Antimicrobial Peptides in Farm Animals: An Updated Review on Its Diversity, Function, Modes of Action and Therapeutic Prospects
Abstract
:1. Introduction
2. Structural Diversity Among AMPs
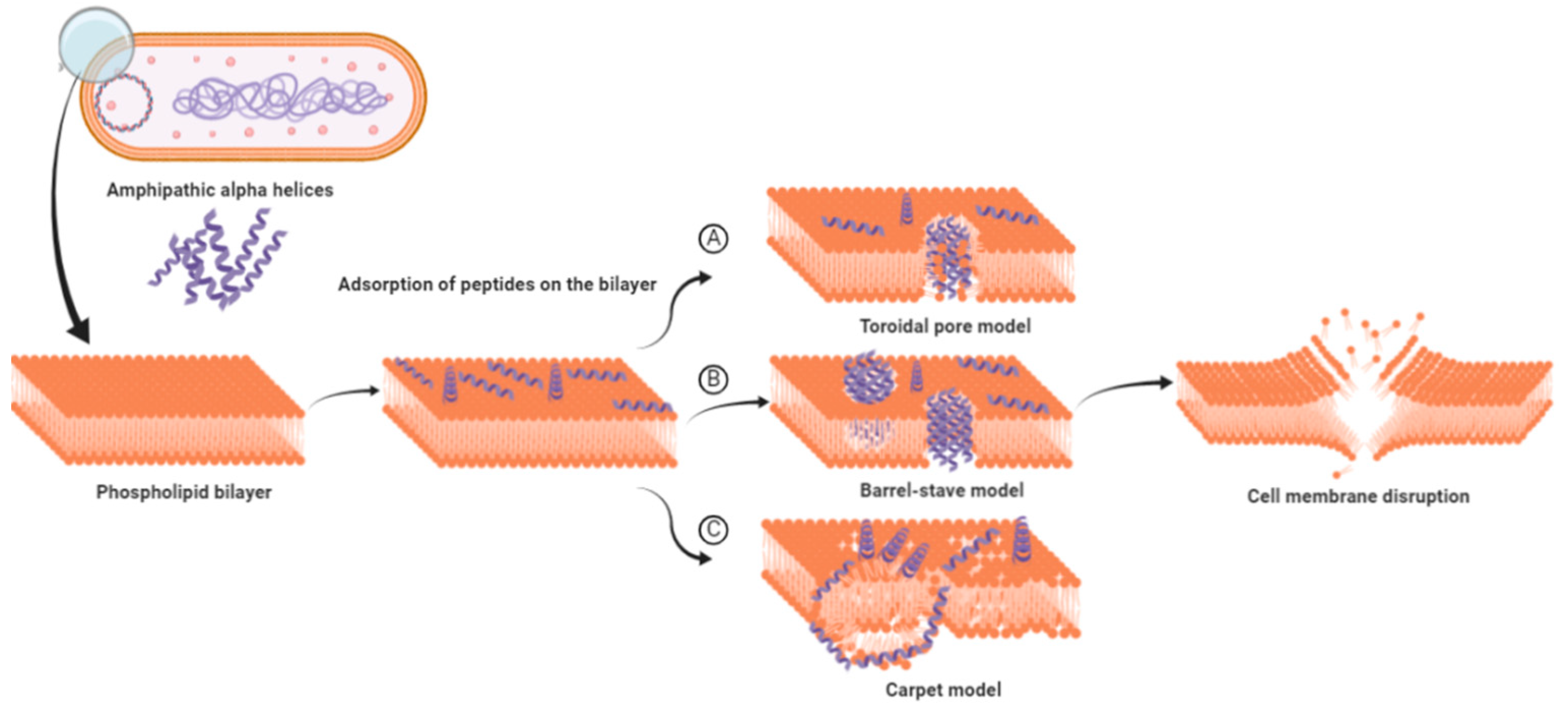
3. Biological Function and Mode of Action
4. Bovine AMPs
4.1. Bovine Cathelicidins
4.1.1. Indolicidin
4.1.2. Bovine MyeloidAntimicrobialPeptides (BMAPs)
BMAP-27
BMAP-28
BMAP-18
BMAP-34
4.2. Bovine β-Defensins
4.2.1. Tracheal Antimicrobial Peptide (TAP)
4.2.2. Lingual Antimicrobial Peptide (LAP)
4.2.3. BNBD (Bovine Neutrophil β-Defensins)
4.2.4. Enteric β–Defensins
4.2.5. Bovine β-Defensin 1
4.3. Bovine Psoriasin
4.4. Proline-Rich AMPs: Bac-5 and Bac-7
5. Equine AMPs
5.1. Equine Cathelicidin
5.2. Equine Neutrophil Antimicrobial Peptides
5.3. Equine Hepcidin
5.4. Equine α and β Defensin
6. Porcine AMPs
6.1. Porcine Cathelicidins
6.1.1. Protegrins
6.1.2. PR39
6.1.3. Prophenin 1
6.1.4. Cecropin P1
6.1.5. Porcine Myeloid Antimicrobial Peptide
6.2. Porcine β Defensin
7. CaprineAMPs
8. Ovine AMPs
8.1. Ovine Cathelicidins
8.2. Ovine β-Defensins
9. Milk-Derived AMPs
| AMPs | Source | Activity | Structure | Mode of Action |
|---|---|---|---|---|
| CATHELICIDIN | ||||
| Indolicidin | Bovine neutrophils [58] | Gram-positive and Gram-negative bacteria, yeast, and fungi [59,62,63] | Extended helix [24,25] | Membrane thinning, disruption of the membrane by channel formation, inhibition of DNA synthesis, and topoisomerase 1 [24,41,42,43,44,45] |
| BMAP-18, BMAP-27, BMAP-28,and BMAP-34 | Bovine myeloid cells [40] | Gram-positive and Gram-negative bacteria, viruses, fungi, trypanosomes, and tumor cells [40,64,65,66,67,68,71,72,75,76] | α-helix [9,76] | Formation of small channels in the bacterial membrane resulting in the release of small ions, transition pores in mitochondria, and LPS neutralization [9,40,69,73,74], |
| Bac-5 and Bac-7 | Bovine neutrophil [101] | Gram-positive and Gram-negative bacteria [101] | Combination of α-helix and β-sheet [27] | Inhibits protein synthesis by entering via bacterial inner membrane transporter SbmA and YjiL/MdtM [102,103] |
| eCATH1, eCATH2, and eCATH3 | Equine neutrophils [14] | Broad-spectrum [14,106,107] | α-helix [14] | N.A. |
| Protegrin | Porcine Lung and intestine [121] | Gram-positive and Gram-negative bacteria, PRRSV, and yeast [18,119,121,122,124,125,126,127,128,135] | β-sheet [17,18,19,20] | Pore formation in the bacterial cell membrane and immunomodulation [120,129] |
| PR-39 | Porcine intestine, upper and lower respiratory tract [137] | Gram-negative bacteria and Mycobacterium tuberculosis [144,145,146] | Combination of α-helix and β-sheet [27] | Halts DNA and protein synthesis by exerting proteolytic activity and acts as a calcium-dependent chemoattractant for neutrophil [40,143,144] |
| Prophenin 1 | Porcine leukocytes [147] | More effective against Gram-negative bacteria and less effective against Gram-positive bacteria [147,148] | β-sheet (homology modeling) | N.A. |
| Cecropin P1 | Porcine small intestine [150] | Gram-positive, Gram-negative bacteria and viruses [152,153,154] | α-helix [12,13] | Disruption of lipid bilayer using the carpet model [12,34] |
| PMAPs | Porcine bone marrow [155] | Gram-positive and Gram-negative bacteria, fungi, and nematodes [155,156,157,158] | α-helix [11] | Permeabilize bacterial membrane [155,156,157] |
| ChBac3 and ChBac5 | Caprine leucocytes [164] | Broad-spectrum [164,166] | N.A. | Membrane disruption [165] |
| OaBac5, OaBac7, and variants | Ovine leucocytes [164] | Broad-spectrum [164,168] | N.A. | depolarization of the cytoplasmic membranes [168] |
| SMAP29 | Ovine myeloid cells [10] | Gram-positive and Gram-negative bacteria and yeast [169] | α-helix [10] | Permeabilize bacterial membrane [35] |
| DEFENSINS | ||||
| TAP | Bovine mucosal epithelial cells and mammary epithelial cells [77,78] | Broad-spectrum [77,81,82,83] | β-sheet (homology modeling) | N.A. |
| LAP | Bovine squamous epithelial cells tongue, esophagus, rumen reticulum, omasum, and chief cells of gastric glands [85,86,87] | Broad-spectrum [84] | β-sheet (homology modeling) | N.A. |
| BNBDs | Bovine neutrophils alveolar tissue and pulmonary macrophages [96,97] | Broad-spectrum [94,96] | β-sheet (homology modeling) | N.A. |
| EBD | Bovine epithelial cells of intestine and colon [99] | Cryptosporidium parvum [99] | N.A. | N.A. |
| Bovine β-defensin 1 | Urogenital tract [100] | Strong response against Gram-negative bacteria as compared to Gram-positive bacteria [100] | β-sheet (homology modeling) | |
| Equine β defensin 1 | Hepatic tissue and respiratory epithelial tissue [114,115] | Broad-spectrum [115] | N.A | N.A |
| Equine α-defensin DEFA1 | Small intestine [116] | Gram-positive andgram-negative bacteriaandfungi [116,117] | β-sheet | membrane permeabilization |
| Porcine β Defensin | Tongue, liver, kidney, small intestine, and large intestine [163] | Broad-spectrum [160,161,162] | β-sheet (homology modeling) | N.A. |
| Ovine β-defensin (SBD1 and SBD2) | Trachea, tongue, and gastrointestinal tract [167] | Gram-positive and Gram-negative bacteria, parainfluenza virus, and M. haemolytica [167,171,172] | β-sheet (homology modeling) | N.A. |
| NEUTROPHIL ANTIMICROBIAL PEPTIDE | ||||
| eNAP-1 and eNAP-2 | Equine neutrophils [108] | Broad-spectrum [108,109,110] | N.A. | Selective activity against microbial serine proteases [109,110] |
| PSORIASIN | ||||
| Bovine Psoriasin | Udder | Gram-negative bacteria [47] | N.A. | Reduces bacterial survival by zinc sequestration [47] |
| HEPCIDIN | ||||
| Equine hepcidin | Liver [111] | N.A. | N.A. | Induces hypoferrimia by iron sequestration [46,112] |
10. Therapeutic Potential of Farm Animal-Derived AMPs
11. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Van Boeckel, T.P.; Brower, C.; Gilbert, M.; Grenfell, B.T.; Levin, S.A.; Robinson, T.P.; Teillant, A.; Laxminarayan, R. Global trends in antimicrobial use in food animals. Proc. Natl. Acad. Sci. USA 2015, 112, 5649–5654. [Google Scholar] [CrossRef] [Green Version]
- Fratini, F.; Cilia, G.; Turchi, B.; Felicioli, A. Insects, arachnids and centipedes venom: A powerful weapon against bacteria. A literature review. Toxicon 2017, 130, 91–103. [Google Scholar] [CrossRef]
- Patocka, J.; Nepovimova, E.; Klimova, B.; Wu, Q.; Kuca, K. Antimicrobial Peptides: Amphibian Host Defense Peptides. Curr. Med. Chem. 2018, 26, 5924–5946. [Google Scholar] [CrossRef]
- Van Hoek, M.L. Antimicrobial peptides in reptiles. Pharmaceuticals 2014, 7, 723–753. [Google Scholar] [CrossRef] [Green Version]
