Changes in Serum Lipid Profiles among Canine Patients Suffering from Chronic Hepatitis
Abstract
:1. Introduction
2. Materials and Methods
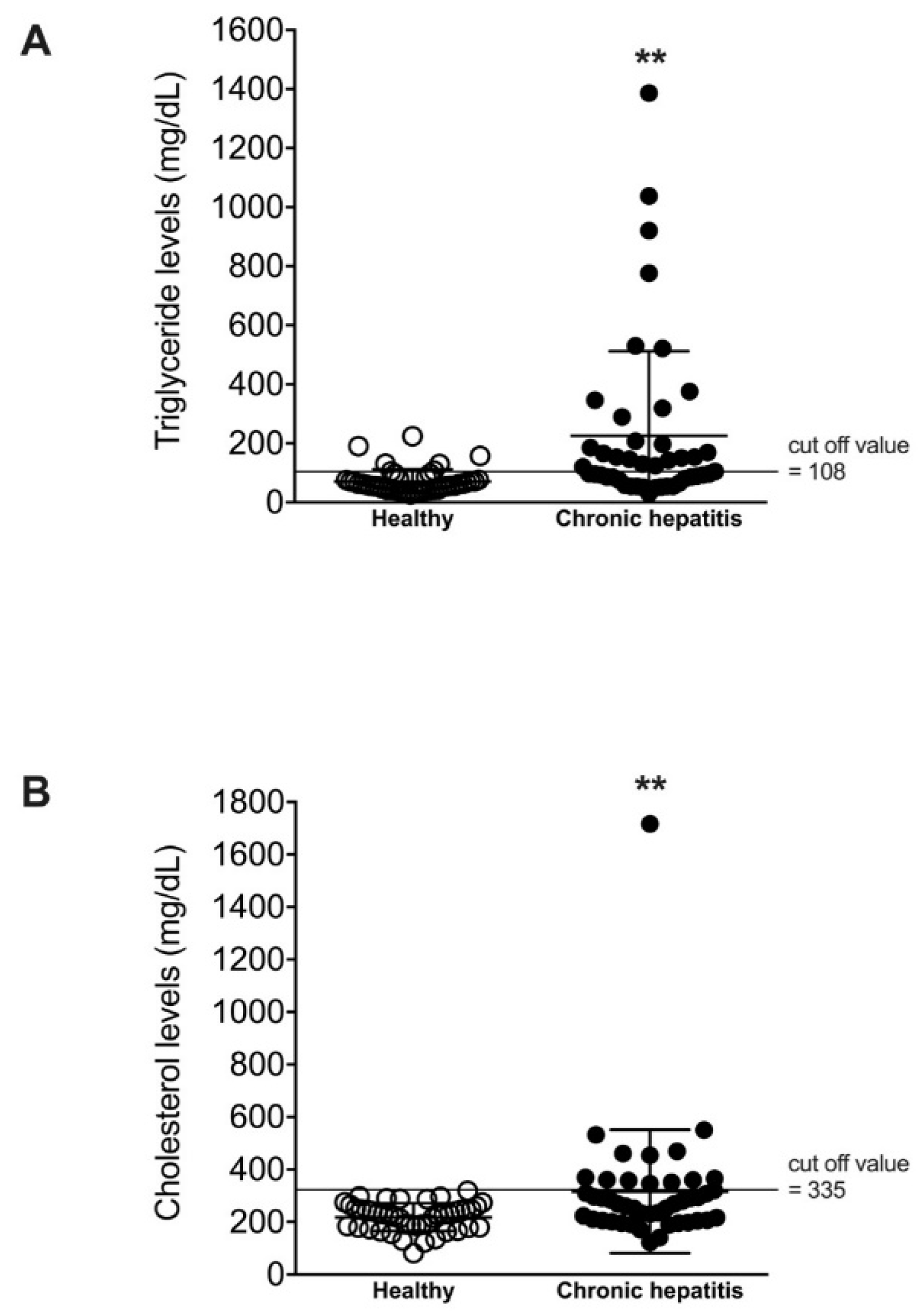
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Assawarachan, S.N.; Maneesaay, P.; Thengchaisri, N. A descriptive study of the histopathologic and biochemical liver test abnormalities in dogs with liver disease in Thailand. Can. J. Vet. Res. 2020, 84, 217–224. [Google Scholar]
- Bexfield, N. Canine Idiopathic Chronic Hepatitis. Vet. Clin. N. Am. Small Anim. Pract. 2017, 47, 645–663. [Google Scholar] [CrossRef] [Green Version]
- Poldervaart, J.H.; Favier, R.P.; Penning, L.C.; van den Ingh, T.S.; Rothuizen, J. Primary hepatitis in dogs: A retrospective review (2002–2006). J. Vet. Intern. Med. 2009, 23, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Webster, C.R.; Center, S.A.; Cullen, J.M.; Penninck, D.G.; Richter, K.P.; Twedt, D.C.; Watson, P.J. ACVIM consensus statement on the diagnosis and treatment of chronic hepatitis in dogs. J. Vet. Intern. Med. 2019, 33, 1173–1200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watson, T.D.G.; Barrie, J. Lipoprotein metabolism and hyperlipidaemia in the clog and cat: A review. J. Small Anim. Pract. 1993, 34, 479–487. [Google Scholar] [CrossRef]
- Lei, S.; Sun, R.Z.; Wang, D.; Gong, M.Z.; Su, X.P.; Yi, F.; Peng, Z.W. Increased Hepatic Fatty Acids Uptake and Oxidation by LRPPRC-Driven Oxidative Phosphorylation Reduces Blood Lipid Levels. Front. Physiol. 2016, 7, 270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ginsberg, H.N. Lipoprotein physiology. Endocrinol. Meta.b Clin. N. Am. 1998, 27, 503–519. [Google Scholar] [CrossRef]
- Nauck, M.; Graziani, M.S.; Jarausch, J.; Bruton, D.; Cobbaert, C.; Cole, T.G.; Colella, F.; Lefevre, F.; Gillery, P.; Haas, B.; et al. A new liquid homogeneous assay for HDL cholesterol determination evaluated in seven laboratories in Europe and the United States. Clin. Chem. Lab. Med. 1999, 37, 1067–1076. [Google Scholar] [CrossRef]
- Xenoulis, P.G.; Steiner, J.M. Lipid metabolism and hyperlipidemia in dogs. Vet. J. 2010, 183, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Xenoulis, P.G.; Steiner, J.M. Canine hyperlipidaemia. J. Small Anim. Pract. 2015, 56, 595–605. [Google Scholar] [CrossRef] [Green Version]
