Suspected Drinking Water Poisoning in a Domestic Kitten with Methemoglobinemia
Abstract
1. Introduction
2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Harvey, J.W. Pathogenesis, laboratory diagnosis, and clinical implications of erythrocyte enzyme deficiencies in dogs, cats, and horses. Veter. Clin. Pathol. 2006, 35, 144–156. [Google Scholar] [CrossRef] [PubMed]
- Jaffey, J.; Harmon, M.; Villani, N.; Creighton, E.; Johnson, G.; Giger, U.; Dodam, J. Long-term Treatment with Methylene Blue in a Dog with Hereditary Methemoglobinemia Caused by Cytochrome b5 Reductase Deficiency. J. Vet. Intern. Med. 2017, 31, 1860–1865. [Google Scholar] [CrossRef] [PubMed]
- Ash-Bernal, R.; Wise, R.; Wright, S.M. Acquired Methemoglobinemia. Medicine 2004, 83, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Finco, D.C.; Duncan, J.R.; Schall, W.D.; Prasse, K.W. Acetaminophen toxicosis in the cat. J. Am. Vet. Med. Assoc. 1975, 166, 469–472. [Google Scholar] [PubMed]
- Jaffey, J.A.; Reading, N.S.; Giger, U.; Abdulmalik, O.; Buckley, R.M.; Johnstone, S.; Lyons, L.A.; The 99 Lives Cat Genome Consortium. Clinical, metabolic, and genetic characterization of hereditary methemoglobinemia caused by cytochrome b 5 reductase deficiency in cats. J. Veter. Intern. Med. 2019, 33, 2725–2731. [Google Scholar] [CrossRef] [PubMed]
- Vasiliadou, E.; Karakitsou, V.; Kazakos, G.; Oikonomidis, I.L.; Tsouloufi, T.K.; Kosmas, P.; Abdulmalik, O.Y.; Kou-tinas, C.; Giger, U.; Mylonakis, M.E. Hereditary methemoglobinemia in a cyanotic cat presented for ovariohysterectomy. Can. Vet. J. 2019, 60, 502–506. [Google Scholar] [PubMed]
- Shihana, F.; Dissanayake, D.M.; Buckley, N.; Dawson, A. A Simple Quantitative Bedside Test to Determine Methemoglobin. Ann. Emerg. Med. 2010, 55, 184–189. [Google Scholar] [CrossRef] [PubMed]
- Rumbeiha, W.K.; Oehme, F.W. Methylene blue can be used to treat methemoglobinemia in cats without induc-ing Heinz body hemolytic anemia. Vet. Hum. Toxicol. 1992, 34, 120–122. [Google Scholar] [PubMed]
- Blanc, P.D. Poisoning and Drug Overdose, 6th ed.; McGraw-Hill: New York, NY, USA, 2011; Chapter 103 Methemoglobinemia. [Google Scholar]
- Nash, S.L.; Savides, M.C.; Oehme, F.W.; Johnson, D.E. The effect of acetaminophen on methemoglobin and blood glutathione parameters in the cat. Toxicology 1984, 31, 329–334. [Google Scholar] [CrossRef]
- Trapp, L.; Will, J. Acquired Methemoglobinemia Revisited. Dent. Clin. N. Am. 2010, 54, 665–675. [Google Scholar] [CrossRef] [PubMed]
- Wilkie, D.A.; Kirby, R. Methemoglobinemia associated with dermal application of benzocaine cream in a cat. J. Am. Vet. Med. Assoc. 1988, 192, 85–86. [Google Scholar] [PubMed]
- Freeman, L.; Wolford, R.W. Methemoglobinemia secondary to cleaning solution ingestion. J. Emerg. Med. 1996, 14, 599–601. [Google Scholar] [CrossRef]
- Matteucci, O.; Diletti, G.; Prencipe, V.; Di Giannatale, E.; Marconi, M.M.; Migliorati, G. Due casi di metaemoglobinemia acuta per sospetto avvelenamento da sodio nitrito. Vet Ital. 2008, 44, 439–445. [Google Scholar] [PubMed]
- Di Bartola, S. Fluid, Electrolyte, and Acid-Base Disorders in Small Animal Practice, 4th ed.; Elsevier Saunders: Columbia, OH, USA, 2011; Chapter 4 Disorders of Chloride: Hyperchloremia and Hypochloremia. [Google Scholar]
- Reference Manual ABL800 FLEX, Radiometer. Available online: healthandcareni.net/stlabs/webhb/poct/documents/poct%20abl800%20man.pdf (accessed on 29 September 2021).