- Dutta, P.; Das, S. Mammalian Antimicrobial Peptides: Promising Therapeutic Targets Against Infection and Chronic Inflammation. Curr. Top. Med. Chem. 2015, 16, 99–129. [Google Scholar] [CrossRef]
- Zhao, X.; Wu, H.; Lu, H.; Li, G.; Huang, Q. LAMP: A Database Linking Antimicrobial Peptides. PLoS ONE 2013, 8, e66557. [Google Scholar] [CrossRef] [Green Version]
- Pasupuleti, M.; Schmidtchen, A.; Malmsten, M. Antimicrobial peptides: Key components of the innate immune system. Crit. Rev. Biotechnol. 2012, 32, 143–171. [Google Scholar] [CrossRef] [Green Version]
- Mahlapuu, M.; Håkansson, J.; Ringstad, L.; Björn, C. Antimicrobial peptides: An emerging category of therapeutic agents. Front. Cell. Infect. Microbiol. 2016, 6, 194. [Google Scholar] [CrossRef] [Green Version]
- Yang, S.; Lee, C.W.; Kim, H.J.; Jung, H.H.; Kim, J.I.; Shin, S.Y.; Shin, S.H. Structural analysis and mode of action of BMAP-27, a cathelicidin-derived antimicrobial peptide. Peptides 2019, 118, 170106. [Google Scholar] [CrossRef]
- Shin, S.Y.; Park, E.J.; Yang, S.T.; Jung, H.J.; Eom, S.H.; Song, W.K.; Kim, Y.; Hahm, K.S.; Kim, J. Il Structure-activity analysis of SMAP-29, a sheep leukocytes-derived antimicrobial peptide. Biochem. Biophys. Res. Commun. 2001, 285, 1046–1051. [Google Scholar] [CrossRef]
- Park, K.; Oh, D.; Kim, Y.; Yub Shin, S.; Hahm, K.S. Structural studies of porcine myeloid antibacterial peptide PMAP-23 and its analogues in DPC micelles by NMR spectroscopy. Biochem. Biophys. Res. Commun. 2002, 290, 204–212. [Google Scholar] [CrossRef]
- Gazit, E.; Boman, A.; Boman, H.G.; Shai, Y. Interaction of the Mammalian Antibacterial Peptide Cecropin PI with Phospholipid Vesicles. Biochemistry 1995, 34, 11479–11488. [Google Scholar] [CrossRef]
- Sipos, D.; Andersson, M.; Ehrenberg, A. The structure of the mammalian antibacterial peptide cecropin P1 in solution, determined by proton-NMR. Eur. J. Biochem. 1992, 209, 163–169. [Google Scholar] [CrossRef]
- Skerlavaj, B.; Scocchi, M.; Gennaro, R.; Risso, A.; Zanetti, M. Structural and functional analysis of horse cathelicidin peptides. Antimicrob. Agents Chemother. 2001, 45, 715–722. [Google Scholar] [CrossRef] [Green Version]
- Yeaman, M.R.; Yount, N.Y. Mechanisms of antimicrobial peptide action and resistance. Pharmacol. Rev. 2003, 55, 27–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lai, Y.; Gallo, R.L. AMPed up immunity: How antimicrobial peptides have multiple roles in immune defense. Trends Immunol. 2009, 30, 131–141. [Google Scholar] [CrossRef] [Green Version]
- Fahrner, R.L.; Dieckmann, T.; Harwig, S.S.L.; Lehrer, R.I.; Eisenberg, D.; Feigon, J. Solution structure of protegrin-1, a broad-spectrum antimicrobial peptide from porcine leukocytes. Chem. Biol. 1996, 3, 543–550. [Google Scholar] [CrossRef] [Green Version]
- Cho, Y.; Turner, J.S.; Dinh, N.N.; Lehrer, R.I. Activity of protegrins against yeast-phase Candida albicans. Infect. Immun. 1998, 66, 2486–2493. [Google Scholar] [CrossRef] [Green Version]
- Usachev, K.S.; Efimov, S.V.; Kolosova, O.A.; Filippov, A.V.; Klochkov, V.V. High-resolution NMR structure of the antimicrobial peptide protegrin-2 in the presence of DPC micelles. J. Biomol. NMR 2015, 61, 227–234. [Google Scholar] [CrossRef]
- Powers, J.P.S.; Hancock, R.E.W. The relationship between peptide structure and antibacterial activity. Peptides 2003. [Google Scholar] [CrossRef]
- Angélique, L.; Frederik, W.J.; Garmi, J.; Hester, D.P.L. The potential use of natural and structural analogues of antimicrobial peptides in the fight against neglected tropical diseases. Molecules 2015, 20, 15392–15433. [Google Scholar]
- Nguyen, L.T.; Haney, E.F.; Vogel, H.J. The expanding scope of antimicrobial peptide structures and their modes of action. Trends Biotechnol. 2011. [Google Scholar] [CrossRef] [PubMed]
- Falla, T.J.; Nedra Karunaratne, D.; Hancock, R.E.W. Mode of action of the antimicrobial peptide indolicidin. J. Biol. Chem. 1996, 271, 19298–19303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rozek, A.; Friedrich, C.L.; Hancock, R.E.W. Structure of the bovine antimicrobial peptide indolicidin bound to dodecylphosphocholine and sodium dodecyl sulfate micelles. Biochemistry 2000, 39, 15765–15774. [Google Scholar] [CrossRef] [PubMed]
- Bahar, A.A.; Ren, D. Antimicrobial peptides. Pharmaceuticals 2013, 6, 1543–1575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anbanandam, A.; Albarado, D.C.; Tirziu, D.C.; Simons, M.; Veeraraghavan, S. Molecular Basis for Proline- and Arginine-Rich Peptide Inhibition of Proteasome. J. Mol. Biol. 2008, 384, 219–227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hein, K.Z.; Takahashi, H.; Tsumori, T.; Yasui, Y.; Nanjoh, Y.; Toga, T.; Wu, Z.; Grötzinger, J.; Jung, S.; Wehkamp, J.; et al. Disulphide-reduced psoriasin is a human apoptosisinducing broad-spectrum fungicide. Proc. Natl. Acad. Sci. USA 2015, 112, 13039–13044. [Google Scholar] [CrossRef] [Green Version]
- Bai, F.; Town, T.; Pradhan, D.; Cox, J.; Ashish; Ledizet, M.; Anderson, J.F.; Flavell, R.A.; Krueger, J.K.; Koski, R.A.; et al. Antiviral Peptides Targeting the West Nile Virus Envelope Protein. J. Virol. 2007, 81, 2047–2055. [Google Scholar] [CrossRef] [Green Version]
- Chang, K.Y.; Yang, J.R. Analysis and Prediction of Highly Effective Antiviral Peptides Based on Random Forests. PLoS ONE 2013, 8, e70166. [Google Scholar] [CrossRef] [Green Version]
- Zhao, H.; Zhou, J.; Zhang, K.; Chu, H.; Liu, D.; Poon, V.K.M.; Chan, C.C.S.; Leung, H.C.; Fai, N.; Lin, Y.P.; et al. A novel peptide with potent and broad-spectrum antiviral activities against multiple respiratory viruses. Sci. Rep. 2016, 6, 22008. [Google Scholar] [CrossRef]
- Sala, A.; Ardizzoni, A.; Ciociola, T.; Magliani, W.; Conti, S.; Blasi, E.; Cermelli, C. Antiviral Activity of Synthetic Peptides Derived from Physiological Proteins. Intervirology 2019, 61, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Varkey, J.; Singh, S.; Nagaraj, R. Antibacterial activity of linear peptides spanning the carboxy-terminal β-sheet domain of arthropod defensins. Peptides 2006, 27, 2614–2623. [Google Scholar] [CrossRef] [PubMed]
- Gazit, E.; Miller, I.R.; Biggin, P.C.; Sansom, M.S.P.; Shai, Y. Structure and orientation of the mammalian antibacterial peptide cecropin P1 within phospholipid membranes. J. Mol. Biol. 1996, 258, 860–870. [Google Scholar] [CrossRef] [PubMed]
- Orioni, B.; Bocchinfuso, G.; Kim, J.Y.; Palleschi, A.; Grande, G.; Bobone, S.; Park, Y.; Kim, J.I.; Hahm, K.S.; Stella, L. Membrane perturbation by the antimicrobial peptide PMAP-23: A fluorescence and molecular dynamics study. Biochim. Biophys. Acta Biomembr. 2009, 1788, 1523–1533. [Google Scholar] [CrossRef]
