Morb. Mortal. Wkly. Rep. 2017, 7, 692–696. [Google Scholar] [CrossRef] [Green Version]
- Dubey, J.P.; Brown, J.; Verma, S.K.; Cerqueira-Cézar, C.K.; Banfield, J.; Kwok, O.C.H.; Ying, Y.; Murata, F.H.A.; Pradhan, A.K.; Su, C. Isolation of viable Toxoplasma gondii, molecular characterization, and seroprevalence in elk (Cervus canadensis) in Pennsylvania, USA. Vet. Parasitol. 2017, 243, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Kamerkar, S.; Davis, P.H. Toxoplasma on the brain: Understanding host-pathogen interactions in chronic CNS infection. J. Parasitol. Res. 2012, 2012, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mosites, E.; Miernyk, K.; Priest, J.W.; Bruden, D.; Hurlburt, D.; Parkinson, A.; Klejka, J.; Hennessy, T.; Bruce, M.G. Giardia and Cryptosporidium antibody prevalence and correlates of exposure among Alaska residents, 2007–2008. Epidemiol. Infect. 2018, 146, 888–894. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shapiro, K.; Bahia-Oliveira, L.; Dixon, B.; Dumètre, A.; de Wit, L.A.; VanWormer, E.; Villena, I. Environmental transmission of Toxoplasma gondii: Oocysts in water, soil and food. Food Waterborne Parasitol. 2019, 15. [Google Scholar] [CrossRef]
- Schellenberg, R.S.; Tan, B.J.K.; Irvine, J.D.; Stockdale, D.R.; Gajadhar, A.A.; Serhir, B.; Botha, J.; Armstrong, C.A.; Woods, S.A.; Blondeau, J.M.; et al. An outbreak of trichinellosis due to consumption of bear meat infected with Trichinella nativa, in 2 northern Saskatchewan communities. J. Infect. Dis. 2003, 188, 835–843. [Google Scholar] [CrossRef] [Green Version]
- Hall, R.L.; Lindsay, A.; Hammond, C.; Montgomery, S.P.; Wilkins, P.P.; da Silva, A.J.; McAuliffe, I.; de Almeida, M.; Bishop, H.; Mathison, B.; et al. Outbreak of human trichinellosis in Northern California caused by Trichinella murrelli. Am. J. Trop Med. Hyg. 2012, 87, 297–302. [Google Scholar] [CrossRef]
- Centers for Disease Control (CDC). Rabies. Available online: https://www.cdc.gov/rabies/location/usa/surveillance/wild_animals.html (accessed on 3 November 2020).
- Velasco-Villa, A.; Reeder, S.A.; Orciari, L.A.; Yager, P.A.; Franka, R.; Blanton, J.D.; Zuckero, L.; Hunt, P.; Oertli, E.H.; Robinson, L.E.; et al. Enzootic rabies elimination from dogs and reemergence in wild terrestrial carnivores, United States. Emerg. Infect. Dis. 2008, 14, 1849–1854. [Google Scholar] [CrossRef]
- Wandeler, A.I.; Matter, H.C.; Kappeler, A.; Budde, A. The ecology of dogs and canine rabies: A selective review. Rev. Sci. Tech. 1993, 12, 51–71. [Google Scholar] [CrossRef]
- Yazaki, Y.; Mizuo, H.; Takahashi, M.; Nishizawa, T.; Sasaki, N.; Gotanda, Y.; Okamoto, H. Sporadic acute or fulminant hepatitis E in Hokkaido, Japan, may be food-borne, as suggested by the presence of hepatitis E virus in pig liver as food. J. Gen. Virol. 2003, 84, 2351–2357. [Google Scholar] [CrossRef]
- Matsuda, H.; Okada, K.; Takahashi, K.; Mishiro, S. Severe hepatitis E virus infection after ingestion of uncooked liver from a wild boar. J. Infect. Dis. 2003, 188, 944. [Google Scholar] [CrossRef] [Green Version]
- Tei, S.; Kitajima, N.; Takahashi, K.; Mishiro, S. Zoonotic transmission of hepatitis E virus from deer to human beings. Lancet 2003, 362, 371–373. [Google Scholar] [CrossRef]
- Roess, A.A.; McCollum, A.M.; Gruszynski, K.; Zhao, H.; Davidson, W.; Lafon, N.; Engelmeyer, T.; Moyer, B.; Godfrey, C.; Kilpatrick, H.; et al. Surveillance of parapoxvirus among ruminants in Virginia and Connecticut. Zoonoses Public Health 2013, 60, 543–548. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.J.; Skelton, H.G.; James, W.D.; Lupton, G.P. Parapoxvirus infections acquired after exposure to wildlife. Arch. Dermatol. 1991, 127, 79–82. [Google Scholar] [CrossRef] [PubMed]
- Huerter, C.J.; Hashish, H. A case of human orf contracted from a deer. Cutis 2003, 71, 288–290. [Google Scholar]
