Multispecies Q Fever Outbreak in a Mixed Dairy Goat and Cattle Farm Based on a New Bovine-Associated Genotype of Coxiella burnetii
Abstract
1. Introduction
2. Materials and Methods
2.1. Goats and Cattle
2.1.1. Background and Sample Collection
2.1.2. Hormone Determination in Goats
2.1.3. Phase-Specific Antibody Detection
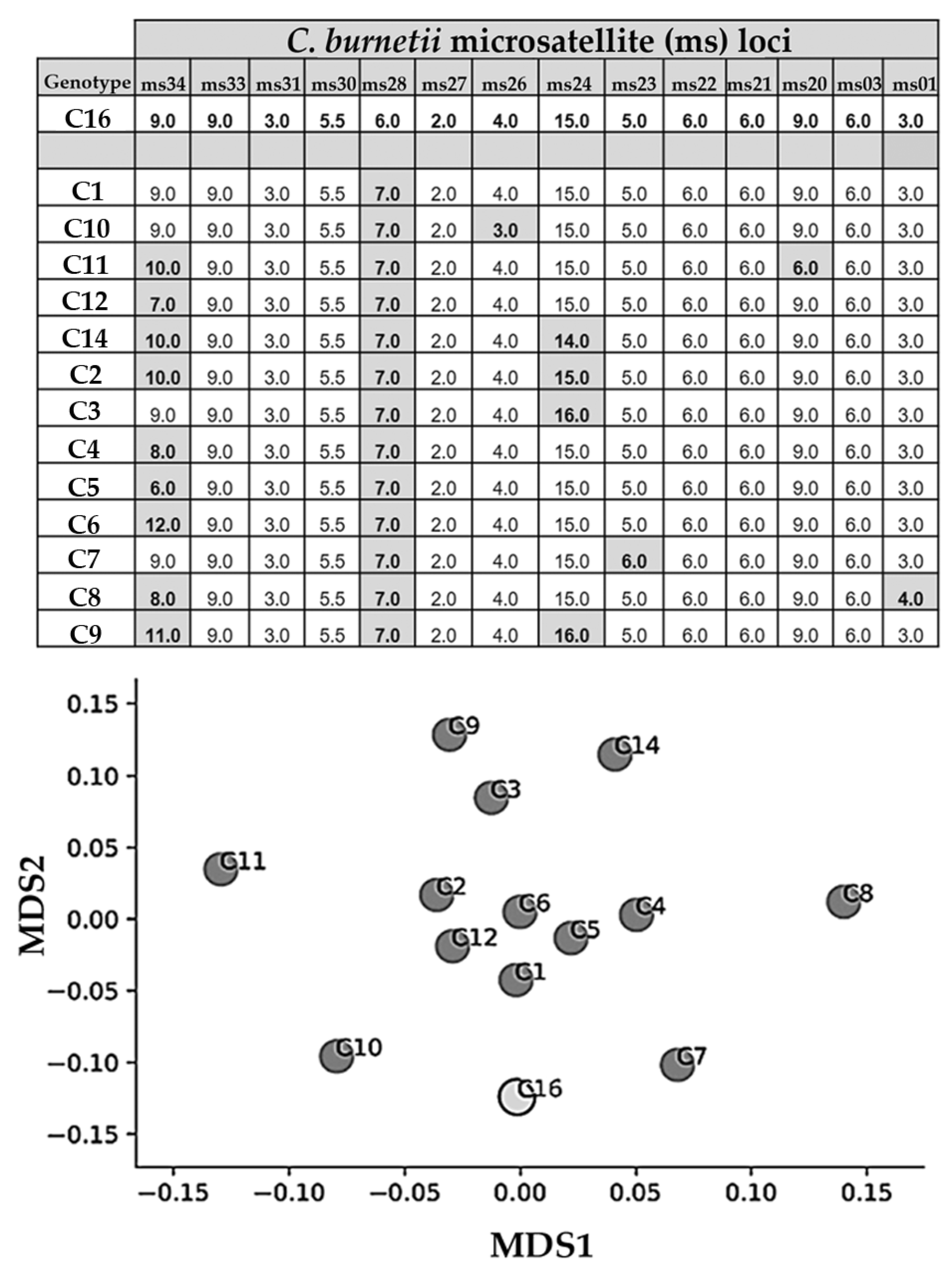
2.1.4. DNA Detection and Genotyping
2.2. Barn Cats and Farm Dog
2.2.1. Background and Sample Collection
2.2.2. Antibody Detection
2.2.3. DNA Detection
2.3. Farmer’s Family
2.4. Statistical Analysis