- Graham, J.M.; Higgins, J.A. Membrane Analysis; BIOS Scientific Publishers: New York, NY, USA, 1997. [Google Scholar]
- Shai, Y. Mechanism of the binding, insertion and destabilization of phospholipid bilayer membranes by α-helical antimicrobial and cell non-selective membrane-lytic peptides. Biochim. Biophys. Acta Biomembr. 1999. [Google Scholar] [CrossRef] [Green Version]
- Matsuzaki, K. Why and how are peptide-lipid interactions utilized for self-defense? Magainins and tachyplesins as archetypes. Biochim. Biophys. Acta Biomembr. 1999, 1462, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.; Weiss, T.M.; Lehrer, R.I.; Huang, H.W. Crystallization of antimicrobial pores in membranes: Magainin and protegrin. Biophys. J. 2000, 79, 2002–2009. [Google Scholar] [CrossRef] [Green Version]
- Boman, H.G.; Agerberth, B.; Boman, A. Mechanisms of action on Escherichia coli of cecropin P1 and PR-39, two antibacterial peptides from pig intestine. Infect. Immun. 1993, 61, 2978–2984. [Google Scholar] [CrossRef] [Green Version]
- Subbalakshmi, C.; Sitaram, N. Mechanism of antimicrobial action of indolicidin. FEMS Microbiol. Lett. 1998, 160, 91–96. [Google Scholar] [CrossRef]
- Hsu, C.H.; Chen, C.; Jou, M.L.; Lee, A.Y.L.; Lin, Y.C.; Yu, Y.P.; Huang, W.T.; Wu, S.H. Structural and DNA-binding studies on the bovine antimicrobial peptide, indolicidin: Evidence for multiple conformations involved in binding to membranes and DNA. Nucleic Acids Res. 2005, 33, 4053–4064. [Google Scholar] [CrossRef] [Green Version]
- Neale, C.; Hsu, J.C.Y.; Yip, C.M.; Pomès, R. Indolicidin binding induces thinning of a lipid bilayer. Biophys. J. 2014, 106, L29–L31. [Google Scholar] [CrossRef] [Green Version]
- Lee, D.G.; Kim, H.K.; Kim, S.A.; Park, Y.; Park, S.C.; Jang, S.H.; Hahm, K.S. Fungicidal effect of indolicidin and its interaction with phospholipid membranes. Biochem. Biophys. Res. Commun. 2003, 305, 305–310. [Google Scholar] [CrossRef]
- Rokitskaya, T.I.; Kolodkin, N.I.; Kotova, E.A.; Antonenko, Y.N. Indolicidin action on membrane permeability: Carrier mechanism versus pore formation. Biochim. Biophys. Acta Biomembr. 2011, 1808, 91–97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oliveira-Filho, J.P.; Badial, P.R.; Cunha, P.H.J.; Bordon, A.P.; Araujo, J.P.; Divers, T.J.; Winand, N.J.; Borges, A.S. Freund’s adjuvant-induced inflammation: Clinical findings and its effect on hepcidin mRNA expression in horses. Pesqui. Vet. Bras. 2014, 34, 51–56. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.C.; Eckert, R.L. S100A7 (Psoriasin) Mechanism of antibacterial action in wounds. J. Investig. Dermatol. 2007, 127, 945–957. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reddy, K.V.R.; Yedery, R.D.; Aranha, C. Antimicrobial peptides: Premises and promises. Int. J. Antimicrob. Agents 2004, 24, 536–547. [Google Scholar] [CrossRef] [PubMed]
- Zuyderduyn, S.; Ninaber, D.K.; Hiemstra, P.S.; Rabe, K.F. The antimicrobial peptide LL-37 enhances IL-8 release by human airway smooth muscle cells. J. Allergy Clin. Immunol. 2006, 117, 1328–1335. [Google Scholar] [CrossRef] [PubMed]
- Paredes-Gamero, E.J.; Martins, M.N.C.; Cappabianco, F.A.M.; Ide, J.S.; Miranda, A. Characterization of dual effects induced by antimicrobial peptides: Regulated cell death or membrane disruption. Biochim. Biophys. Acta Gen. Subj. 2012, 1820, 1062–1072. [Google Scholar] [CrossRef] [Green Version]
- Elsbach, P. What is the real role of antimicrobial polypeptides that can mediate several other inflammatory responses? J. Clin. Investig. 2003, 111, 1643–1645. [Google Scholar] [CrossRef] [Green Version]
- Yang, D.; Biragyn, A.; Kwak, L.W.; Oppenheim, J.J. Mammalian defensins in immunity: More than just microbicidal. Trends Immunol. 2002, 23, 291–296. [Google Scholar] [CrossRef]
- Torres, P.; DÍaz, J.; Arce, M.; Silva, P.; Mendoza, P.; Lois, P.; Molina-Berríos, A.; Owen, G.I.; Palma, V.; Torres, V.A. The salivary peptide histatin-1 promotes endothelial cell adhesion, migration, and angiogenesis. FASEB J. 2017, 31, 4946–4958. [Google Scholar] [CrossRef] [Green Version]
- Kanazawa, K.; Okumura, K.; Ogawa, H.; Niyonsaba, F. An antimicrobial peptide with angiogenic properties, AG-30/5C, activates human mast cells through the MAPK and NF-κB pathways. Immunol. Res. 2016, 64, 594–603. [Google Scholar] [CrossRef] [PubMed]
- Mu, L.; Tang, J.; Liu, H.; Shen, C.; Rong, M.; Zhang, Z.; Lai, R. A potential wound-healing-promoting peptide from salamander skin. FASEB J. 2014, 28, 3919–3929. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, W.; Jia, J.; Li, C.; Duan, Q.; Yang, J.; Wang, X.; Li, R.; Chen, C.; Yan, H.; Zheng, Y. Antimicrobial peptide LL-37 promotes the proliferation and invasion of skin squamous cell carcinoma by upregulating DNA-binding protein A. Oncol. Lett. 2016, 12, 1745–1752. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Zheng, Y.; Jia, J.; Li, C.; Duan, Q.; Li, R.; Wang, X.; Shao, Y.; Chen, C.; Yan, H. Antimicrobial peptide LL-37 promotes the viability and invasion of skin squamous cell carcinoma by upregulating YB-1. Exp. Ther. Med. 2017, 14, 499–506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Selsted, M.E.; Novotny, M.J.; Morris, W.L.; Tang, Y.Q.; Smith, W.; Cullor, J.S. Indolicidin, a novel bactericidal tridecapeptide amide from neutrophils. J. Biol. Chem. 1992. [Google Scholar] [CrossRef]
- Van Abel, R.J.; Tang, Y.-Q.; Rao, V.; Dobbs, C.H.; Tran, D.; Barany, G.; Selsted, M.E. Synthesis and characterization of indolicidin, a tryptophan-rich antimicrobial peptide from bovine neutrophils. Int. J. Pept. Protein Res. 1995, 45, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Tomasinsig, L.; Zanetti, M. The Cathelicidins Structure, Function and Evolution. Curr. Protein Pept. Sci. 2005, 6, 23–34. [Google Scholar] [CrossRef]
- Subbalakshmi, C.; Krishnakumari, V.; Nagaraj, R.; Sitaram, N. Requirements for antibacterial and hemolytic activities in the bovine neutrophil derived 13-residue peptide indolicidin. FEBS Lett. 1996, 395, 48–52. [Google Scholar] [CrossRef] [Green Version]
- De Alteriis, E.; Maselli, V.; Falanga, A.; Galdiero, S.; Di Lella, F.M.; Gesuele, R.; Guida, M.; Galdiero, E. Efficiency of gold nanoparticles coated with the antimicrobial peptide indolicidin against biofilm formation and development of Candida spp. clinical isolates. Infect. Drug Resist. 2018, 11, 915–925. [Google Scholar] [CrossRef] [Green Version]
