Are Anthelminthic Treatments of Captive Ruminants Necessary?
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Prevalence and Abundance of Gastrointestinal Parasites
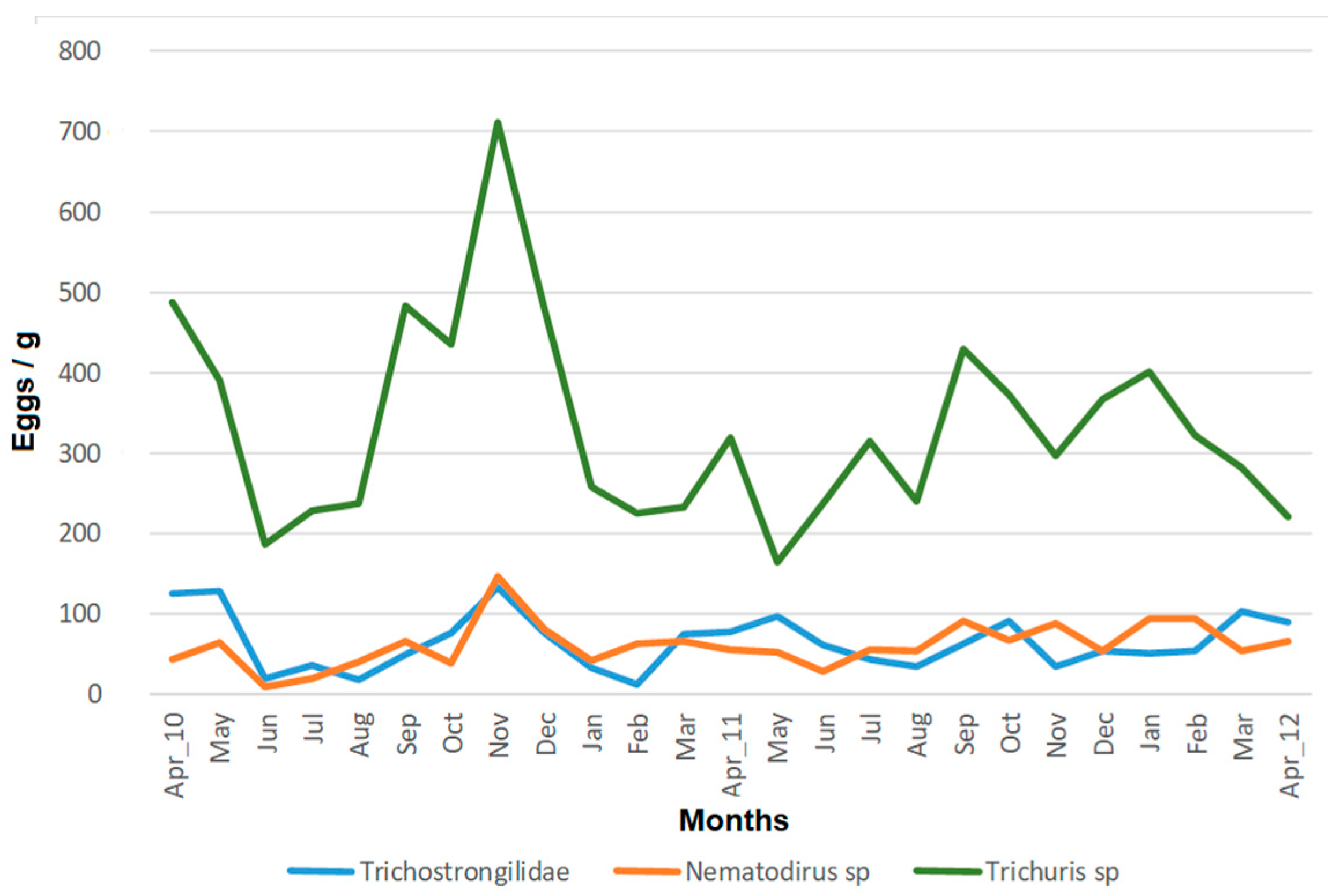
3.2. Temporal Pattern of Egg Shedding and Interindividual Variability
3.3. Factors Influencing the Egg Shedding Pattern
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Moreno, E.; Gonzálvez, M.; Ruiz de Ybáñez, M.R.; Gilbert, T.; Ortiz, J.; Espeso, G.; Benzal, J.; Ibáñez, B.; Valera, F. Survey of husbandry practices for bovidae in zoos: The importance of parasite management for reintroduction programmes. Vet. Rec. 2019, 184, 282. [Google Scholar] [CrossRef]
- Barongi, R.; Fisken, F.A.; Parker, M.; Gusset, M. Committing to Conservation: The World Zoo and Aquarium Conservation Strategy; World Association of Zoos and Aquariums Executive Office: Gland, Switzerland, 2015. [Google Scholar]
- Conde, D.A.; Colchero, F.; Gusset, M.; Pearce-Kelly, P.; Byers, O.; Flesness, N.; Browne, R.K.; Jones, O.R. Zoos through the lens of the IUCN Red List: A global metapopulation approach to support conservation breeding programs. PLoS ONE 2013, 8, e80311. [Google Scholar] [CrossRef] [Green Version]
- Gusset, M.; Fa, J.E.; Sutherland, W.J. A horizon scan for species conservation by zoos and aquariums. Zoo Biol. 2014, 33, 375–380. [Google Scholar] [CrossRef]
- Little, H.A.; Gilbert, T.C.; Athorn, M.L.; Marshall, A.R. Evaluating conservation breeding success for an extinct-in-the-wild antelope. PLoS ONE 2016, 11, e0166912. [Google Scholar] [CrossRef] [PubMed]
- International Union for Conservation of Nature/Species Survival Commission (IUCN/SSC). Guidelines for Reintroductions and Other Conservation Translocations; Version 1.0; International Union for Conservation of Nature/Species Survival Commission: Gland, Switzerland, 2013. [Google Scholar]
- Woodford, M.H. Quarantine and Health Screening Protocols for Wildlife Prior to Translocation and Release into the Wild; IUCN Species Survival Commission’s Veterinary Specialist Group: Gland, Switzerland; The Office International des Epizooties (OIE): Paris, France; Care for the Wild: Sussex, UK; The European Association of Zoo and Wildlife Veterinarians: Schaffhausen, Switzerland, 2000. [Google Scholar]