- Zelber-Sagi, S.; Nitzan-Kaluski, D.; Halpern, Z.; Oren, R. Prevalence of primary non-alcoholic fatty liver disease in a population-based study and its association with biochemical and anthropometric measures. Liver Int. Off. J. Int. Assoc. Study Liver 2006, 26, 856–863. [Google Scholar] [CrossRef]
- Reid, A.E. Nonalcoholic steatohepatitis. Gastroenterology 2001, 121, 710–723. [Google Scholar] [CrossRef]
- Harrison, J.L.; Turek, B.J.; Brown, D.C.; Bradley, C.; Callahan Clark, J. Cholangitis and Cholangiohepatitis in Dogs: A Descriptive Study of 54 Cases Based on Histopathologic Diagnosis (2004–2014). J. Vet. Intern. Med. 2018, 32, 172–180. [Google Scholar] [CrossRef] [Green Version]
- Allerton, F.; Swinbourne, F.; Barker, L.; Black, V.; Kathrani, A.; Tivers, M.; Henriques, T.; Kisielewicz, C.; Dunning, M.; Kent, A. Gall bladder mucoceles in Border terriers. J. Vet. Intern. Med. 2018, 32, 1618–1628. [Google Scholar] [CrossRef]
- Lee, S.; Kweon, O.K.; Kim, W.H. Associations between serum leptin levels, hyperlipidemia, and cholelithiasis in dogs. PLoS ONE 2017, 12, e0187315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van den Ingh, T.S.G.A.M.; Van Winkle, T.; Cullen, J.M.; Charles, J.A.; Desmet, V.J. Morphological Classification of Parenchymal Disorders of the Canine and Feline Liver. In WSAVA Standards for Clinical and Histological Diagnosis of Canine and Feline Liver Diseases; Rothuizen, J., Bunch, S.E., Charles, J.A., Cullen, J.M., Desmet, V.J., Szatmari, V., Twedt, D.C., van den Ingh, T.S.G.A.M., van Winkle, T., Washabau, R.J., Eds.; Saunders Elsevier: Philadelphia, PA, USA, 2009; pp. 85–101. [Google Scholar]
- Assawarachan, S.N.; Chuchalermporn, P.; Maneesaay, P.; Thengchaisri, N. Evaluation of hepatobiliary ultrasound scores in healthy dogs and dogs with liver diseases. Vet. World 2019, 12, 1266–1272. [Google Scholar] [CrossRef]
- Kawasumi, K.; Kashiwado, N.; Okada, Y.; Sawamura, M.; Sasaki, Y.; Iwazaki, E.; Mori, N.; Yamamoto, I.; Arai, T. Age effects on plasma cholesterol and triglyceride profiles and metabolite concentrations in dogs. BMC Vet. Res. 2014, 10, 57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, G.; Al-Shali, K.Z.; Hegele, R.A. Hypertriglyceridemia: Its etiology, effects and treatment. Cmaj 2007, 176, 1113–1120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jahn, C.E.; Schaefer, E.J.; Taam, L.A.; Hoofnagle, J.H.; Lindgren, F.T.; Albers, J.J.; Jones, E.A.; Brewer, H.B., Jr. Lipoprotein abnormalities in primary biliary cirrhosis. Association with hepatic lipase inhibition as well as altered cholesterol esterification. Gastroenterology 1985, 89, 1266–1278. [Google Scholar] [CrossRef]