- Ghosh, A.; Kar, R.K.; Jana, J.; Saha, A.; Jana, B.; Krishnamoorthy, J.; Kumar, D.; Ghosh, S.; Chatterjee, S.; Bhunia, A. Indolicidin targets duplex DNA: Structural and mechanistic insight through a combination of spectroscopy and microscopy. ChemMedChem 2014, 9, 2052–2058. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benincasa, M.; Skerlavaj, B.; Gennaro, R.; Pellegrini, A.; Zanetti, M. In vitro and in vivo antimicrobial activity of two α-helical cathelicidin peptides and of their synthetic analogs. Peptides 2003, 24, 1723–1731. [Google Scholar] [CrossRef] [PubMed]
- Benincasa, M.; Scocchi, M.; Pacor, S.; Tossi, A.; Nobili, D.; Basaglia, G.; Busetti, M.; Gennaro, R. Fungicidal activity of five cathelicidin peptides against clinically isolated yeasts. J. Antimicrob. Chemother. 2006, 58, 950–959. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pompilio, A.; Scocchi, M.; Pomponio, S.; Guida, F.; Di Primio, A.; Fiscarelli, E.; Gennaro, R.; Di Bonaventura, G. Antibacterial and anti-biofilm effects of cathelicidin peptides against pathogens isolated from cystic fibrosis patients. Peptides 2011, 32, 1807–1814. [Google Scholar] [CrossRef]
- Ciornei, C.D.; Sigurdardóttir, T.; Schmidtchen, A.; Bodelsson, M. Antimicrobial and chemoattractant activity, lipopolysaccharide neutralization, cytotoxicity, and inhibition by serum of analogs of human cathelicidin LL-37. Antimicrob. Agents Chemother. 2005, 49, 2845–2850. [Google Scholar] [CrossRef] [Green Version]
- Mookherjee, N.; Wilson, H.L.; Doria, S.; Popowych, Y.; Falsafi, R.; Yu, J.J.; Li, Y.; Veatch, S.; Roche, F.M.; Brown, K.L.; et al. Bovine and human cathelicidin cationic host defense peptides similarly suppress transcriptional responses to bacterial lipopolysaccharide. J. Leukoc. Biol. 2006, 80, 1563–1574. [Google Scholar] [CrossRef]
- Risso, A.; Zanetti, M.; Gennaro, R. Cytotoxicity and apoptosis mediated by two peptides of innate immunity. Cell. Immunol. 1998, 189, 107–115. [Google Scholar] [CrossRef]
- Ahmad, A.; Azmi, S.; Srivastava, R.M.; Srivastava, S.; Pandey, B.K.; Saxena, R.; Bajpai, V.K.; Ghosh, J.K. Design of nontoxic analogues of cathelicidin-derived bovine antimicrobial peptide BMAP-27: The role of leucine as well as phenylalanine zipper sequences in determining its toxicity. Biochemistry 2009, 48, 10905–10917. [Google Scholar] [CrossRef]
- Guo, Y.; Xun, M.; Han, J. A bovine myeloid antimicrobial peptide (BMAP-28) and its analogs kill pan-drug-resistant Acinetobacter baumannii by interacting with outer membrane protein A (OmpA). Medicine 2018, 97, e12832. [Google Scholar] [CrossRef]
- Takagi, S.; Hayashi, S.; Takahashi, K.; Isogai, H.; Bai, L.; Yoneyama, H.; Ando, T.; Ito, K.; Isogai, E. Antimicrobial activity of a bovine myeloid antimicrobial peptide (BMAP-28) against methicillin-susceptible and methicillin-resistant Staphylococcus aureus. Anim. Sci. J. 2012, 83, 482–486. [Google Scholar] [CrossRef]
- D’Este, F.; Tomasinsig, L.; Skerlavaj, B.; Zanetti, M. Modulation of cytokine gene expression by cathelicidin BMAP-28 in LPS-stimulated and -unstimulated macrophages. Immunobiology 2012, 217, 962–971. [Google Scholar] [CrossRef] [PubMed]
- Risso, A.; Braidot, E.; Sordano, M.C.; Vianello, A.; Macrì, F.; Skerlavaj, B.; Zanetti, M.; Gennaro, R.; Bernardi, P. BMAP-28, an Antibiotic Peptide of Innate Immunity, Induces Cell Death through Opening of the Mitochondrial Permeability Transition Pore. Mol. Cell. Biol. 2002, 22, 1926–1935. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haines, L.R.; Thomas, J.M.; Jackson, A.M.; Eyford, B.A.; Razavi, M.; Watson, C.N.; Gowen, B.; Hancock, R.E.W.; Pearson, T.W. Killing of trypanosomatid parasites by a modified bovine host defense peptide, BMAP-18. PLoS Negl. Trop. Dis. 2009, 3, e373. [Google Scholar] [CrossRef] [PubMed]
- Gennaro, R.; Scocchi, M.; Merluzzi, L.; Zanetti, M. Biological characterization of a novel mammalian antimicrobial peptide. Biochim. Biophys. Acta Gen. Subj. 1998, 1425, 361–368. [Google Scholar] [CrossRef]
- Diamond, G.; Zasloff, M.; Eck, H.; Brasseur, M.; Lee Maloy, W.; Bevins, C.L. Tracheal antimicrobial peptide, a cysteine-rich peptide from mammalian tracheal mucosa: Peptide isolation and cloning of a cDNA. Proc. Natl. Acad. Sci. USA 1991, 88, 3952–3956. [Google Scholar] [CrossRef] [Green Version]
- López-Meza, J.E.; Gutiérrez-Barroso, A.; Ochoa-Zarzosa, A. Expression of tracheal antimicrobial peptide in bovine mammary epithelial cells. Res. Vet. Sci. 2009, 87, 59–63. [Google Scholar] [CrossRef]
- Berghuis, L.; Abdelaziz, K.T.; Bierworth, J.; Wyer, L.; Jacob, G.; Karrow, N.A.; Sharif, S.; Clark, M.E.; Caswell, J.L. Comparison of innate immune agonists for induction of tracheal antimicrobial peptide gene expression in tracheal epithelial cells of cattle. Vet. Res. 2014, 45, 1–10. [Google Scholar] [CrossRef]
- Taha-Abdelaziz, K.; Wyer, L.; Berghuis, L.; Bassel, L.L.; Clark, M.E.; Caswell, J.L. Regulation of tracheal antimicrobial peptide gene expression in airway epithelial cells of cattle. Vet. Res. 2016, 47, 44. [Google Scholar] [CrossRef] [Green Version]
- Caverly, J.M.; Diamond, G.; Gallup, J.M.; Brogden, K.A.; Dixon, R.A.; Ackermann, M.R. Coordinated expression of tracheal antimicrobial peptide and inflammatory-response elements in the lungs of neonatal calves with acute bacterial pneumonia. Infect. Immun. 2003, 71, 2950–2955. [Google Scholar] [CrossRef] [Green Version]
- Yarus, S.; Rosen, J.M.; Cole, A.M.; Diamond, G. Production of active bovine tracheal antimicrobial peptide in milk of transgenic mice. Proc. Natl. Acad. Sci. USA 1996, 93, 14118–14121. [Google Scholar] [CrossRef] [Green Version]
- Schonwetter, B.S.; Stolzenberg, E.D.; Zasloff, M.A. Epithelial antibiotics induced at sites of inflammation. Science (80-.) 1995, 267, 1645–1648. [Google Scholar] [CrossRef] [PubMed]
- Isobe, N.; Morimoto, K.; Nakamura, J.; Yamasaki, A.; Yoshimura, Y. Intramammary challenge of lipopolysaccharide stimulates secretion of lingual antimicrobial peptide into milk of dairy cows. J. Dairy Sci. 2009, 92, 6046–6051. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Isobe, N.; Sugino, T.; Taniguchi, K.; Moriya, N.; Hosoda, K.; Yoshimura, Y. Differential localization of lingual antimicrobial peptide in the digestive tract mucosal epithelium of calves. Vet. Immunol. Immunopathol. 2011, 142, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Tetens, J.; Friedrich, J.J.; Hartmann, A.; Schwerin, M.; Kalm, E.; Thaller, G. The spatial expression pattern of antimicrobial peptides across the healthy bovine udder. J. Dairy Sci. 2010, 93, 775–783. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kościuczuk, E.M.; Lisowski, P.; Jarczak, J.; Krzyzewski, J.; Zwierzchowski, L.; Bagnicka, E. Expression patterns of β-defensin and cathelicidin genes in parenchyma of bovine mammary gland infected with coagulase-positive or coagulase-negative Staphylococci. BMC Vet. Res. 2014, 10, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Téllez-Pérez, A.D.; Alva-Murillo, N.; Ochoa-Zarzosa, A.; López-Meza, J.E. Cholecalciferol (vitamin D) differentially regulates antimicrobial peptide expression in bovine mammary epithelial cells: Implications during Staphylococcus aureus internalization. Vet. Microbiol. 2012, 160, 91–98. [Google Scholar] [CrossRef]