- Pain, D.J.; Mateo, R.; Green, R.E. Effects of lead from ammunition on birds and other wildlife: A review and update. AMBIO 2019, 48, 935–953. [Google Scholar] [CrossRef] [Green Version]
- Bannon, D.I.; Drexler, J.W.; Fent, G.M.; Casteel, S.W.; Hunter, P.J.; Brattin, W.J.; Major, M.A. Evaluation of small arms range soils for metal contamination and lead bioavailability. Environ. Sci. Technol. 2009, 43, 9071–9076. [Google Scholar] [CrossRef]
- Mateo, R.; Kanstrup, N. Regulations on lead ammunition adopted in Europe and evidence of compliance. Ambio 2019, 48, 989–998. [Google Scholar] [CrossRef]
- Arnemo, J.M.; Andersen, O.; Stokke, S.; Thomas, V.G.; Krone, O.; Pain, D.J.; Mateo, R. Health and environmental risks from lead-based ammunition: Science versus socio-politics. Ecohealth 2016, 13, 618–622. [Google Scholar] [CrossRef] [Green Version]
- Treble, R.G.; Thompson, T.S. Elevated blood lead levels resulting from the ingestion of air rifle pellets. J. Anal. Toxicol. 2002, 26, 370–373. [Google Scholar] [CrossRef]
- Haldimann, M.; Baumgartner, A.; Zimmerli, B. Intake of lead from game meat—A risk to consumers’ health? Eur. Food Res. Technol. 2002, 215, 375–379. [Google Scholar] [CrossRef]
- Iqbal, S.; Blumenthal, W.; Kennedy, C.; Yip, F.Y.; Pickard, S.; Flanders, W.D.; Loringer, K.; Kruger, K.; Caldwell, K.L.; Jean Brown, M. Hunting with lead: Association between blood lead levels and wild game consumption. Environ. Res. 2009, 109, 952–959. [Google Scholar] [CrossRef] [PubMed]
- Buenz, E.J. Lead exposure through eating wild game. Am. J. Med. 2016, 129, 457–458. [Google Scholar] [CrossRef] [Green Version]
- Hunt, W.G.; Burnham, W.; Parish, C.N.; Burnham, K.K.; Mutch, B.R.; Oaks, J.L. Bullet fragments in deer remains: Implications for lead exposure in avian scavengers. Wildl. Soc. Bull. 2006, 34, 167–170. [Google Scholar] [CrossRef]
- Fisher, I.J.; Pain, D.J.; Thomas, V.G. A review of lead poisoning from ammunition sources in terrestrial birds. Biol. Conserv. 2006, 131, 421–432. [Google Scholar] [CrossRef]
| Geographic Region | Pathogen | Species | Transmission Pathway | Reference |
|---|---|---|---|---|
| Oregon, USA | Escherichia coli 0157:H7 | Black-tailed deer (Odocoileus hemionus) | Communal consumption of homemade venison jerky | [17] |
| Connecticut, USA | Escherichia coli 0157:H7 | White-tailed deer (Odocoileus virginianus) | Consumption of undercooked meat | [84] |
| Oregon, USA | Escherichia coli 0157:H7 | Black-tailed deer (Odocoileus hemionus) | Consumption of strawberries contaminated by deer feces | [85] |
| Hawaiian island of Lana’I, USA | Salmonella Birkenhead | Axis deer (Axis axis) | Consumption of undercooked meat | [86] |
| Illinois, USA | Toxoplasma gondii | White-tailed deer (Odocoileus virginianus) | Consumption of undercooked meat | [20] |
| Saskatchewan, CAN | Trichinella nativa | Black bear (Ursus americanus) | Consumption of undercooked meat | [109] |
| California, USA | Trichinella murrelli | Black bear (Ursus americanus) | Consumption of undercooked meat | [110] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hedman, H.D.; Varga, C.; Duquette, J.; Novakofski, J.; Mateus-Pinilla, N.E. Food Safety Considerations Related to the Consumption and Handling of Game Meat in North America. Vet. Sci. 2020, 7, 188. https://doi.org/10.3390/vetsci7040188
Hedman HD, Varga C, Duquette J, Novakofski J, Mateus-Pinilla NE. Food Safety Considerations Related to the Consumption and Handling of Game Meat in North America. Veterinary Sciences. 2020; 7(4):188. https://doi.org/10.3390/vetsci7040188
Chicago/Turabian StyleHedman, Hayden D., Csaba Varga, Jared Duquette, Jan Novakofski, and Nohra E. Mateus-Pinilla. 2020. "Food Safety Considerations Related to the Consumption and Handling of Game Meat in North America" Veterinary Sciences 7, no. 4: 188. https://doi.org/10.3390/vetsci7040188