- Goossens, E.; Dorny, P.; Boomker, J.; Vercammen, F.; Vercruysse, J. A 12-month survey of the gastro-intestinal helminths of antelopes, gazelles and giraffids kept al two zoos in Belgium. Vet. Parasitol. 2005, 127, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, O.B.; Omer, S.A.; Sandouka, M.A. The efficacy of ivermectin and levamisole against natural Nematodirus spathiger infection in the Arabian sand gazelle (Gazella subgutturosa marica) and the Arabian mountain gazelle (Gazella gazella) in Saudi Arabia. Vet. Parasitol. 2007, 150, 170–173. [Google Scholar] [CrossRef] [PubMed]
- Mathews, F.; Moro, D.; Strachan, R.; Gelling, M.; Buller, N. Health surveillance in wildlife reintroductions. Biol. Conserv. 2006, 131, 338–347. [Google Scholar] [CrossRef]
- Hudson, P.J.; Rizzoli, A.; Grenfell, B.T.; Heesterbeek, H.; Dobson, A. The Ecology of Wildlife Disease, 1st ed.; Oxford University press: New York, NY, USA, 2007; pp. 1–6. [Google Scholar]
- Page, K.R.; Scot, A.L.; Manabe, Y.C. The expanding realm of heterologous immunity: Friend or foe? Cell Microbiol. 2006, 8, 185–196. [Google Scholar] [CrossRef]
- Fenton, A.; Perkins, S.E. Applying predator-prey theory to modelling immune-mediated, within host interspecific parasite interactions. Parasitology 2010, 137, 1027–1038. [Google Scholar] [CrossRef] [Green Version]
- Sangster, N.C.; Cowling, A.; Woodgate, R.G. Ten events that defined anthelmintic resistance research. Trends Parasitol. 2018, 34, 553–563. [Google Scholar] [CrossRef]
- Kaplan, R.M. Drug resistance in nematodes of veterinary importance: A status report. Trends Parasitol. 2004, 20, 477–481. [Google Scholar] [CrossRef] [PubMed]
- International Union for Conservation of Nature (IUCN). Nueva Estrategia para la Conservación de la Gacela de Cuvier en el Norte de África. Available online: www.uicnmed.org/newsletter/2018/nueva_estrategia_para_la_conservacion_de_la_gacela_de_cuvier_en_el_norte_de_africa_.htm (accessed on 22 July 2021).