- Swanson, K.; Gorodetsky, S.; Good, L.; Davis, S.; Musgrave, D.; Stelwagen, K.; Farr, V.; Molenaar, A. Expression of a β-defensin mRNA, lingual antimicrobial peptide, in bovine mammary epithelial tissue is induced by mastitis. Infect. Immun. 2004, 72, 7311–7314. [Google Scholar] [CrossRef] [Green Version]
- Kawai, K.; Akamatsu, H.; Obayashi, T.; Nagahata, H.; Higuchi, H.; Iwano, H.; Oshida, T.; Yoshimura, Y.; Isobe, N. Relationship between concentration of lingual antimicrobial peptide and somatic cell count in milk of dairy cows. Vet. Immunol. Immunopathol. 2013, 153, 298–301. [Google Scholar] [CrossRef]
- Jin, D.; Chang, G.; Zhang, K.; Guo, J.; Xu, T.; Shen, X. Rumen-derived lipopolysaccharide enhances the expression of lingual antimicrobial peptide in mammary glands of dairy cows fed a high-concentrate diet. BMC Vet. Res. 2016, 12, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Isobe, N.; Hosoda, K.; Yoshimura, Y. Immunolocalization of lingual antimicrobial peptide (LAP) in the bovine mammary gland. Anim. Sci. J. 2009, 80, 446–450. [Google Scholar] [CrossRef]
- Selsted, M.E.; Tang, Y.Q.; Morris, W.L.; McGuire, P.A.; Novotny, M.J.; Smith, W.; Henschen, A.H.; Cullor, J.S. Purification, primary structures, and antibacterial activities of β- defensins, a new family of antimicrobial peptides from bovine neutrophils. J. Biol. Chem. 1993, 271, 16430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mackenzie-Dyck, S.; Kovacs-Nolan, J.; Snider, M.; Babiuk, L.A.; Van Drunen Littel-Van Den Hurk, S. Inclusion of the bovine neutrophil beta-defensin 3 with glycoprotein D of bovine herpesvirus 1 in a DNA vaccine modulates immune responses of mice and cattle. Clin. Vaccine Immunol. 2014, 21, 463–477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gurao, A.; Kashyap, S.K.; Singh, R. β-defensins: An innate defense for bovine mastitis. Vet. World 2017, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ryan, L.K.; Rhodes, J.; Bhat, M.; Diamond, G. Expression of β-defensin genes in bovine alveolar macrophages. Infect. Immun. 1998, 66, 878–881. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yount, N.Y.; Yuan, J.; Tarver, A.; Castro, T.; Diamond, G.; Tran, P.A.; Levy, J.N.; McCullough, C.; Cullor, J.S.; Bevins, C.L.; et al. Cloning and expression of bovine neutrophil β-defensins: Biosynthetic profile during neutrophilic maturation and localization of mature peptide to novel cytoplasmic dense granules. J. Biol. Chem. 1999, 274, 26249–26258. [Google Scholar] [CrossRef] [Green Version]
- Tarver, A.P.; Clark, D.P.; Diamond, G.; Russell, J.P.; Erdjument-Bromage, H.; Tempst, P.; Cohen, K.S.; Jones, D.E.; Sweeney, R.W.; Wines, M.; et al. Enteric β-defensin: Molecular cloning and characterization of a gene with inducible intestinal epithelial cell expression associated with Cryptosporidium parvum infection. Infect. Immun. 1998, 66, 1045–1056. [Google Scholar] [CrossRef] [Green Version]
- Aono, S.; Li, C.; Zhang, G.; Kemppainen, R.J.; Gard, J.; Lu, W.; Hu, X.; Schwartz, D.D.; Morrison, E.E.; Dykstra, C.; et al. Molecular and functional characterization of bovine β-defensin-1. Vet. Immunol. Immunopathol. 2006, 113, 181–190. [Google Scholar] [CrossRef]
- Gennaro, R.; Skerlavaj, B.; Romeo, D. Purification, composition, and activity of two bactenecins, antibacterial peptides of bovine neutrophils. Infect. Immun. 1989, 57, 3142–3146. [Google Scholar] [CrossRef] [Green Version]
- Mattiuzzo, M.; Bandiera, A.; Gennaro, R.; Benincasa, M.; Pacor, S.; Antcheva, N.; Scocchi, M. Role of the Escherichia coli SbmA in the antimicrobial activity of proline-rich peptides. Mol. Microbiol. 2007, 66, 151–163. [Google Scholar] [CrossRef]
- Mardirossian, M.; Barrière, Q.; Timchenko, T.; Müller, C.; Pacor, S.; Mergaert, P.; Scocchi, M.; Wilsona, D.N. Fragments of the nonlytic proline-rich antimicrobial peptide Bac5 kill Escherichia coli cells by inhibiting protein synthesis. Antimicrob. Agents Chemother. 2018, 62. [Google Scholar] [CrossRef] [Green Version]
- Podda, E.; Benincasa, M.; Pacor, S.; Micali, F.; Mattiuzzo, M.; Gennaro, R.; Scocchi, M. Dual mode of action of Bac7, a proline-rich antibacterial peptide. Biochim. Biophys. Acta Gen. Subj. 2006, 1760, 1732–1740. [Google Scholar] [CrossRef]
- Schlusselhuber, M.; Guldbech, K.; Sevin, C.; Leippe, M.; Petry, S.; Grötzinger, J.; Giguère, S.; Cauchard, J. In vitro effectiveness of the antimicrobial peptide eCATH1 against antibiotic-resistant bacterial pathogens of horses. FEMS Microbiol. Lett. 2014, 350, 216–222. [Google Scholar] [CrossRef] [Green Version]
- Cauchard, S.; Van Reet, N.; Büscher, P.; Goux, D.; Grötzinger, J.; Leippe, M.; Cattoir, V.; Laugier, C.; Cauchard, J. Killing of trypanozoon parasites by the equine cathelicidin eCATH1. Antimicrob. Agents Chemother. 2016, 60, 2610–2619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, Z.; Wang, Y.; Zhai, L.; Che, Q.; Wang, H.; Du, S.; Wang, D.; Feng, F.; Liu, J.; Lai, R.; et al. Novel cathelicidin-derived antimicrobial peptides from Equus asinus. FEBS J. 2010, 277, 2329–2339. [Google Scholar] [CrossRef]
- Couto, M.A.; Harwig, S.S.L.; Cullor, J.S.; Hughes, J.P.; Lehrer, R.I. Identification of eNAP-1, an antimicrobial peptide from equine neutrophils. Infect. Immun. 1992, 60, 3065–3071. [Google Scholar] [CrossRef] [Green Version]
- Couto, M.A.; Harwig, S.S.; Cullor, J.S.; Hughes, J.P.; Lehrer, R.I. eNAP-2, a novel cysteine-rich bactericidal peptide from equine leukocytes. Infect. Immun. 1992, 60, 5042–5047. [Google Scholar] [CrossRef] [Green Version]
- Couto, M.A.; Harwig, S.S.L.; Lehrer, R.I. Selective inhibition of microbial serine proteases by eNAP-2, an antimicrobial peptide from equine neutrophils. Infect. Immun. 1993, 61, 2991–2994. [Google Scholar] [CrossRef] [Green Version]
