Intestinal Strongyle Genera in Different Typology of Donkey Farms in Tuscany, Central Italy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Farms
2.2. Parasitological Analysis
2.3. Statistical Analysis
2.4. Ethical Statement
3. Results
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Camillo, F.; Rota, A.; Biagini, L.; Tesi, M.; Fanelli, D.; Panzani, D. The current situation and trend of donkey industry in Europe. Equine Vet. Sci. 2018, 65, 44–49. [Google Scholar] [CrossRef]
- Altomonte, I.; Salari, F.; Licitra, R.; Martini, M. Donkey and human milk: Insights into their compositional similarities. Int. Dairy J. 2019, 89, 111–118. [Google Scholar] [CrossRef]
- Souroullas, K.; Aspri, M.; Papademas, P. Donkey milk as a supplement in infant formula: Benefits and technological challenges. Int. Food Res. J. 2018, 109, 416–425. [Google Scholar] [CrossRef]
- Raglio. La Comunità Dedicate Agli Asini. Available online: http://www.raglio.com/allevamenti/index.php?regione=Toscana (accessed on 5 November 2020).
- Rete Rurale Nazionale 2014–2020. Le Bandite di Scarlino. Italian Ministry of Agricultural Policies. Available online: https://www.reterurale.it/flex/cm/pages/ServeBLOB.php/L/IT/IDPagina/18035 (accessed on 5 November 2020).
- Matthews, J.B.; Burden, F.A. Common helminth infections of donkeys and their control in temperate regions. Equine Vet. Educ. 2013, 25, 461–467. [Google Scholar] [CrossRef]
- Ragona, G.; Corrias, F.; Benedetti, M.; Paladini, I.; Salari, F.; Altomonte, I.; Martini, M. Amiata donkey milk chain: Animal health evaluation and milk. Ital. J. Food Saf. 2016, 5, 5951. [Google Scholar] [CrossRef] [Green Version]
- Nielsen, M.K.; Reinemeyer, C.R. Biology and Life Cycles of Equine Parasites. In Handbook of Equine Parasite Control, 2nd ed.; Nielsen, M.K., Reinemeyer, C.R., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2018; Chapter 1; pp. 1–23. [Google Scholar] [CrossRef]
- Morriss, C.; Trawford, A.; Svendsen, E. Donkey: Hero or villain of the parasite world? Past, present and future. Vet. Parasitol. 2004, 125, 43–58. [Google Scholar]