- Fewtrell, L. Drinking-Water Nitrate, Methemoglobinemia, and Global Burden of Disease: A Discussion. Environ. Health Perspect. 2004, 112, 1371–1374. [Google Scholar] [CrossRef] [PubMed]
- Sadeq, M.; Abouqal, R.; Attarassi, B.; Lakranbi, M.; ElAouad, R.; Idrissi, L. Does Exposure to Nitrate in Drinking Water Contribute Anything the Effect of Water Chlorination on Children Methemoglobin Levels? J. Environ. Prot. 2012, 3, 169–176. [Google Scholar] [CrossRef][Green Version]
- Manassaram, D.M.; Backer, L.C.; Messing, R.; Fleming, L.E.; Luke, B.; Monteilh, C.P. Nitrates in drinking water and methemoglobin levels in pregnancy: A longitudinal study. Environ. Health 2010, 9, 1–12. [Google Scholar] [CrossRef] [PubMed]

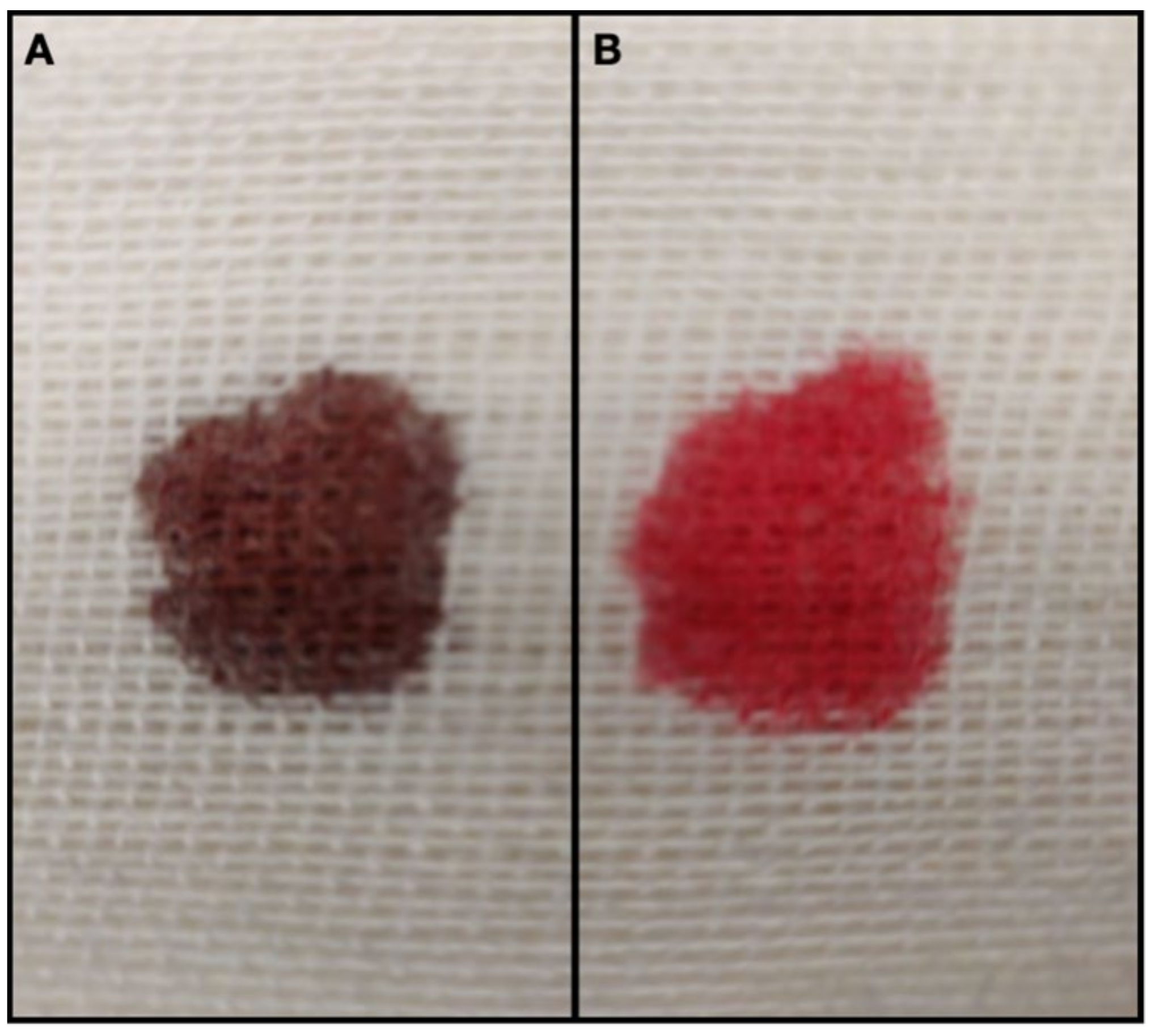
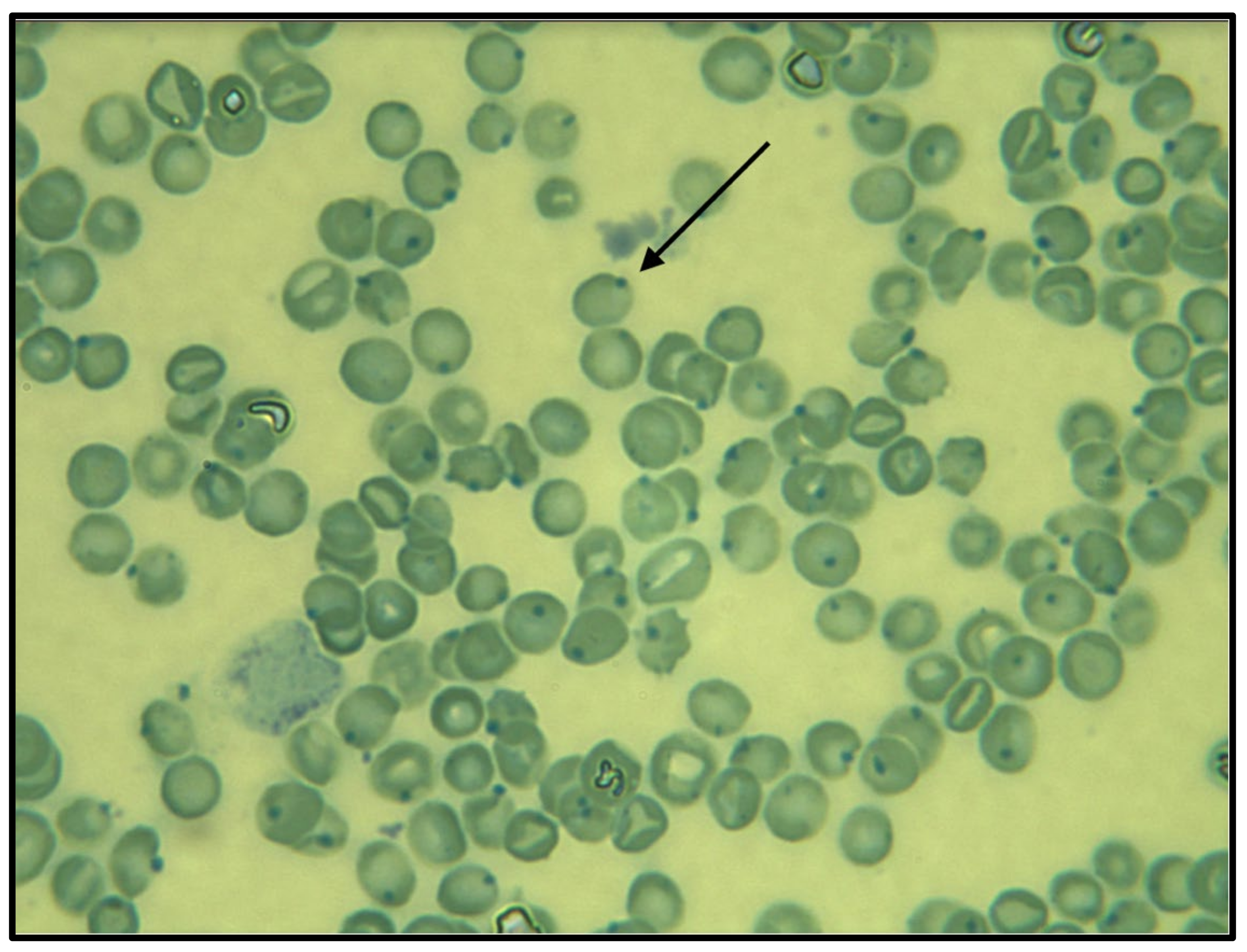
| Day | 1 | 1 | 2 | 3 | Reference Interval |
|---|---|---|---|---|---|
| Time | 07:02 p.m. | 11:57 p.m. * | 07.37 a.m. | 07:25 a.m. | |