- Valore, E.V.; Ganz, T. Posttranslational processing of hepcidin in human hepatocytes is mediated by the prohormone convertase furin. Blood Cells, Mol. Dis. 2008, 40, 132–138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nicolas, G.; Bennoun, M.; Porteu, A.; Mativet, S.; Beaumont, C.; Grandchamp, B.; Sirito, M.; Sawadogo, M.; Kahn, A.; Vaulont, S. Severe iron deficiency anemia in transgenic mice expressing liver hepcidin. Proc. Natl. Acad. Sci. USA 2002, 99, 4596–4601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oliveira-Filho, J.P.; Marques, J.A.; Cunha, P.H.J.; Medeiros, G.X.; Riet-Correa, F.; Machado, V.M. V.; Borges, A.S. Sequencing and expression analysis of hepcidin mrna in donkey (Equus asinus) liver. Pesqui. Vet. Bras. 2012, 32, 1050–1054. [Google Scholar] [CrossRef] [Green Version]
- Quintana, A.M.; Landolt, G.A.; Annis, K.M.; Hussey, G.S. Immunological characterization of the equine airway epithelium and of a primary equine airway epithelial cell culture model. Vet. Immunol. Immunopathol. 2011, 140, 226–236. [Google Scholar] [CrossRef] [PubMed]
- Davis, E.G.; Sang, Y.; Blecha, F. Equine β-defensin-1: Full-length cDNA sequence and tissue expression. Vet. Immunol. Immunopathol. 2004, 99, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Bruhn, O.; Paul, S.; Tetens, J.; Thaller, G. The repertoire of equine intestinal α-defensins. BMC Genomics 2009, 10, 631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shomali, M.R. Recombinant Expression of the NOD2 CARD2 Domain and Determination of the Solution Structure of Equine Alpha-Defensin 1 (DEFA1). Ph.D. Thesis, Kiel University, Kiel, Germany, 2012. [Google Scholar]
- Kościuczuk, E.M.; Lisowski, P.; Jarczak, J.; Strzałkowska, N.; Jóźwik, A.; Horbańczuk, J.; Krzyżewski, J.; Zwierzchowski, L.; Bagnicka, E. Cathelicidins: Family of antimicrobial peptides. A review. Mol. Biol. Rep. 2012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panyutich, A.; Shi, J.; Boutz, P.L.; Zhao, C.; Ganz, T. Porcine polymorphonuclear leukocytes generate extracellular microbicidal activity by elastase-mediated activation of secreted proprotegrins. Infect. Immun. 1997, 65, 978–985. [Google Scholar] [CrossRef] [Green Version]
- Penney, J.; Li, J. Protegrin 1 Enhances Innate Cellular Defense via the Insulin-Like Growth Factor 1 Receptor Pathway. Front. Cell. Infect. Microbiol. 2018, 8, 331. [Google Scholar] [CrossRef]
- Liu, C.L.; Jiao, H.J.; Xu, M.J.; Ding, Z.Q.; Li, H.Q.; Jiang, J.B. Infection of porcine reproductive and respiratory syndrome virus suppressing expression of protegrin-1 gene in swine lung. Chin. J. Vet. Med. 2013, 49, 28–31. [Google Scholar]
- Guo, C.; Cong, P.; He, Z.; Mo, D.; Zhang, W.; Chen, Y.; Liu, X. Inhibitory activity and molecular mechanism of protegrin-1 against porcine reproductive and respiratory syndrome virus in vitro. Antivir. Ther. 2015, 20, 573–582. [Google Scholar] [CrossRef] [Green Version]
- Han, F.F.; Wang, Y.Z.; Feng, J.; Guo, J.; Xu, Z.R. Developmental gene expression of antimicrobial peptide Protegrin-1 and effect of weaning on gene regulation of Protegrin-1 in piglets. J. Anim. Feed Sci. 2007, 16, 86–95. [Google Scholar] [CrossRef] [Green Version]
- Yan, J.; Zhang, C.; Tang, L.; Kuang, S. Effect of dietary copper sources and concentrations on serum lysozyme concentration and protegrin-1 gene expression in weaning piglets. Ital. J. Anim. Sci. 2015, 14, 3709. [Google Scholar] [CrossRef]
- Miyasaki, K.T.; Iofel, R.; Lehrer, R.I. Sensitivity of periodontal pathogens to the bactericidal activity of synthetic protegrins, antibiotic peptides derived from porcine leukocytes. J. Dent. Res. 1997, 76, 1453–1459. [Google Scholar] [CrossRef] [PubMed]
- Qu, X.D.; Harwig, S.S.L.; Oren, A.; Shafer, W.M.; Lehrer, R.I. Susceptibility of Neisseria gonorrhoeae to protegrins. Infect. Immun. 1996, 64, 1240–1245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yasin, B.; Harwig, S.S.L.; Lehrer, R.I.; Wagar, E.A. Susceptibility of Chlamydia trachomatis to protegrins and defensins. Infect. Immun. 1996, 64, 709–713. [Google Scholar] [CrossRef] [Green Version]
- Steinberg, D.A.; Hurst, M.A.; Fujii, C.A.; Kung, A.H.C.; Ho, J.F.; Cheng, F.C.; Loury, D.J.; Fiddes, J.C. Protegrin-1: A broad-spectrum, rapidly microbicidal peptide with in vivo activity. Antimicrob. Agents Chemother. 1997, 41, 1738–1742. [Google Scholar] [CrossRef] [Green Version]
- Lam, K.L.H.; Ishitsuka, Y.; Cheng, Y.; Chien, K.; Waring, A.J.; Lehrer, R.I.; Lee, K.Y.C. Mechanism of supported membrane disruption by antimicrobial peptide protegrin-1. J. Phys. Chem. B 2006, 110, 21282–21286. [Google Scholar] [CrossRef]
- Harwig, S.S.L. Intramolecular disulfide bonds enhance the antimicrobial and lytic activities of protegrins at physiological sodium chloride concentrations. Eur. J. Biochem. 1996, 240, 352–357. [Google Scholar] [CrossRef]
- Ishitsuka, Y.; Pham, D.S.; Waring, A.J.; Lehrer, R.I.; Lee, K.Y.C. Insertion selectivity of antimicrobial peptide protegrin-1 into lipid monolayers: Effect of head group electrostatics and tail group packing. Biochim. Biophys. Acta Biomembr. 2006, 1758, 1450–1460. [Google Scholar] [CrossRef] [Green Version]
- Gidalevitz, D.; Ishitsuka, Y.; Muresan, A.S.; Konovalov, O.; Waring, A.J.; Lehrer, R.I.; Lee, K.Y.C. Interaction of antimicrobial peptide protegrin with biomembranes. Proc. Natl. Acad. Sci. USA 2003, 100, 6302–6307. [Google Scholar] [CrossRef] [Green Version]
- Niu, M.; Chai, S.; You, X.; Wang, W.; Qin, C.; Gong, Q.; Zhang, T.; Wan, P. Expression of porcine protegrin-1 in Pichia pastoris and its anticancer activity in vitro. Exp. Ther. Med. 2015, 9, 1075–1079. [Google Scholar] [CrossRef]
- Hill, E.K. Production of Protegrin-1 with a Matrix Metalloproteinase/Elastase Cleavage Site and Its Therapeutic Potential for Skin Wound Infections. Ph.D. Thesis, The University of Guelph, Guelph, ON, Canada, 2017. [Google Scholar]
- Cheung, Q.C.K.; Turner, P.V.; Song, C.; Wu, D.; Cai, H.Y.; MacInnes, J.I.; Li, J. Enhanced resistance to bacterial infection in protegrin-1 transgenic mice. Antimicrob. Agents Chemother. 2008, 52, 1812–1819. [Google Scholar] [CrossRef] [Green Version]
- Gudmundsson, G.H.; Magnusson, K.P.; Chowdhary, B.P.; Johansson, M.; Andersson, L.; Boman, H.G. Structure of the gene for porcine peptide antibiotic PR-39, a cathelin gene family member: Comparative mapping of the locus for the human peptide antibiotic FALL-39. Proc. Natl. Acad. Sci. USA 1995, 92, 7085–7089. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hennig-Pauka, I.; Koch, R.; Hoeltig, D.; Gerlach, G.F.; Waldmann, K.H.; Blecha, F.; Brauer, C.; Gasse, H. PR-39, a porcine host defence peptide, is prominent in mucosa and lymphatic tissue of the respiratory tract in healthy pigs and pigs infected with Actinobacillus pleuropneumoniae. BMC Res. Notes 2012, 5, 539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chan, Y.R.; Zanetti, M.; Gennaro, R.; Gallo, R.L. Anti-microbial activity and cell binding are controled by sequence determinants in the anti-microbial peptide PR-39. J. Investig. Dermatol. 2001, 116, 230–235. [Google Scholar] [CrossRef] [PubMed]