- Ortiz, J.; Ruiz de Ybáñez, M.R.; Garijo, M.M.; Goyena, M.; Espeso, G.; Abaigar, T.; Cano, M. Abomasal and small intestinal nematodes from captive gazelles in Spain. J. Helminthol. 2001, 75, 363–365. [Google Scholar] [CrossRef] [PubMed]
- Cassinello, J.; Gomendio, M.; Roldán, E.R.S. Relationship between coefficient of inbreeding and parasite burden in endangered gazelles. Conserv. Biol. 2001, 5, 1171–1174. [Google Scholar] [CrossRef]
- Ibáñez, B.; De Andrés Cara, D.F.; Moreno, E. Haematology and serum biochemistry parameters in vaccinated vs. unvaccinated captive Cuvier’s gazelles: Implications for zoo management practices. JZAR 2016, 4, 1–6. [Google Scholar]
- Moreno, E.; Espeso, G. International Studbook. Cuvier’s Gazelle (Gazella Cuvieri); Excelentísimo Ayuntamiento de Roquetas de Mar: Almería, Spain, 2008. [Google Scholar]
- Waid, D.D.; Pence, D.B.; Warren, R.J. Effects of season and physical condition on the gastrointestinal helminth community of white-tailed deer from the Texas Edwards Plateau. J. Wildl. Dis. 1985, 21, 264–273. [Google Scholar] [CrossRef] [PubMed]
- Belem, A.M.G.; Couvillion, C.E.; Siefker, C.; Griffin, R.N. Evidence of arrested development of abomasal nematodes in white-tailed deer. J. Wildl. Dis. 1993, 29, 261–265. [Google Scholar] [CrossRef]
- Rossanigo, C.E.; Gruner, L. Moisture and temperature requirements in faeces for the development of free-living stages of gastrointestinal nematodes of sheep, cattle and deer. J. Helminthol. 1995, 9, 357–362. [Google Scholar] [CrossRef]
- Ortiz, J.; Ruiz de Ybáñez, M.R.; Abaigar, T.; Goyena, M.; Garijo, M.M.; Espeso, G.; Cano, M. Output of gastrointestinal nematode eggs in the feces of captive gezelles (Gazella dama mhorr, Gazella cuvieri and Gazella dorcas neglecta) in a semiarid region of southeastern Spain. J. Zoo Wildl. Med. 2006, 37, 249–254. [Google Scholar] [CrossRef]
- Andrade, O.; Orihuela, A.; Solano, J.; Galina, C.S. Some effects of a mask on stress responses in zebu cattle during restraint. Appl. Anim. Behav. Sci. 2001, 71, 175–181. [Google Scholar] [CrossRef]
- Davies, E.T. Parasitología. Recuento de Huevos de Nematodos en Heces. In Manual de Investigación Veterinaria. Técnicas de Laboratorio; Acribia: Zaragoza, Spain, 1990; Volume 2, pp. 202–203. [Google Scholar]
- Soulsby, E.J.L. Parasitología y Enfermedades Parasitarias de los Animales Domésticos, 7th ed.; Nueva Editorial Interamericana: México, México, 1987. [Google Scholar]
- International Species Information System (ISIS): SPARK Single Population Animal Record Keeping System Software, version 1.54; Traylor-Holzer, K. (Ed.) ISIS: Eagan, MN, USA, 2004. [Google Scholar]
- Bush, A.O.; Fernández, J.C.; Esch, G.W.; Seed, J.R. Parasitism. In The Diversity and Ecology of Animal Parasites; Cambridge University Press: Cambridge, UK, 2001; pp. 314–319. [Google Scholar]
- RStudio Team. RStudio: Integrated Development for R; RStudio, Inc.: Boston, MA, USA, 2018; Available online: http://www.rstudio.com/ (accessed on 20 April 2021).