- Proudman, C.J.; Matthews, J.B. Control of equine intestinal helminths. In Pract. 2000, 22, 90–97. [Google Scholar] [CrossRef]
- Nielsen, M.K.; Jacobsen, S.; Olsen, S.N.; Bousquet, E.; Pihl, T. Non strangulating intestinal infarction associated with Strongylus vulgaris in referred Danish equine cases. Equine Vet. J. 2016, 48, 376–379. [Google Scholar] [CrossRef]
- Hung, G.C.; Jacobs, D.E.; Krecek, R.C.; Gasser, R.B.; Chilton, N.B. Strongylus asini (Nematoda, Strongyloidea): Genetic relationships with other Strongylus species determined by ribosomal DNA. Int. J. Parasitol. 1996, 26, 1407–1411. [Google Scholar] [CrossRef]
- Malan, F.S.; Vos, V.; de Reinecke, R.K.; Pletcher, J.M. Studies on Strongylus asini. I. Experimental infestation of equines. Onderstepoort J. Vet. Res. 1982, 49, 151–153. [Google Scholar]
- Gokbulut, C.; Aksit, D.; Smaldone, G.; Mariani, U.; Veneziano, V. Plasma pharmacokinetics, faecal excretion and efficacy of pyrantel pamoate paste and granule formulations following per os administration in donkeys naturally infected with intestinal strongylidae. Vet. Parasitol. 2014, 205, 186–192. [Google Scholar] [CrossRef] [Green Version]
- Traversa, D.; Iorio, R.; Klei, T.R.; Kharchenko, V.A.; Gawor, J.; Otranto, D.; Sparagano, O.A.E. New method for simultaneous species-specific identification of equine strongyles (Nematoda, Strongylida) by reverse line blot hybridization. J. Clin. Microbiol. 2007, 45, 2937–2942. [Google Scholar] [CrossRef] [Green Version]
- Ionita, M.; Howe, D.K.; Lyons, E.T.; Tolliver, S.C.; Kaplan, R.M.; Mitrea, I.L.; Yeargan, M. Use of a reverse line blot assay to survey small strongyle (Strongylida: Cyathostominae) populations in horses before and after treatment with ivermectin. Vet. Parasitol. 2010, 168, 332–337. [Google Scholar] [CrossRef] [PubMed]
- Bredtmann, C.M.; Krücken, J.; Murugaiyan, J.; Kuzmina, T.; von Samson-Himmelstjerna, G. Nematode species identification, current status, challenges and future perspectives for cyathostomins. Front. Cell Infect. Microbiol. 2017, 7, 283. [Google Scholar] [CrossRef]