- Veldhuizen, E.J.A.; Schneider, V.A.F.; Agustiandari, H.; Van Dijk, A.; Tjeerdsma-van Bokhoven, J.L.M.; Bikker, F.J.; Haagsman, H.P. Antimicrobial and immunomodulatory activities of PR-39 derived peptides. PLoS ONE 2014, 9, e95939. [Google Scholar] [CrossRef]
- Li, J.; Post, M.; Volk, R.; Gao, Y.; Li, M.; Metais, C.; Sato, K.; Tsai, J.; Aird, W.; Rosenberg, R.D.; et al. PR39, a peptide regulator of angiogenesis. Nat. Med. 2000, 6, 49–55. [Google Scholar] [CrossRef]
- Ramanathan, B.; Wu, H.; Ross, C.R.; Blecha, F. PR-39, a porcine antimicrobial peptide, inhibits apoptosis: Involvement of caspase-3. Dev. Comp. Immunol. 2004, 28, 163–169. [Google Scholar] [CrossRef]
- Shi, J.; Ross, C.R.; Leto, T.L.; Blecha, F. PR-39, a proline-rich antibacterial peptide that inhibits phagocyte NADPH oxidase activity by binding to Src homology 3 domains of p47phox. Proc. Natl. Acad. Sci. USA 1996, 93, 6014–6018. [Google Scholar] [CrossRef] [Green Version]
- Huang, H.-J.; Ross, C.R.; Blecha, F. Chemoattractant properties of PR-39, a neutrophil antibacterial peptide. J. Leukoc. Biol. 1997, 61, 624–629. [Google Scholar] [CrossRef]
- Zhang, G.; Ross, C.R.; Dritz, S.S.; Nietfeld, J.C.; Blecha, F. Salmonella infection increases porcine antibacterial peptide concentrations in serum. Clin. Diagn. Lab. Immunol. 1997, 4. [Google Scholar] [CrossRef] [Green Version]
- Linde, C.M.A.; Hoffner, S.E.; Refai, E.; Andersson, M. In vitro activity of PR-39, a proline-arginine-rich peptide, against susceptible and multi-drug-resistant Mycobacterium tuberculosis. J. Antimicrob. Chemother. 2001, 47, 575–580. [Google Scholar] [CrossRef] [Green Version]
- Zeng, F.; Dong, R.; Zhao, C.; Liu, D.; Zheng, E.; Song, C.; Wu, Z.; Li, Z. Constitutive expression of antimicrobial peptide PR-39 in transgenic mice significantly enhances resistance to bacterial infection and promotes growth. Transgenic Res. 2018, 27, 409–422. [Google Scholar] [CrossRef] [PubMed]
- Harwig, S.S.L.; Kokryakov, V.N.; Swiderek, K.M.; Aleshina, G.M.; Zhao, C.; Lehrer, R.I. Prophenin-1, an exceptionally proline-rich antimicrobial peptide from porcine leukocytes. FEBS Lett. 1995, 362, 65–69. [Google Scholar] [CrossRef] [Green Version]
- Hernandez-Flores, J.L.; Rodriguez, M.C.; Gastelum Arellanez, A.; Alvarez-Morales, A.; Avila, E.E. Effect of recombinant prophenin 2 on the integrity and viability of Trichomonas vaginalis. Biomed Res. Int. 2015, 2015, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Ramanathan, B.; Minton, J.E.; Ross, C.R.; Blecha, F. PU. 1-mediated transcriptional regulation of prophenin-2 in primary bone marrow cells. Gene 2005, 352, 1–9. [Google Scholar] [CrossRef]
- Lee, J.Y.; Boman, A.; Chuanxin, S.; Andersson, M.; Jornvall, H.; Mutt, V.; Boman, H.G. Antibacterial peptides from pig intestine: Isolation of a mammalian cecropin. Proc. Natl. Acad. Sci. USA 1989, 86, 9159–9162. [Google Scholar] [CrossRef] [Green Version]
- Baek, M.H.; Kamiya, M.; Kushibiki, T.; Nakazumi, T.; Tomisawa, S.; Abe, C.; Kumaki, Y.; Kikukawa, T.; Demura, M.; Kawano, K.; et al. Lipopolysaccharide-bound structure of the antimicrobial peptide cecropin P1 determined by nuclear magnetic resonance spectroscopy. J. Pept. Sci. 2016, 22, 214–221. [Google Scholar] [CrossRef] [Green Version]
- Guo, C.; Huang, Y.; Cong, P.; Liu, X.; Chen, Y.; He, Z. Cecropin P1 inhibits porcine reproductive and respiratory syndrome virus by blocking attachment. BMC Microbiol. 2014, 14, 273. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Zhong, F.; Zhang, Y.; Zhang, J.; Huo, S.; Lin, H.; Wang, L.; Cui, D.; Li, X. Construction of Bacillus subtilis strain engineered for expression of porcine β-defensin-2/cecropin P1 fusion antimicrobial peptides and its growth-promoting effect and antimicrobial activity. Asian-Australas J. Anim. Sci. 2017, 30, 576–584. [Google Scholar] [CrossRef] [Green Version]
- Sarmasik, A.; Chen, T.T. Bactericidal activity of cecropin B and cecropin P1 expressed in fish cells (CHSE-214): Application in controlling fish bacterial pathogens. Aquaculture 2003, 220, 183–194. [Google Scholar] [CrossRef]
- Zanetti, M.; Storici, P.; Tossi, A.; Scocchi, M.; Gennaro, R. Molecular cloning and chemical synthesis of a novel antibacterial peptide derived from pig myeloid cells. J. Biol. Chem. 1994, 269. [Google Scholar] [CrossRef]
- Lee, D.G.; Kim, D.H.; Park, Y.; Kim, H.K.; Kim, H.N.; Shin, Y.K.; Choi, C.H.; Hahm, K.S. Fungicidal effect of antimicrobial peptide, PMAP-23, isolated from porcine myeloid against Candida albicans. Biochem. Biophys. Res. Commun. 2001, 282, 570–574. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Jang, S.H.; Lee, D.G.; Hahm, K.S. Antinematodal effect of antimicrobial peptide, PMAP-23, isolated from porcine myeloid against Caenorhabditis elegans. J. Pept. Sci. 2004, 10, 304–311. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, H.; Jia, Z.; Ma, Q.; Dong, N.; Shan, A. In vitro and in vivo activity of the dimer of PMAP-36 expressed in Pichia pastoris. J. Mol. Microbiol. Biotechnol. 2014, 24, 234–240. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Hiraiwa, H.; Yasue, H.; Wu, H.; Ross, C.R.; Troyer, D.; Blecha, F. Cloning and characterization of the gene for a new epithelial β- defensin. Genomic structure, chromosomal localization, and evidence for its constitutive expression. J. Biol. Chem. 1999, 274, 24031–24037. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, Z.; Wang, A.; Feng, Q.; Wang, Z.; Ivanova, I.V.; He, X.; Zhang, B.; Song, W. High-level expression, purification and characterisation of porcine β-defensin 2 in Pichia pastoris and its potential as a cost-efficient growth promoter in porcine feed. Appl. Microbiol. Biotechnol. 2014, 98, 5487–5497. [Google Scholar] [CrossRef]
- Veldhuizen, E.J.A.; Rijnders, M.; Claassen, E.A.; van Dijk, A.; Haagsman, H.P. Porcine β-defensin 2 displays broad antimicrobial activity against pathogenic intestinal bacteria. Mol. Immunol. 2008, 45, 386–394. [Google Scholar] [CrossRef]
- Peng, Z.; Wang, A.; Xie, L.; Song, W.; Wang, J.; Yin, Z.; Zhou, D.; Li, F. Use of recombinant porcine β-defensin 2 as a medicated feed additive for weaned piglets. Sci. Rep. 2016, 6, 26790. [Google Scholar] [CrossRef]
- Bao, Y.Y.; Li, L.; Zhang, H.; Gao, C.Y.; Xiao, C.B.; Li, C.L. Preparation of polyclonal antibody against porcine beta defensin 2 and identification of its distribution in tissues of pig. Genet. Mol. Res. 2015, 14, 18863–18871. [Google Scholar] [CrossRef]