- El-Azazy, O.M.E. Seasonal changes and inhibited development of the abomasal nematodes of sheep and goats in Saudi Arabia. Vet. Parasitol. 1995, 58, 91–98. [Google Scholar] [CrossRef]
- Hunter, R.P. Zoological Pharmacology. In Veterinary Pharmacology and Therapeutics, 9th ed.; Riviere, J.E., Papich, M.G., Eds.; Wiley-Blackwell: Ames, IA, USA, 2013; pp. 1343–1352. [Google Scholar]
- Moudgil, A.D.; Singla, L.D. Molecular confirmation and anthelmintic efficacy assessment against natural trichurid infections in zoo-housed non-human primates. J. Med. Primatol. 2018, 47, 388–392. [Google Scholar] [CrossRef]
- Silvestre, A.; Leignel, V.; Berrag, B.; Gasnier, N.; Humbert, J.F.; Chartiere, C.; Cabaret, J. Sheep and goat nematode resistance to anthelmintics; pro and cons among breeding management factors. Vet. Res. 2002, 33, 465–480. [Google Scholar] [CrossRef] [Green Version]
- Citino, S.B. Bovidae (except sheep and goat) and antilocapridae. In Zoo & Wild Animal Medicine, 5th ed.; Fowler, E.M., Miller, E.R., Eds.; Saunders: Philadelphia, PA, USA, 2003; pp. 649–674. [Google Scholar]
- Reichard, M.V.; Wolf, R.F.; Carey, D.W.; Garrett, J.J.; Briscoe, H.A. Efficacy of fenbendazole and milbemycin oxime for treating baboons (Papio cynocephalus anubis) infected with Trichuris trichiura. J. Am. Assoc. Lab. Anim 2007, 46, 42–45. [Google Scholar]
- Georgi, J.R.; Whitlock, R.H.; Flinton, J.H. Fatal Trichuris discolor infection in a Holstein-Freisian heifer. Report of a case. Cornell Vet. 1972, 62, 58–60. [Google Scholar]
- Palomero, A.; Cazapal-Monteiro, C.; Valderrábano, E.; Paz-Silva, A.; Sánchez-Andrade, R.; Arias, M.S. Soil fungi enable the control of gastrointestinal nematodes in wild bovidae captive in a zoological park: A 4-year trial. Parasitology 2020, 147, 791–798. [Google Scholar] [CrossRef]
- Wisser, J.; Tscherner, W.; Jantschke, B. Infestation with Camelostrongylus mentulatus in Dorcas gazelles (Gazella dorcas neglecta) caused lethal abomasitis. Verh. Ber. Erkrg. Zootiere 2001, 40, 81–86. [Google Scholar]
- González-Tokman, D.; Martínez, M.I.; Villalobos-Ávalos, Y.; Munguía-Steyer, R.; Ortiz-Zayas, M.R.; Cruz-Rosales, M.; Lumaret, J.P. Ivermectin alters reproductive success, body condition and sexual trait expression in dung beetles. Chemosphere 2017, 178, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Verdú, J.R.; Lobo, J.M.; Sánchez-Piñero, F.; Gallego, B.; Numa, C.; Lumaret, J.P.; Cortez, V.; Ortiz, A.J.; Tonelli, M.; García-Teba, J.P.; et al. Ivermectin residues disrupt dung beetle diversity, soil properties and ecosystem functioning: An interdisciplinary field study. Sci. Total Environ. 2018, 618, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Schulte-Hostedde, A.I.; Mastromonaco, G.F. Integrating evolution in the management of captive zoo populations. Evol. Appl. 2015, 8, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Weinstock, J.V.; Elliott, D.E. Helminths and the IBD hygiene hypothesis. Inflamm. Bowel Dis. 2009, 15, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Rook, G.A.W. Review series on helminths, immune modulation and the hygiene hypothesis: The broader implications of the hygiene hypothesis. Immunology 2009, 126, 3–11. [Google Scholar] [CrossRef]
