Autologous Bone Marrow Mononuclear Cells (BMMCs) for the Treatment of Uncomplicated Grade 2 Ununited Anconeal Process (UAP) in Six Dogs: Preliminary Results
Abstract
:1. Introduction
2. Materials and Methods
2.1. Inclusion Criteria
2.2. Clinical Examination
2.3. X-ray and CT Evaluation
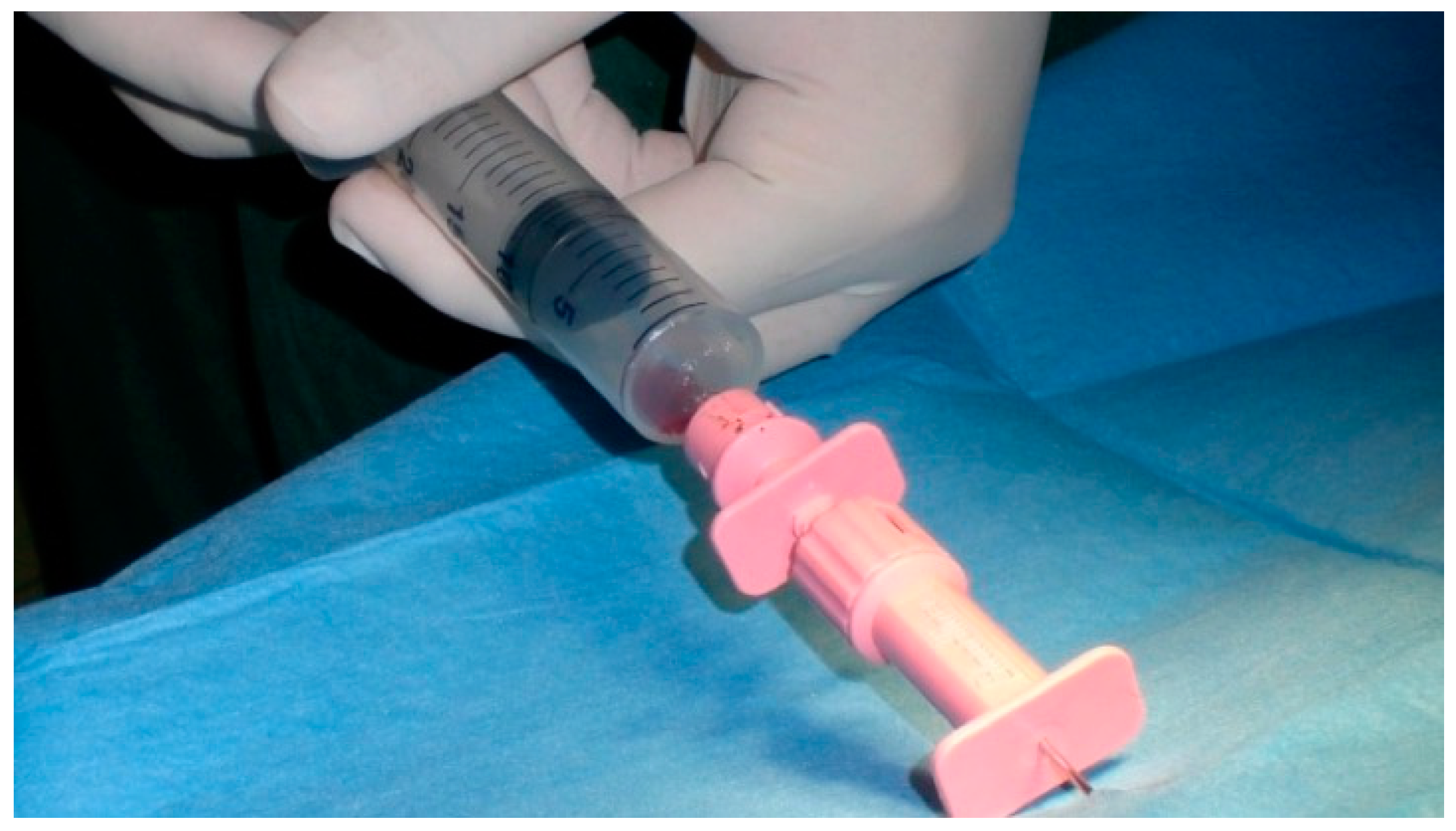
2.4. Bone Marrow Harvesting
2.5. Bone Marrow Processing and BMMC Isolation
2.6. BMMC Injection
2.7. Post-Surgical Regimen
2.8. Complications
3. Results
3.1. X-ray Results
3.2. CT Results
3.3. Bone Marrow Results
3.4. Follow-Up
3.5. Microradiographic Assessment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stiern, R.A. Ectopic sesamoid bones at the elbow (Patella cubiti) of the dog. J. Am. Veter-Med. Assoc. 1956, 128, 498–501. [Google Scholar]
- Lenehan, T.M.; Van Sickle, D.C. Ununited Anconeal Process, Ununited Coroneal Process, Ununited Medial Epicondyle, Patella Cubiti and Sesamoidale Fragments of the Elbow; JB Lippincott Company: Philadelphia, PA, USA, 1985. [Google Scholar]
- Corley, E.A.; Sutherland, T.M.; Carlson, W.D. Genetic aspects of canine elbow dysplasia. J. Am. Veter-Med. Assoc. 1968, 153, 543–547. [Google Scholar]
- Harasen, G. Orthopedics: Ununited anconeal process. Can. Vet. J. 2009, 50, 877–878. [Google Scholar] [PubMed]
- Swenson, L.; Audell, L.; Hedhammar, A. Prevalence and inheritance of and selection for elbow arthrosis in Bernese mountain dogs and Rottweilers in Sweden and benefit: Cost analysis of a screening and control program. J. Am. Veter-Med. Assoc. 1997, 210, 215–221. [Google Scholar]
- Olsson, S.E. Osteochondrosis of the canine elbow joint. Pathogenesis and a new approach to the surgical treatment of UAP. In Proceedings of the 18th Congress-European Society of Veterinary Surgery, Uppsala, Sweden, 8–9 June 1990. [Google Scholar]
- Hazewinkel, H.A.W. Nutrition in relation to skeletal growth deformities. J. Small Anim. Pr. 1989, 30, 625–630. [Google Scholar] [CrossRef]
- Hazewinkel, H.A.W. Nutrition in Orthopedics. In Disease Mechanisms in Small Animal Surgery; Febiger, B.M.e.L.a.: Philadelphia, PA, USA, 1993. [Google Scholar]
- Grøndalen, J.; Lingaas, F. Arthrosis of the elbow joint among Rottweiler dogs. Results from investigations into hereditary disposition. Tijdschr. Voor Diergeneeskd. 1988, 113, 49S–51S. [Google Scholar]
- Wind, A.P.; Packard, M.E. Elbow incongruity and development elbow disease in the dog: Part I. J. Am. Hosp. Assoc. 1986, 22, 711–724. [Google Scholar]