- Shamova, O.; Brogden, K.A.; Zhao, C.; Nguyen, T.; Kokryakov, V.N.; Lehrer, R.I. Purification and properties of proline-rich antimicrobial peptides from sheep and goat leukocytes. Infect. Immun. 1999, 67, 4106–4111. [Google Scholar] [CrossRef] [Green Version]
- Shamova, O.; Orlov, D.; Stegemann, C.; Czihal, P.; Hoffmann, R.; Brogden, K.; Kolodkin, N.; Sakuta, G.; Tossi, A.; Sahl, H.G.; et al. ChBac3.4: A novel proline-rich antimicrobial peptide from goat leukocytes. Int. J. Pept. Res. Ther. 2009, 15, 31–42. [Google Scholar] [CrossRef]
- Shamova, O.V.; Orlov, D.S.; Zharkova, M.S.; Balandin, S.V.; Yamschikova, E.V.; Knappe, D.; Hoffmann, R.; Kokryakov, V.N.; Ovchinnikova, T.V. Minibactenecins ChBac7.Nα and ChBac7. Nβ Antimicrobial Peptides from leukocytes of the goat Capra hircus. Acta Naturae 2016, 8. [Google Scholar] [CrossRef]
- Huttner, K.M.; Brezinski-Caliguri, D.J.; Mahoney, M.M.; Diamond, G. Antimicrobial Peptide Expression Is Developmentally Regulated in the Ovine Gastrointestinal Tract. J. Nutr. 1998, 128, 297S–299S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anderson, R.C.; Hancock, R.E.W.; Yu, P.L. Antimicrobial Activity and Bacterial-Membrane Interaction of Ovine-Derived Cathelicidins. Antimicrob. Agents Chemother. 2004, 48, 673–676. [Google Scholar] [CrossRef] [Green Version]
- Skerlavaj, B.; Benincasa, M.; Risso, A.; Zanetti, M.; Gennaro, R. SMAP-29: A potent antibacterial and antifungal peptide from sheep leukocytes. FEBS Lett. 1999, 463, 58–62. [Google Scholar] [CrossRef] [Green Version]
- Huttner, K.M.; Lambeth, M.R.; Burkin, H.R.; Burkin, D.J.; Broad, T.E. Localization and genomic organization of sheep antimicrobial peptide. Gene 1998, 206, 85–91. [Google Scholar] [CrossRef]
- Ackermann, M.R.; Gallup, J.M.; Zabner, J.; Evans, R.B.; Brockus, C.W.; Meyerholz, D.K.; Grubor, B.; Brogden, K.A. Differential expression of sheep beta-defensin-1 and -2 and interleukin 8 during acute Mannheimia haemolytica pneumonia. Microb. Pathog. 2004, 37, 21–27. [Google Scholar] [CrossRef] [Green Version]
- Zhao, P.; Cao, G. Production of bioactive sheep β-defensin-1 in Pichia pastoris. J. Ind. Microbiol. Biotechnol. 2012, 39, 11–17. [Google Scholar] [CrossRef]
- Thomä-Worringer, C.; Sørensen, J.; López-Fandiño, R. Health effects and technological features of caseinomacropeptide. Int. Dairy J. 2006, 16, 1324–1333. [Google Scholar] [CrossRef]
- Malkoski, M.; Dashper, S.G.; O’Brien-Simpson, N.M.; Talbo, G.H.; Macris, M.; Cross, K.J.; Reynolds, E.C. Kappacin, a novel antibacterial peptide from bovine milk. Antimicrob. Agents Chemother. 2001, 45, 2723–2732. [Google Scholar] [CrossRef] [Green Version]
- Haque, E.; Chand, R. Antihypertensive and antimicrobial bioactive peptides from milk proteins. Eur. Food Res. Technol. 2008, 227, 7–15. [Google Scholar] [CrossRef]
- Hayes, M.; Ross, R.P.; Fitzgerald, G.F.; Hill, C.; Stanton, C. Casein-derived antimicrobial peptides generated by Lactobacillus acidophilus DPC6026. Appl. Environ. Microbiol. 2006, 72, 2260–2264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zucht, H.D.; Raida, M.; Adermann, K.; Mägert, H.J.; Forssmann, W.G. Casocidin-I: A casein-αs2 derived peptide exhibits antibacterial activity. FEBS Lett. 1995, 372, 185–188. [Google Scholar] [CrossRef] [Green Version]
- Pellegrini, A.; Thomas, U.; Bramaz, N.; Hunziker, P.; Von Fellenberg, R. Isolation and identification of three bactericidal domains in the bovine α-lactalbumin molecule. Biochim. Biophys. Acta Gen. Subj. 1999, 1426, 439–448. [Google Scholar] [CrossRef]
- Pellegrini, A.; Dettling, C.; Thomas, U.; Hunziker, P. Isolation and characterization of four bactericidal domains in the bovine β-lactoglobulin. Biochim. Biophys. Acta Gen. Subj. 2001, 1526, 131–140. [Google Scholar] [CrossRef]
- Tidona, F.; Criscione, A.; Guastella, A.M.; Zuccaro, A.; Bordonaro, S.; Marletta, D. Bioactive peptides in dairy products. Ital. J. Anim. Sci. 2009, 8, 315–340. [Google Scholar] [CrossRef]
- Van Der Kraan, M.I.A.; Groenink, J.; Nazmi, K.; Veerman, E.C.I.; Bolscher, J.G.M.; Nieuw Amerongen, A.V. Lactoferrampin: A novel antimicrobial peptide in the N1-domain of bovine lactoferrin. Peptides 2004, 25, 177–183. [Google Scholar] [CrossRef]
- Wang, J.; Liu, Q.; Tian, Y.; Jian, Z.; Li, H.; Wang, K. Biodegradable hydrophilic polyurethane PEGU25 loading antimicrobial peptide Bmap-28: A sustained-release membrane able to inhibit bacterial biofilm formation in vitro. Sci. Rep. 2015, 5, 8634. [Google Scholar] [CrossRef] [Green Version]
- Zhang, D.; Wan, L.; Zhang, J.; Liu, C.; Sun, H. Effect of BMAP-28 on human thyroid cancer TT cells is mediated by inducing apoptosis. Oncol. Lett. 2015, 10, 2620–2626. [Google Scholar] [CrossRef] [Green Version]



| AMPs | Source | Clinical Phase | Indication | Administration | Clinical Identifier |
|---|---|---|---|---|---|
| MBI-226Omignan | Indolicidin(Bovine) | Phase III | Prevention of local catheter site infection and colonization in patients with central venous catheters | Topical gel | NCT00231153 |
| CLS001Omignan | Phase III | Severe papulopustular rosacea | Topical gel | NCT02576860 | |
| MX942 (SGX942) | Phase III | Immunomodulation during oral mucositis | Intravenous infusion | NCT03237325 | |
| Omignan | Phase II | Seborrheic Dermatitis | Topical Gel | NCT03688971 | |
| IB-367Isegnan | Protegrin-1 Derivative | Phase III | Oral mucositis in the patient receiving radiation therapy for head and neck cancer | Oral solution | NCT00022373 |
| POL7080 | Protegrin-1Analog | Phase II | Non-cystic fibrosis bronchiectasis caused by Pseudomonas aeruginosa infection. | Intravenous infusion | NCT02096328 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, R.; Ali, S.A.; Singh, S.K.; Bhushan, V.; Mathur, M.; Jamwal, S.; Mohanty, A.K.; Kaushik, J.K.; Kumar, S. Antimicrobial Peptides in Farm Animals: An Updated Review on Its Diversity, Function, Modes of Action and Therapeutic Prospects. Vet. Sci. 2020, 7, 206. https://doi.org/10.3390/vetsci7040206
Kumar R, Ali SA, Singh SK, Bhushan V, Mathur M, Jamwal S, Mohanty AK, Kaushik JK, Kumar S. Antimicrobial Peptides in Farm Animals: An Updated Review on Its Diversity, Function, Modes of Action and Therapeutic Prospects. Veterinary Sciences. 2020; 7(4):206. https://doi.org/10.3390/vetsci7040206
Chicago/Turabian StyleKumar, Rohit, Syed Azmal Ali, Sumit Kumar Singh, Vanya Bhushan, Manya Mathur, Shradha Jamwal, Ashok Kumar Mohanty, Jai Kumar Kaushik, and Sudarshan Kumar. 2020. "Antimicrobial Peptides in Farm Animals: An Updated Review on Its Diversity, Function, Modes of Action and Therapeutic Prospects" Veterinary Sciences 7, no. 4: 206. https://doi.org/10.3390/vetsci7040206