| pH | 7.249 | 7.332 | 7.343 | 7.418 | 7.26–7.46 |
| pCO2 | 40.2 | 30 | 35.5 | 25.5 | 32.7–42.7 mmHg |
| HCO3 | 16.9 | 15.7 | 18.7 | 18.3 | 18–23.5 mmol/L |
| Hematocrit | 40.9 | 40.8 | 41.8 | 39.5 | 39–54% |
| MetHb | 81.4 | 4.2 | 2.9 | 3.7 | 0–1% |
| Sodium | 142 | 147 | 151 | 151 | 151–158 mmol/L |
| Chloride | 321 | 254 | 216 | 132 | 105–116 mmol/L |
| Laboratory Variables | Results | Reference Interval |
|---|---|---|
| AST | 15 | 14–41 U/l |
| ALT | 17 | 5–45 U/l |
| CK | 196 | 91–326 U/l |
| ALP | 46 | 0–120 U/l |
| Creatinine | 0.62 | 0.8–1.80 mg/dl |
| Urea | 43.30 | 15–60 mg/dl |
| Glucose | 108 | 75–160 mg/dl |
| Total proteins | 6.55 | 6.0–8.0 g/dl |
| Albumin | 2.87 | 2.10–3.30 g/dl |
| Hematocrit | 40.1 | 24–45% |
| Platelets | 400,000 | 300,000–700,000/mm3 |
| Leucocytes | 7340 | 5000–19,000/mm3 |
| USG | 1036 | >1032 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fidanzio, F.; Corsini, A.; Spindler, K.P.; Crosara, S. Suspected Drinking Water Poisoning in a Domestic Kitten with Methemoglobinemia. Vet. Sci. 2021, 8, 243. https://doi.org/10.3390/vetsci8110243
Fidanzio F, Corsini A, Spindler KP, Crosara S. Suspected Drinking Water Poisoning in a Domestic Kitten with Methemoglobinemia. Veterinary Sciences. 2021; 8(11):243. https://doi.org/10.3390/vetsci8110243
Chicago/Turabian StyleFidanzio, Francesca, Andrea Corsini, Kevin Pascal Spindler, and Serena Crosara. 2021. "Suspected Drinking Water Poisoning in a Domestic Kitten with Methemoglobinemia" Veterinary Sciences 8, no. 11: 243. https://doi.org/10.3390/vetsci8110243
APA StyleFidanzio, F., Corsini, A., Spindler, K. P., & Crosara, S. (2021). Suspected Drinking Water Poisoning in a Domestic Kitten with Methemoglobinemia. Veterinary Sciences, 8(11), 243. https://doi.org/10.3390/vetsci8110243