- Müller, P.; Felin, R.; Lambrecht, J.; Agostini, B.; Wieland, H.; Rost, W.; Seidel, D. Hypertriglyceridaemia secondary to liver disease. Eur. J. Clin. Investig. 1974, 4, 419–428. [Google Scholar] [CrossRef]
- Havel, R.J. Functional activities of hepatic lipoprotein receptors. Annu. Rev. Physiol. 1986, 48, 119–134. [Google Scholar] [CrossRef]
- Boisclair, J.; Doré, M.; Beauchamp, G.; Chouinard, L.; Girard, C. Characterization of the inflammatory infiltrate in canine chronic hepatitis. Vet. Pathol. 2001, 38, 628–635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McIntyre, N.; Harry, D.S.; Pearson, A.J. The hypercholesterolaemia of obstructive jaundice. Gut 1975, 16, 379–391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Danielsson, B.; Ekman, R.; Johansson, B.G.; Petersson, B.G. Plasma lipoprotein changes in experimental cholestasis in the dog. Clin. Chim. Acta 1977, 80, 157–170. [Google Scholar] [CrossRef]
- Whitney, M.S. Evaluation of hyperlipidemias in dogs and cats. Semin. Vet. Med. Surg. Small Anim. 1992, 7, 292–300. [Google Scholar] [PubMed]
- Chuang, J.H.; Shieh, C.S.; Chang, N.K.; Chen, W.J.; Lo, S.K. Metabolic effect of parenteral nutrition in dogs with obstructive jaundice. J. Am. Coll. Nutr. 1995, 14, 197–201. [Google Scholar] [CrossRef] [PubMed]
- Walli, A.K.; Seidel, D. Role of lipoprotein-X in the pathogenesis of cholestatic hypercholesterolemia. Uptake of lipoprotein-X and its effect on 3-hydroxy-3-methylglutaryl coenzyme A reductase and chylomicron remnant removal in human fibroblasts, lymphocytes, and in the rat. J. Clin. Investig. 1984, 74, 867–879. [Google Scholar] [CrossRef] [Green Version]
- Blomhoff, J.P.; Holme, R.; Ostrem, T. Plasma cholesterol esterification and plasma lipoproteins in bile-duct-ligated dogs. Scand. J. Gastroenterol. 1978, 13, 693–702. [Google Scholar] [CrossRef]
- Unger, L.W.; Forstner, B.; Schneglberger, S.; Muckenhuber, M.; Eigenbauer, E.; Scheiner, B.; Mandorfer, M.; Trauner, M.; Reiberger, T. Patterns and prevalence of dyslipidemia in patients with different etiologies of chronic liver disease. Wien. Klin. Wochenschr. 2019, 131, 395–403. [Google Scholar] [CrossRef] [Green Version]