- Mason, G.J. Species differences in responses to captivity: Stress, welfare and the comparative method. Trends Ecol. Evol. 2010, 25, 713–721. [Google Scholar] [CrossRef] [Green Version]
- Amaradasa, B.S.; Lane, R.A.; Manage, A. Vertical migration of Haemonchus contortus infective larvae on Cynodon dactylon and Paspalum notatum pastures in response to climatic conditions. Vet. Parasitol. 2010, 170, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Manfredi, M.T. Biologia dei nematodi gastrointestinali dei ruminanti. Parassitologia 2006, 48, 397–401. [Google Scholar] [PubMed]
- Thornhill, N.W. The Natural History of Inbreeding and Outbreeding; The University of Chicago Press: Chicago, IL, USA, 1993. [Google Scholar]
- Coltman, D.W.; Pilkington, J.; Kruuk, L.E.; Wilson, K.; Pemberton, J.M. Positive genetic correlation between parasite resistance and body size in a free-living ungulate population. Evolution 2001, 55, 2116–2125. [Google Scholar] [CrossRef]
- Mitchell, J.; Vitikainen, E.I.K.; Wells, D.A.; Cant, M.A.; Nichols, H.J. Heterozygosity but not inbreeding coefficient predicts parasite burdens in the banded mongoose. J. Zool. 2016, 302, 32–39. [Google Scholar] [CrossRef]
- Aguilar-Marcelino, L.; Mendoza-de-Gives, P.; Torres-Hernández, G.; López-Arellano, M.E.; Becerril-Pérez, C.M.; Orihuela-Trujillo, A.; Torres-Acosta, J.F.J.; Olmedo-Juárez, A. Consumption of nutritional pellets with Duddingtonia flagrans fungal chlamydospores reduces infective nematode larvae of Haemonchus contortus in faeces of Saint Croix lambs. J. Helminthol. 2017, 91, 665–671. [Google Scholar] [CrossRef]
- Palomero, A.M.; Hernández, J.A.; Cazapal-Monteiro, C.F.; Arroyo Balán, F.; Silva, M.I.; Paz-Silva, A.; Sánchez-Andrade, R.; Arias Vázquez, M.S. Implementation of biological control to the integrated control of Strongyle infection among wild Captive equids in a zoological park. BioMed Res. Int. 2018, 4267683. [Google Scholar] [CrossRef] [Green Version]
| Species | Prevalence | Minimum Median | Maximum Median | Overall Median | Overall Q1 | Overall Q2 |
|---|---|---|---|---|---|---|
| Strongylid-like | 100% | 0 1 | 118.5 | 42.3 | 13.15 | 87 |
| Nematodirus | 100% | 6.35 | 140.4 | 40.4 | 13.9 | 81.8 |
| Trichuris | 100% | 97.3 | 573.5 | 208.3 | 118.3 | 412.2 |
| Strongylid-like | Nematodirus | Trichuris | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Animal ID | Prevalence | Median 1 | Range 2 | Prevalence | Median | Range | Prevalence | Median | Range |
| 42 | 71% (24) | 14.4 | 168.2 | 71% (24) | 28.6 | 155.6 | 100% (24) | 118.2 | 726.3 |
| 45 | 91% (22) | 48.6 | 228.6 | 50% (22) | 6.35 | 122.2 | 95% (22) | 210.6 | 733.5 |
| 47 | 90% (21) | 118.5 | 472.4 | 95% (21) | 140.4 | 493.8 | 100% (21) | 166.7 | 1406.5 |
| 48 | 70% (20) | 51.1 | 246.9 | 90% (20) | 59.2 | 193.2 | 100% (20) | 322.8 | 1481.5 |