- Sjöström, L.; Kasström, H.; Källberg, M. Ununited anconeal process in the dog. Pathogenesis and treatment by osteotomy of the ulna. Vet. Comp. Orthop. Traumatol. 1995, 8, 170–176. [Google Scholar]
- Vezzoni, A.; Benjamino, K. Canine Elbow Dysplasia. Veter-Clin. North Am. Small Anim. Pract. 2021, 51, 439–474. [Google Scholar] [CrossRef]
- Bardet, J. Classification and treatment of ununited anconeal process in dogs. In Proceedings of the 9th ESVOT Congress, Munich, Germany, 16–19 April 1998. [Google Scholar]
- Turner, B.M.; Abercromby, R.H.; Innes, J.; McKee, W.M.; Ness, M.G. Dynamic Proximal Ulnar Osteotomy for the Treatment of Ununited Anconeal Process in 17 Dogs. Veter-Comp. Orthop. Traumatol. 1998, 11, 76–79. [Google Scholar] [CrossRef]
- Ferrigno, C.R.; Schmaedecke, A.; Sterman, F.A.; Lincoln, J. Treatment of ununited anconeal process in 8 dogs by osteotomy and dynamic distraction of the proximal part of the ulna. Pesqui. Veterinária Bras. 2007, 27, 352–356. [Google Scholar] [CrossRef]
- Fox, S.M.; Burbidge, H.M.; Bray, J.C.; Guerin, S.R. Ununited anconeal process: Lag-screw fixation. J. Am. Anim. Hosp. Assoc. 1996, 32, 52–56. [Google Scholar] [CrossRef]
- Pettitt, R.A.; Tattersall, J.; Gemmill, T.; Butterworth, S.J.; O’Neill, T.J.; Langley-Hobbs, S.J.; Comerford, E.J.; Innes, J.F. Effect of surgical technique on radiographic fusion of the anconeus in the treatment of ununited anconeal process. J. Small Anim. Pract. 2009, 50, 545–548. [Google Scholar] [CrossRef]
- Hulse, D.A.; Bähr, A.; Jerram, R.M.; Krotscheck, U. Ununited anconeal process: Lag-screw fixation with proximal ulnar osteotomy. Veter-Comp. Orthop. Traumatol. 2000, 13, 212–216. [Google Scholar] [CrossRef]
- Gangji, V.; Hauzeur, J.-P.; Matos, C.; De Maertelaer, V.; Toungouz, M.; Lambermont, M. Treatment of Osteonecrosis of the Femoral Head with Implantation of Autologous Bone-Marrow Cells. JBJS 2004, 86, 1153–1160. [Google Scholar] [CrossRef] [Green Version]
- Körbling, M.; Estrov, Z. Adult Stem Cells for Tissue Repair—A New Therapeutic Concept? N. Engl. J. Med. 2003, 349, 570–582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cabrolier, J.; Molina, M. Is instillation of bone marrow stem cells at the time of core decompression useful for osteonecrosis of the femoral head? Medwave 2016, 16, e6406. [Google Scholar] [CrossRef]