- Chapman, S.E.; Hostutler, R.A. A laboratory diagnostic approach to hepatobiliary disease in small animals. Vet. Clin. N. Am. Small Anim. Pract. 2013, 43, 1209–1225, v. [Google Scholar] [CrossRef]
- Björnsson, E.; Angulo, P. Hepatitis C and steatosis. Arch. Med. Res. 2007, 38, 621–627. [Google Scholar] [CrossRef]
- Kushner, P.A.; Cobble, M.E. Hypertriglyceridemia: The importance of identifying patients at risk. Postgrad. Med. 2016, 128, 848–858. [Google Scholar] [CrossRef]
- Kutsunai, M.; Kanemoto, H.; Fukushima, K.; Fujino, Y.; Ohno, K.; Tsujimoto, H. The association between gall bladder mucoceles and hyperlipidaemia in dogs: A retrospective case control study. Vet. J. 2014, 199, 76–79. [Google Scholar] [CrossRef] [PubMed]
- Center, S.A. Interpretation of liver enzymes. Vet. Clin. N. Am. Small Anim. Pract. 2007, 37, 297–333, vii. [Google Scholar] [CrossRef] [PubMed]
- Xenoulis, P.G.; Suchodolski, J.S.; Levinski, M.D.; Steiner, J.M. Serum liver enzyme activities in healthy Miniature Schnauzers with and without hypertriglyceridemia. J. Am. Vet. Med. Assoc. 2008, 232, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Jonkers, I.J.; Smelt, A.H.; Princen, H.M.; Kuipers, F.; Romijn, J.A.; Boverhof, R.; Masclee, A.A.; Stellaard, F. Fish oil increases bile acid synthesis in male patients with hypertriglyceridemia. J. Nutr. 2006, 136, 987–991. [Google Scholar] [CrossRef] [Green Version]
- Bexfield, N.H.; Buxton, R.J.; Vicek, T.J.; Day, M.J.; Bailey, S.M.; Haugland, S.P.; Morrison, L.R.; Else, R.W.; Constantino-Casas, F.; Watson, P.J. Breed, age and gender distribution of dogs with chronic hepatitis in the United Kingdom. Vet. J. 2012, 193, 124–128. [Google Scholar] [CrossRef] [Green Version]
- Patnaik, A.K.; Hurvitz, A.I.; Lieberman, P.H. Canine hepatic neoplasms: A clinicopathologic study. Vet. Pathol. 1980, 17, 553–564. [Google Scholar] [CrossRef]
- Patnaik, A.K.; Hurvitz, A.I.; Lieberman, P.H.; Johnson, G.F. Canine hepatocellular carcinoma. Vet. Pathol. 1981, 18, 427–438. [Google Scholar] [CrossRef] [Green Version]
- Sepesy, L.M.; Center, S.A.; Randolph, J.F.; Warner, K.L.; Erb, H.N. Vacuolar hepatopathy in dogs: 336 cases (1993–2005). J. Am. Vet. Med. Assoc. 2006, 229, 246–252. [Google Scholar] [CrossRef]