| 50 | 83% (24) | 42.7 | 257.2 | 54% (24) | 13.9 | 44.4 | 100% (24) | 260.9 | 666.1 |
| 51 | 85% (13) | 44.2 | 223.7 | 58% (13) | 14.7 | 89.5 | 92% (13) | 227.5 | 1804.6 |
| 52 | 70% (10) | 24.2 | 310.9 | 50% (10) | 7.15 | 74.1 | 100% (10) | 97.3 | 1940.8 |
| 53 | 94% (16) | 107.1 | 429.6 | 81% (16) | 43.7 | 255.3 | 100% (16) | 492.1 | 1128.0 |
| 54 | 91% (23) | 58.3 | 308.0 | 91% (23) | 29.6 | 239.7 | 100% (23) | 184.1 | 1436.8 |
| 56 | 71% (24) | 2.9 | 277.8 | 88% (24) | 40.4 | 277.8 | 100% (24) | 246.6 | 800.5 |
| 60 | 41% (22) | 0.0 | 80.3 | 73% (22) | 47.0 | 167.5 | 91% (22) | 118.1 | 318.6 |
| 61 | 61% (23) | 2.9 | 173.0 | 87% (23) | 44.0 | 213.3 | 100% (23) | 332.6 | 1248.3 |
| 62 | 82% (22) | 44.7 | 244.4 | 82% (22) | 44.4 | 132.9 | 100% (22) | 233.2 | 1352.6 |
| 65 | 61% (23) | 28.7 | 241.7 | 91% (23) | 88.9 | 555.6 | 100% (23) | 573.5 | 1859.3 |
| 67 | 79% (24) | 31.2 | 103.4 | 78% (24) | 43.6 | 192.0 | 100% (24) | 133.3 | 518.7 |
| Strongylid-like Eggs—AIC = 3547 − Marginal R2 = 0.09/Conditional R2 = 0.19 | |||||
|---|---|---|---|---|---|
| Estimate | Std. Error | df | t value | Pr(>|t|) | |
| (Intercept) * | −265.962 | 114.965 | 11.877 | −2.313 | 0.039 |
| Precipitation | 0.513 | 0.197 | 297.148 | 2.610 | 0.010 |
| inbreeding | 1399.020 | 498.618 | 11.888 | 2.806 | 0.016 |
| Nematodirus—AIC = 3469 − Marginal R2 = 0.11/Conditional R2 = 0.34 | |||||
| Estimate | Std. Error | df | t value | Pr(>|t|) | |
| (Intercept) | −255.723 | 157.156 | 12.555 | −1.627 | 0.129 |
| precipitation | 0.769 | 0.177 | 294.397 | 4.344 | 0.000 |
| inbreeding | 1309.332 | 681.496 | 12.557 | 1.921 | 0.078 |
| Trichuris—AIC = 4463 − Marginal R2 = 0.03/Conditional R2 = 0.20 | |||||
| Estimate | Std. Error | df | t value | Pr(>|t|) | |
| (Intercept) | 172.3653 | 618.7681 | 12.8382 | 0.2790 | 0.7850 |
| precipitation | 2.6999 | 0.8483 | 297.0684 | 3.1830 | 0.0016 |
| inbreeding | 506.9516 | 2683.4574 | 12.8453 | 0.1890 | 0.8531 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lahat, L.; Ortiz, J.M.; Tizzani, P.; Ibáñez, B.; Valera, F.; Moreno, E.; Espeso, G.; Ruiz de Ybáñez, R. Are Anthelminthic Treatments of Captive Ruminants Necessary? Vet. Sci. 2021, 8, 240. https://doi.org/10.3390/vetsci8100240
Lahat L, Ortiz JM, Tizzani P, Ibáñez B, Valera F, Moreno E, Espeso G, Ruiz de Ybáñez R. Are Anthelminthic Treatments of Captive Ruminants Necessary? Veterinary Sciences. 2021; 8(10):240. https://doi.org/10.3390/vetsci8100240
Chicago/Turabian StyleLahat, Liron, Juana M. Ortiz, Paolo Tizzani, Belén Ibáñez, Francisco Valera, Eulalia Moreno, Gerardo Espeso, and Rocío Ruiz de Ybáñez. 2021. "Are Anthelminthic Treatments of Captive Ruminants Necessary?" Veterinary Sciences 8, no. 10: 240. https://doi.org/10.3390/vetsci8100240
APA StyleLahat, L., Ortiz, J. M., Tizzani, P., Ibáñez, B., Valera, F., Moreno, E., Espeso, G., & Ruiz de Ybáñez, R. (2021). Are Anthelminthic Treatments of Captive Ruminants Necessary? Veterinary Sciences, 8(10), 240. https://doi.org/10.3390/vetsci8100240