- Jin, H.; Xia, B.; Yu, N.; He, B.; Shen, Y.; Xiao, L.; Tong, P. The effects of autologous bone marrow mesenchymal stem cell arterial perfusion on vascular repair and angiogenesis in osteonecrosis of the femoral head in dogs. Int. Orthop. 2012, 36, 2589–2596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, B.-L.; Sun, W.; Shi, Z.-C.; Zhang, N.-F.; Yue, D.-B.; Guo, W.-S.; Xu, S.-Q.; Lou, J.-N.; Li, Z.-R. Treatment of nontraumatic osteonecrosis of the femoral head with the implantation of core decompression and concentrated autologous bone marrow containing mononuclear cells. Arch. Orthop. Trauma Surg. 2009, 130, 859–865. [Google Scholar] [CrossRef] [PubMed]
- Crovace, A.; Favia, A.; Lacitignola, L.; Di Comite, M.S.; Staffieri, F.; Francioso, E. Use of autologous bone marrow mononuclear cells and cultured bone marrow stromal cells in dogs with orthopaedic lesions. Veter-Res. Commun. 2008, 32, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Crovace, A.; Lacitignola, L.; Rossi, G.; Francioso, E. Histological and Immunohistochemical Evaluation of Autologous Cultured Bone Marrow Mesenchymal Stem Cells and Bone Marrow Mononucleated Cells in Collagenase-Induced Tendinitis of Equine Superficial Digital Flexor Tendon. Veter-Med. Int. 2010, 2010, 250978. [Google Scholar] [CrossRef] [Green Version]
- Luzzi, S.; Crovace, A.M.; Del Maestro, M.; Lucifero, A.G.; Elbabaa, S.K.; Cinque, B.; Palumbo, P.; Lombardi, F.; Cimini, A.; Cifone, M.G.; et al. The cell-based approach in neurosurgery: Ongoing trends and future perspectives. Heliyon 2019, 5, e02818. [Google Scholar] [CrossRef] [PubMed]
- Sabino, L.; Maria, C.; Luca, L.; Valerio, V.; Edda, F.; Giacomo, R.; Gloria, I.; Juan, G.; Antonio, C. Engraftment, neuroglial transdifferentiation and behavioral recovery after complete spinal cord transection in rats. Surg. Neurol. Int. 2018, 9, 19. [Google Scholar] [CrossRef]
- Crovace, A.M.; Lacitignola, L.; Staffieri, F.; Francioso, E.; Rossi, G.; Crovace, A. Treatment of Monolateral Legg-Calvé-Perthes Disease with Autologous Bone Marrow Mononuclear Cells in 32 Dogs. VCOT Open 2020, 3, e1–e10. [Google Scholar] [CrossRef] [Green Version]
- Hazewinkel, H.A.W. Screening for Elbow Dysplasia, grading according to the IEWG. In Proceedings of the 30th Annual Meeting IEWG, Vienna, Austria, 23 June 2006; pp. 8–13. [Google Scholar]
- Ohlerth, S.; Tellhelm, B.; Amort, K.; Ondreka, N. Explanation of the IEWG grading system. In Proceedings of the 30th Annual Meeting IEWG, Vienna, Austria, 23 June 2006; pp. 14–16. [Google Scholar]
- Samoy, Y.; Gielen, I.; van Bree, H.; Van Ryssen, B. Dysplastic elbow diseases in dogs. Vlaams Diergeneeskd. Tijdschr. 2011, 80, 327–338. [Google Scholar]