- Lichtenfels, J.R.; Kharchenko, V.A.; Dvojnos, G.M. Illustrated identification keys to strongylid parasites (Strongylidae: Nematoda) of horses, zebras and asses (Equidae). Vet. Parasitol. 2008, 156, 4–161. [Google Scholar] [CrossRef] [PubMed]
- Bevilaqua, C.M.L.; Rodrigues, M.L.; Concordet, D. Identification of infective larvae of some common nematode strongylids of horses. Rev. Med. Vet. 1993, 144, 989–995. [Google Scholar]
- Brianti, E.; Giannetto, S.; Traversa, D.; Chirgwin, S.R.; Shakya, K.; Klei, T.R. In vitro development of cyathostomin larvae from the third stage larvae to the fourth stage: Morphologic characterization, effects of refrigeration, and species-specific patterns. Vet. Parasitol. 2009, 163, 348–356. [Google Scholar] [CrossRef]
- Santos, D.W.; Madeira de Carvalho, L.M.; Molento, M.B. Identification of third stage larval types of cyathostomins of equids: An improved perspective. Vet. Parasitol. 2018, 260, 49–52. [Google Scholar] [CrossRef]
- Gokbulut, C.; Aksit, D.; Santoro, M.; Roncoroni, C.; Mariani, U.; Buono, F.; Rufrano, D.; Fagiolo, A.; Veneziano, V. Plasma disposition, milk excretion and parasitological efficacy of mebendazole in donkeys naturally infected by Cyathostominae. Vet. Parasitol. 2016, 217, 95–100. [Google Scholar] [CrossRef]
- Gianfaldoni, C.; Barlozzari, G.; Mancini, S.; Di Domenico, E.; Maestrini, M.; Perrucci, S. Parasitological investigation in an organic dairy donkey farm. Large Anim. Rev. 2020, 26, 25–30. [Google Scholar]
- Arfuso, F.; Bazzano, M.; Brianti, E.; Gaglio, G.; Passantino, A.; Tesei, B.; Laus, F. Nutritional Supplements Containing Cardus mariano, Eucalyptus globulus, Gentiana lutea, Urtica urens, and Mallotus philippinensis extracts are effective in reducing egg shedding in dairy jennies (Equus asinus) naturally infected by cyathostomins. Front. Vet. Sci. 2020, 7, 556270. [Google Scholar] [CrossRef]
- Burden, F. Practical feeding and condition scoring for donkeys and mules. Equine Vet. Educ. 2012, 24, 589–596. [Google Scholar] [CrossRef]