| Parameters | Healthy | Liver Disease |
|---|---|---|
| N | 45 | 45 |
| Bodyweight (kg, mean ± SD) | 23.6 ± 16.4 | 12.4 ± 9.4 ** |
| 9-point BCS (mean ± SD) | 6.2 ± 1.6 | 6.8 ± 1.8 |
| Age (years, mean ± SD) | 5.1 ± 2.8 | 10.1 ± 3.6 ** |
| Sex (N) | ||
| Male | 24 | 21 |
| Female | 21 | 24 |
| Size (N) | ||
| Small | 17 | 28 |
| Medium | 3 | 13 |
| Large | 25 | 4 |
| Triglyceride (mg/dL, mean ± SD) | 70.9 ± 40.9 | 225.5 ± 286.5 ** |
| Cholesterol (mg/dL, mean ± SD) | 218.0 ± 52.7 | 316.3 ± 234.8 ** |
| ALT (IU/L, mean ± SD) | 37.7 ± 12.2 | 361.4 ± 735.3 ** |
| ALP (IU/L, mean ± SD) | 36.0 ± 16.7 | 1020.5 ± 1946.3 ** |
| GGT (IU/L, mean ± SD) | 2.5 ± 1.6 | 14.7 ± 33.7 * |
| Bile acid (µmol/L, mean ± SD) | 5.5 ± 2.3 | 13.8 ± 10.0 ** |
| Hyperlipidemia | Healthy | Chronic Hepatitis |
|---|---|---|
| N (%) | N (%) | |
| Normal lipid levels | 40 (88.9%) | 17 (37.8%) |
| Hypercholesterolemia | 0 (0.0%) | 5 (11.1%) |
| Hypertriglyceridemia | 5 (11.1%) | 15 (33.3%) |
| Both hypercholesterolemia and hypertriglyceridemia | 0 (0.0%) | 8 (17.8%) |
| ALT | ALP | GGT | Bile Acid | Cholesterol | |
|---|---|---|---|---|---|
| Triglyceride | 0.0016 | 0.2582 * | −0.0325 | 0.0855 | 0.2442 * |
| Cholesterol | 0.8287 ** | 0.8436 ** | 0.5640 ** | 0.3310 ** | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Assawarachan, S.N.; Chuchalermporn, P.; Maneesaay, P.; Thengchaisri, N. Changes in Serum Lipid Profiles among Canine Patients Suffering from Chronic Hepatitis. Vet. Sci. 2021, 8, 221. https://doi.org/10.3390/vetsci8100221
Assawarachan SN, Chuchalermporn P, Maneesaay P, Thengchaisri N. Changes in Serum Lipid Profiles among Canine Patients Suffering from Chronic Hepatitis. Veterinary Sciences. 2021; 8(10):221. https://doi.org/10.3390/vetsci8100221
Chicago/Turabian StyleAssawarachan, Sathidpak Nantasanti, Piyathip Chuchalermporn, Phudit Maneesaay, and Naris Thengchaisri. 2021. "Changes in Serum Lipid Profiles among Canine Patients Suffering from Chronic Hepatitis" Veterinary Sciences 8, no. 10: 221. https://doi.org/10.3390/vetsci8100221
APA StyleAssawarachan, S. N., Chuchalermporn, P., Maneesaay, P., & Thengchaisri, N. (2021). Changes in Serum Lipid Profiles among Canine Patients Suffering from Chronic Hepatitis. Veterinary Sciences, 8(10), 221. https://doi.org/10.3390/vetsci8100221