- Wind, A.P.; Packard, M. Elbow incongruity and developmental elbow diseases in the dog: Part II. J. Am. Anim. Hosp. Assoc. 1986, 22, 725–730. [Google Scholar]
- Wang, D.; Zhang, H.; Liang, J.; Li, X.; Feng, X.; Wang, H.; Hua, B.; Liu, B.; Lu, L.; Gilkeson, G.S.; et al. Allogeneic Mesenchymal Stem Cell Transplantation in Severe and Refractory Systemic Lupus Erythematosus: 4 Years of Experience. Cell Transplant. 2013, 22, 2267–2277. [Google Scholar] [CrossRef]
- Wang, M.; Liao, Q.; Zhou, B.; Qiu, Z.-Q.; Cheng, L.-M. Preliminary study of influence of bone tissue from osteonecrosis of femoral head on the proliferation and differentiation of canine bone marrow mesenchymal stem cells. Zhonghua Yi Xue Za Zhi 2013, 93, 856–859. [Google Scholar]
- Murphy, M.B.; Moncivais, K.; Caplan, A. Mesenchymal stem cells: Environmentally responsive therapeutics for regenerative medicine. Exp. Mol. Med. 2013, 45, e54. [Google Scholar] [CrossRef] [Green Version]
- Jiang, T.; Xu, G.; Wang, Q.; Yang, L.; Zheng, L.; Zhao, J.; Zhang, X. Correction: In vitro expansion impaired the stemness of early passage mesenchymal stem cells for treatment of cartilage defects. Cell Death Dis. 2019, 10, 716. [Google Scholar] [CrossRef] [Green Version]
- Imam, M.A.; Holton, J.; Ernstbrunner, L.; Pepke, W.; Grubhofer, F.; Narvani, A.; Snow, M. A systematic review of the clinical applications and complications of bone marrow aspirate concentrate in management of bone defects and nonunions. Int. Orthop. 2017, 41, 2213–2220. [Google Scholar] [CrossRef]
- Hernigou, J.; Picard, L.; Alves, A.; Silvera, J.; Homma, Y.; Hernigou, P. Understanding bone safety zones during bone marrow aspiration from the iliac crest: The sector rule. Int. Orthop. 2014, 38, 2377–2384. [Google Scholar] [CrossRef]
- Bain, B.J. Bone marrow biopsy morbidity and mortality. Br. J. Haematol. 2003, 121, 949–951. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burkle, C.M.; Harrison, B.A.; Koenig, L.F.; Decker, P.A.; Warner, D.O.; Gastineau, D.A. Morbidity and mortality of deep sedation in outpatient bone marrow biopsy. Am. J. Hematol. 2004, 77, 250–256. [Google Scholar] [CrossRef]
- Husebye, E.E.; Lyberg, T.; Røise, O. Bone marrow fat in the circulation: Clinical entities and pathophysiological mechanisms. Injury 2006, 37, S8–S18. [Google Scholar] [CrossRef]