- Traversa, D.; Milillo, P.; Barnes, H.; Von Samson-Himmelstjerna, G.; Schurmann, S.; Demeler, J.; Otranto, D.; Lia, R.P.; Perrucci, S.; Frangipane di Regalbono, A.; et al. Distribution and species-specific occurrence of cyathostomins (Nematoda, Strongylida) in naturally infected horses from Italy, United Kingdom and Germany. Vet. Parasitol. 2010, 168, 84–92. [Google Scholar] [CrossRef]
- Kaspar, A.; Pfister, K.; Nielsen, M.K.; Silaghi, C.; Fink, H.; Scheuerle, M.C. Detection of Strongylus vulgaris in equine faecal samples by real-time PCR and larval culture - method comparison and occurrence assessment. BMC Vet. Res. 2017, 13, 19. [Google Scholar] [CrossRef] [Green Version]
- Steuer, A.E.; Loynachan, A.T.; Nielsen, M.K. Evaluation of the mucosal inflammatory responses to equine cyathostomins in response to anthelmintic treatment. Vet. Immunol. Immunopathol. 2018, 199, 1–7. [Google Scholar] [CrossRef]
- Love, S.; Murphy, D.; Mellor, D. Pathogenicity of cyathostome infection. Vet. Parasitol. 1999, 85, 113–225. [Google Scholar] [CrossRef]
- Peregrine, A.S.; McEwen, B.; Bienzle, D.; Koch, T.G.; Weese, J.S. Larval cyathostominosis in horses in Ontario: An emerging disease? Can. Vet. J. 2006, 47, 80–82. [Google Scholar]
- Mezgebu, T.; Tafess, K.; Tamiru, F. Prevalence of gastrointestinal parasites of horses and donkeys in and around Gondar Town, Ethiopia. Open J. Vet. Med. 2013, 3, 267–272. [Google Scholar] [CrossRef] [Green Version]
- Eysker, M.; Pandey, V.S. Overwintering of non-migrating strongyles in donkeys in the highveld of Zimbabwe. Res. Vet. Sci. 1987, 42, 262–263. [Google Scholar] [CrossRef]
- Oryan, A.; Kish, G.F.; Rajabloo, M. Larval cyathostominosis in a working donkey. J. Parasit. Dis. 2015, 39, 324–327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thiemann, A.K.; Sullivan, R.J.E. Intestinal disorders of donkeys and mules. Vet. Clin. N. Am. Equine Pract. 2019, 35, 419–432. [Google Scholar] [CrossRef] [PubMed]
- Matthee, S.; Dreyer, F.H.; Hoffmann, W.A.; van Niekerk, F.E. An introductory survey of helminth control practices in south Africa and anthelmintic resistance on Thoroughbred stud farms in the Western Cape Province. J. S. Afr. Vet. Assoc. 2002, 73, 195–200. [Google Scholar] [CrossRef] [Green Version]