- Orlowski, J.P.; Julius, C.J.; E Petras, R.; Porembka, D.T.; Gallagher, J.M. The safety of intraosseous infusions: Risks of fat and bone marrow emboli to the lungs. Ann. Emerg. Med. 1989, 18, 1062–1067. [Google Scholar] [CrossRef]
- Hernigou, P.; Beaujean, F.; Lambotte, J.C. Decrease in the mesenchymal stem-cell pool in the proximal femur in corticosteroid-induced osteonecrosis. J. Bone Jt. Surgery. Br. Vol. 1999, 81, 349–355. [Google Scholar] [CrossRef]




| X-ray | Surgery | |
|---|---|---|
| Grade 1 (1°) | Opaque fissure | Completely attached anconeal process |
| Grades 2 and 3 (2–3°) | Transparent fissure | Low mobility anconeal process |
| Grades 4 and 5 (4–5°) | Incongruous anconeal process with dislocation and bone reabsorption | Detached anconeal process |
| Variable | Mean | St Dev | Median | Interquantile Range |
|---|---|---|---|---|
| Age (months) | 7.7 | 0.8 | 7.5 | 2.0 |
| Bone marrow volume (ml) | 34.33 | 5.96 | 35.00 | 16.00 |
| No. BMMCs | 2.57 × 107 | 1.30 × 107 | 2.30 × 107 | 3.70 × 107 |
| UFC/ml | 528.5 | 224.3 | 495 | 500 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Crovace, A.M.; Lacitignola, L.; Di Comite, M.; Esposito, C.; Guarracino, A.; Francioso, E.; Staffieri, F.; Crovace, A. Autologous Bone Marrow Mononuclear Cells (BMMCs) for the Treatment of Uncomplicated Grade 2 Ununited Anconeal Process (UAP) in Six Dogs: Preliminary Results. Vet. Sci. 2021, 8, 214. https://doi.org/10.3390/vetsci8100214
Crovace AM, Lacitignola L, Di Comite M, Esposito C, Guarracino A, Francioso E, Staffieri F, Crovace A. Autologous Bone Marrow Mononuclear Cells (BMMCs) for the Treatment of Uncomplicated Grade 2 Ununited Anconeal Process (UAP) in Six Dogs: Preliminary Results. Veterinary Sciences. 2021; 8(10):214. https://doi.org/10.3390/vetsci8100214
Chicago/Turabian StyleCrovace, Alberto Maria, Luca Lacitignola, Mariasevera Di Comite, Cosimo Esposito, Alessandro Guarracino, Edda Francioso, Francesco Staffieri, and Antonio Crovace. 2021. "Autologous Bone Marrow Mononuclear Cells (BMMCs) for the Treatment of Uncomplicated Grade 2 Ununited Anconeal Process (UAP) in Six Dogs: Preliminary Results" Veterinary Sciences 8, no. 10: 214. https://doi.org/10.3390/vetsci8100214
APA StyleCrovace, A. M., Lacitignola, L., Di Comite, M., Esposito, C., Guarracino, A., Francioso, E., Staffieri, F., & Crovace, A. (2021). Autologous Bone Marrow Mononuclear Cells (BMMCs) for the Treatment of Uncomplicated Grade 2 Ununited Anconeal Process (UAP) in Six Dogs: Preliminary Results. Veterinary Sciences, 8(10), 214. https://doi.org/10.3390/vetsci8100214