- Attia, M.M.; Khalifa, M.M.; Atwa, M.T. The prevalence and intensity of external and internal parasites in working donkeys (Equus asinus) in Egypt. Vet. World 2018, 11, 1298–1306. [Google Scholar] [CrossRef] [Green Version]
- Getachew, M.; Trawford, A.; Feseha, G.; Reid, S.W.J. Intestinal parasites of working donkeys of Ethiopia. Trop. Anim. Health 2010, 42, 27–33. [Google Scholar] [CrossRef]
- Ismail, A.A.; Ahmed, N.K.; Bashar, A.E.; Seri, H.I.; El Tigani-Asil, T.A.; Abakar, A.D. A survey of seasonal gastrointestinal parasitic infections in donkeys from a semiarid sub-Saharan region, Sudan. J. Pathog. 2016, 4602751. [Google Scholar] [CrossRef] [Green Version]
- Sallé, G.; Guillot, J.; Tapprest, J.; Foucher, N.; Sevin, C.; Laugier, C. Compilation of 29 years of post-mortem examinations identifies major shifts in equine parasite prevalence from 2000 onwards. Int. J. Parasitol. 2020, 50, 125–132. [Google Scholar] [CrossRef]
- Love, S.; Duncan, J.L. Could the worms have turned? Equine Vet. J. 1991, 23, 152–154. [Google Scholar] [CrossRef]
- Canever, R.J.; Braga, P.R.C.; Boeck, A.; Grycajuck, M.; Bier, D.; Molento, M.B. Lack of cyathostomin sp. reduction after anthelmintic treatment in horses in Brazil. Vet. Parasitol. 2013, 194, 9–39. [Google Scholar] [CrossRef] [Green Version]
- Buono, F.; Roncoroni, C.; Pacifico, L.; Piantedosi, D.; Neola, B.; Barile, V.L.; Fagiolo, A.; Várady, M.; Veneziano, V. Cyathostominae egg reappearance period after treatment with major horse anthelmintics in donkeys. J. Equine Vet. Sci. 2018, 65, 6–11. [Google Scholar] [CrossRef]
- Stancampiano, L.; Usai, F. The role of density-dependent arrested larval stages on parasite dynamics and stability: Lessons from nematodes and donkeys. Ecol. Model. 2015, 297, 69–79. [Google Scholar] [CrossRef]
- European Union Regulation N. 834, 2007. Council Regulation (EC) No 834/2007 of 28 June 2007 on Organic Production and Labelling of Organic Products and Repealing Regulation (EEC) N. 2092/91. Available online: https://eur-lex.europa.eu/legalcontent/EN/TXT/PDF/?uri=CELEX:32007R0834&from=EN (accessed on 28 October 2020).
- European Union Regulation N. 889, 2008. Commission Regulation (EC) No 889/2008 of 5 September 2008 Laying Down Detailed Rules for the Implementation of Council Regulation (EC) No 834/2007 on Organic Production and Labelling of Organic Products with Regard to Organic Production, Labelling and Control. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32008R0889&from=EN (accessed on 28 October 2020).
- European Union Regulation N. 853, 2004. Regulation (EC) of the European Parliament and of the Council of 29 April 2004 Laying Down Specific Hygiene Rules for on the Hygiene of Foodstuffs. Official Journal of the European Union. Available online: https://eur-lex.europa.eu/legalcontent/EN/TXT/PDF/?uri=CELEX:32004R0853&from=EN (accessed on 28 October 2020).
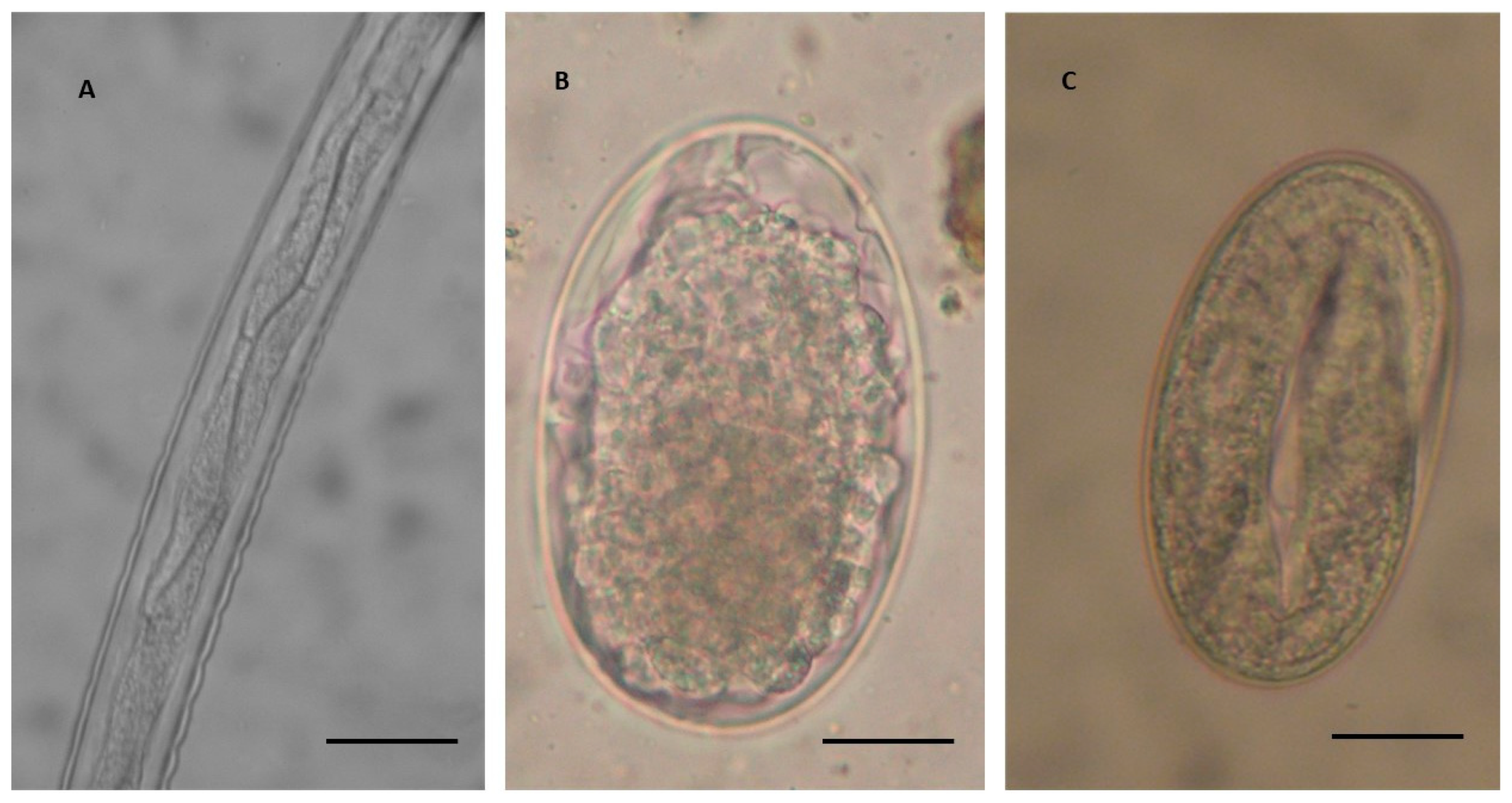
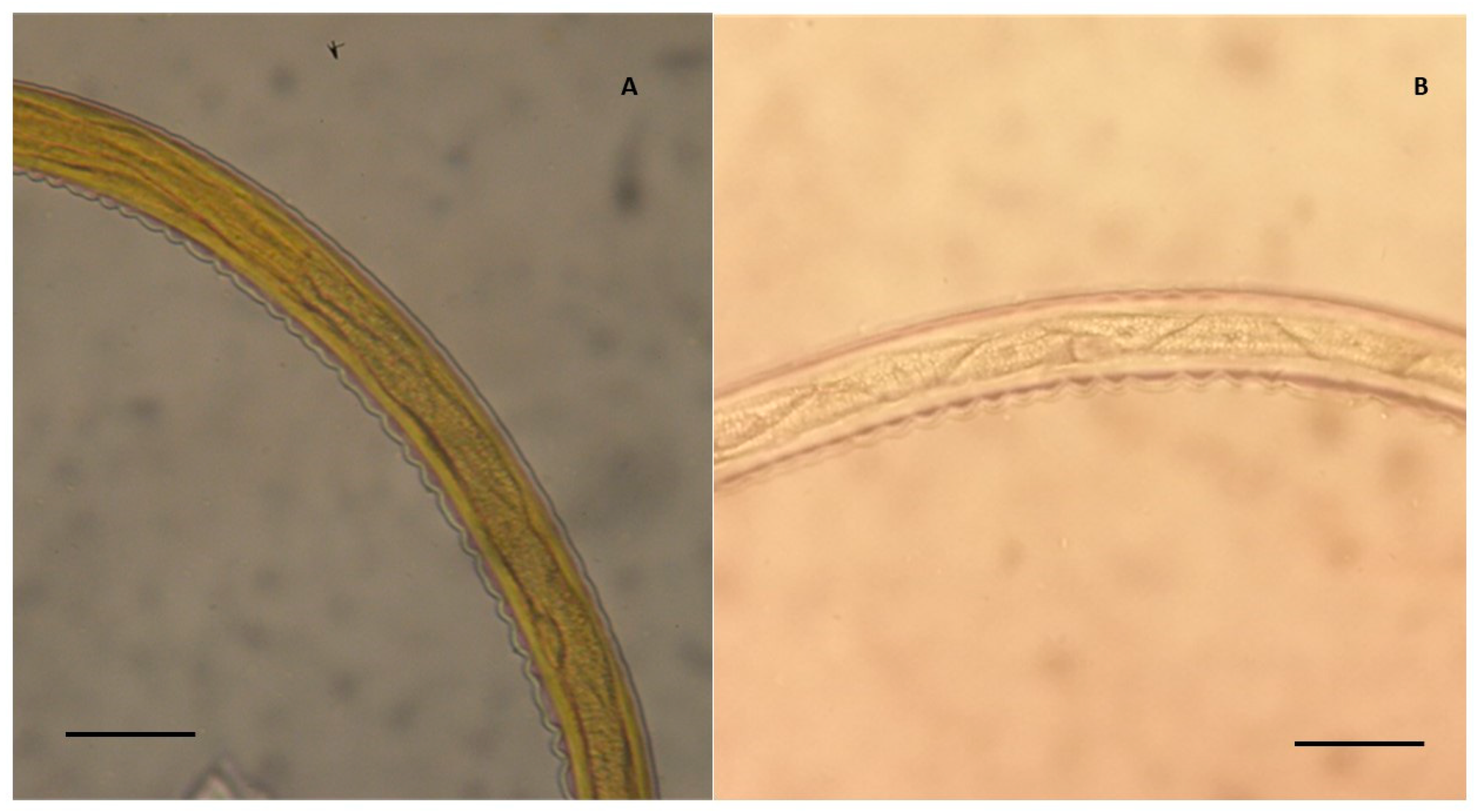
| Province/Group of L3 | Grosseto a | Arezzo b | Pisa c |
|---|---|---|---|
| Group 1 C. hybridus, C. calicatus, and C. longibursatus | 25 | 24 | 34 |
| Group 2 Cy. nassatus, Cy. radiatus, Cy. insigne, C. minutus, and C. poculatus | 75 | 68 | 60 |
| Group 3 Cy. ultrajectinus, Cy. brevicapsulatus, and C. bicoronatus | - | - | 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maestrini, M.; Molento, M.B.; Mancini, S.; Martini, M.; Angeletti, F.G.S.; Perrucci, S. Intestinal Strongyle Genera in Different Typology of Donkey Farms in Tuscany, Central Italy. Vet. Sci. 2020, 7, 195. https://doi.org/10.3390/vetsci7040195
Maestrini M, Molento MB, Mancini S, Martini M, Angeletti FGS, Perrucci S. Intestinal Strongyle Genera in Different Typology of Donkey Farms in Tuscany, Central Italy. Veterinary Sciences. 2020; 7(4):195. https://doi.org/10.3390/vetsci7040195
Chicago/Turabian StyleMaestrini, Michela, Marcelo Beltrão Molento, Simone Mancini, Mina Martini, Francesco Giovanni Salvo Angeletti, and Stefania Perrucci. 2020. "Intestinal Strongyle Genera in Different Typology of Donkey Farms in Tuscany, Central Italy" Veterinary Sciences 7, no. 4: 195. https://doi.org/10.3390/vetsci7040195