3. Results
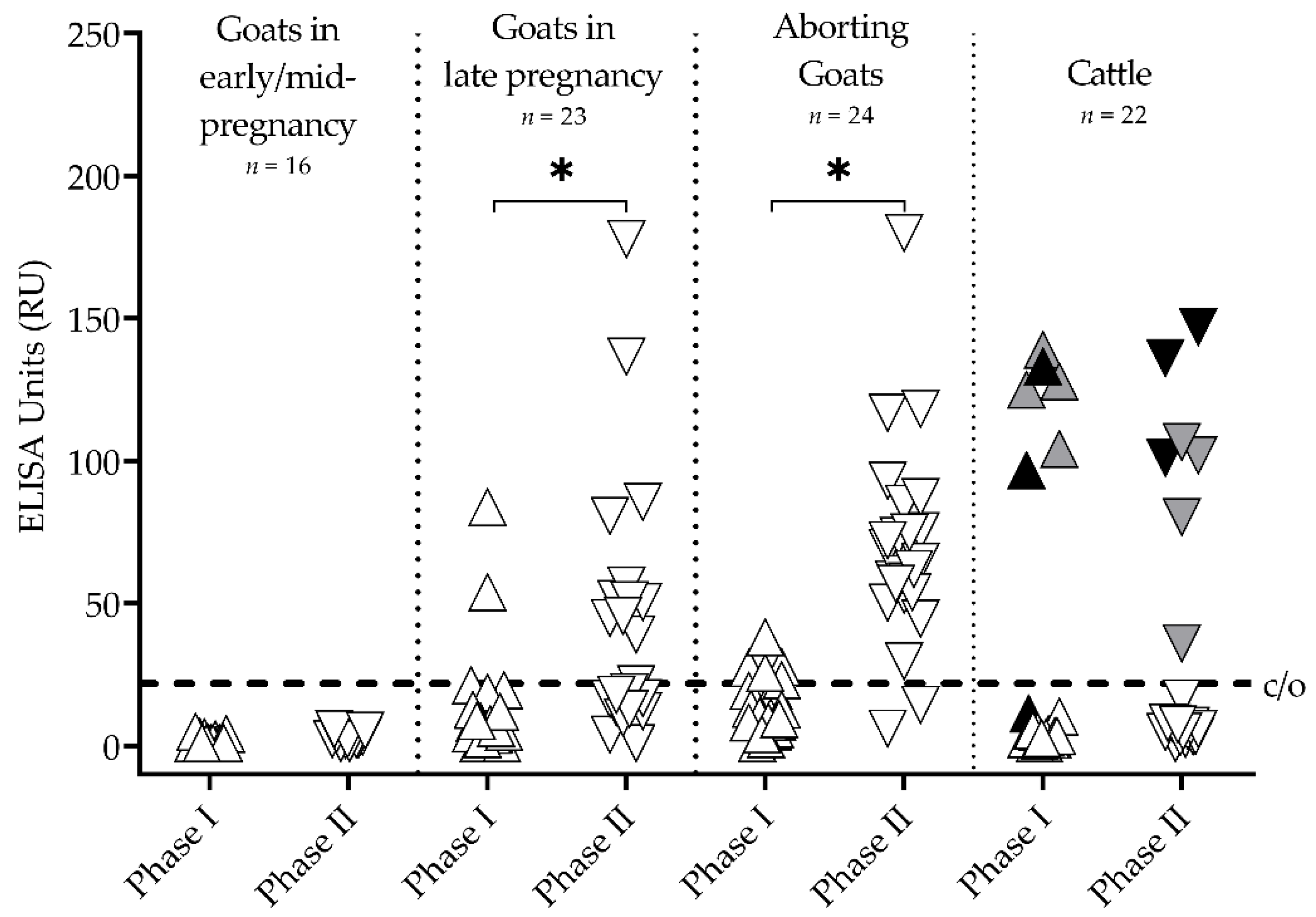
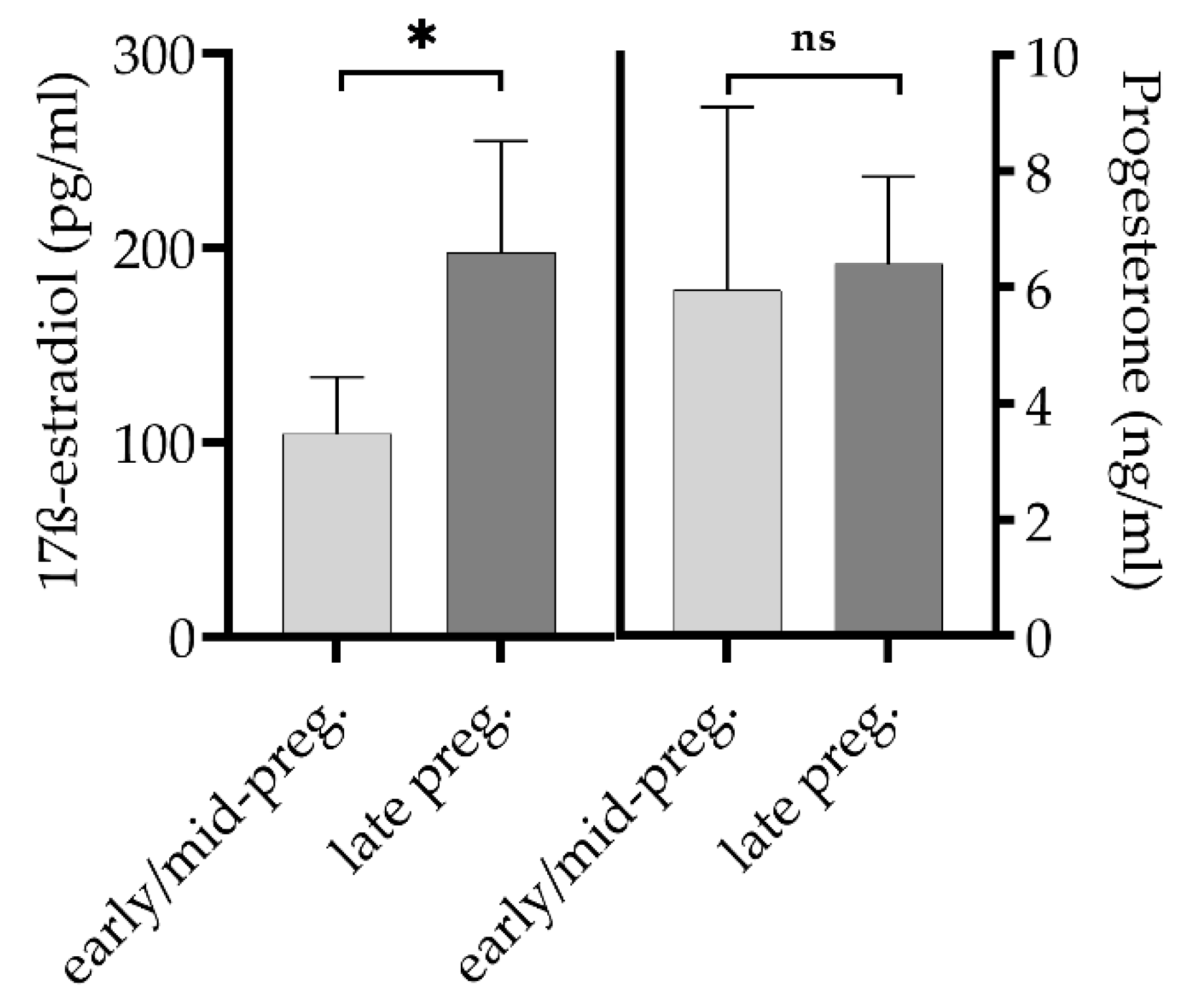
3.1. Goats and Cattle
3.2. Barn Cats and Farm Dog
3.3. Farmer’s Family
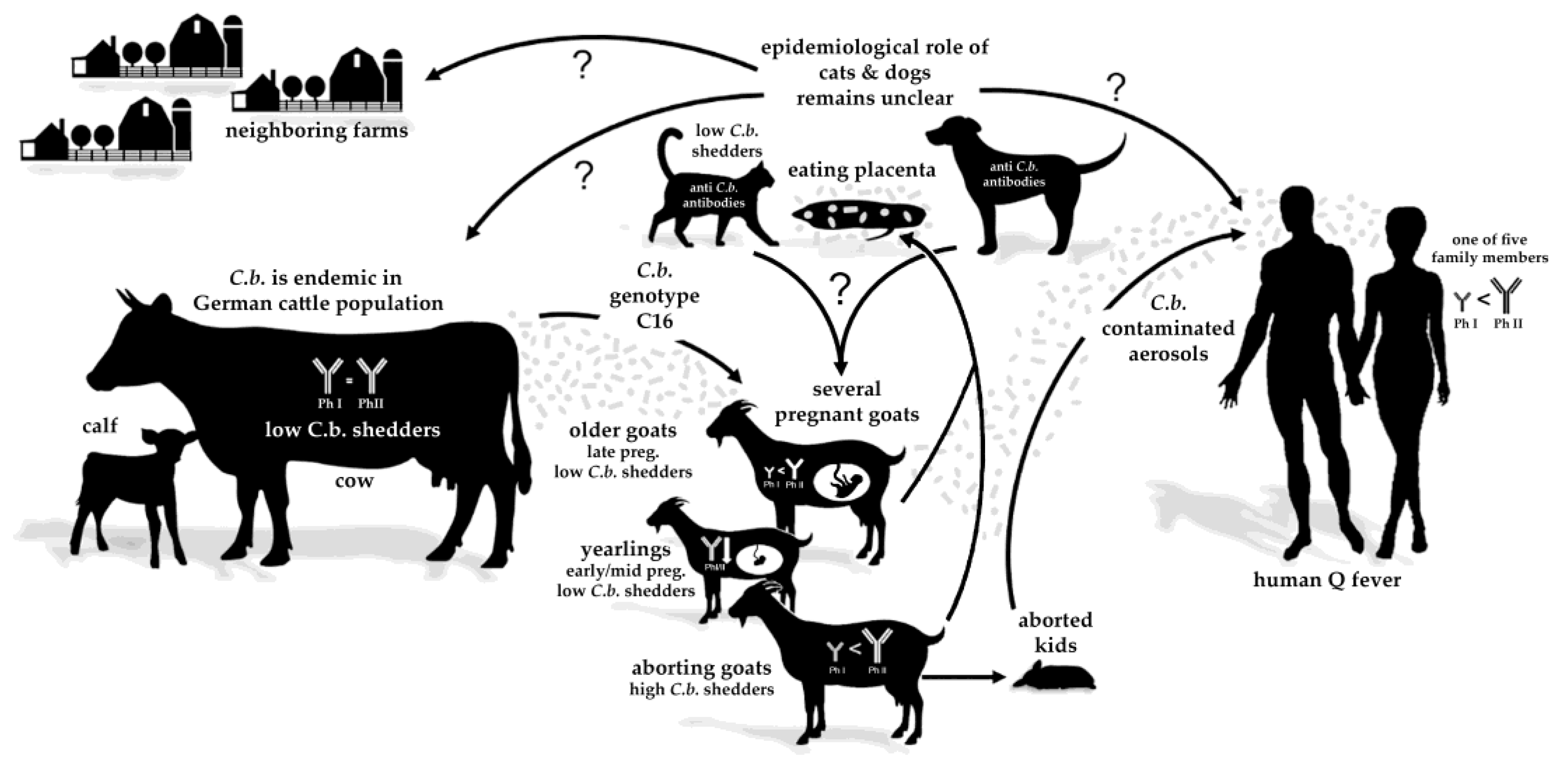
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sidi-Boumedine, K.; Rousset, E.; Henning, K.; Ziller, M.; Niemczuck, K.; Roest, H.; Thiéry, R. Development of harmonised schemes for the monitoring and reporting of Q-fever in animals in the European Union. Sci. Rep. Submitt. EFSA 2010, 7, 48E. [Google Scholar] [CrossRef]
- Roest, H.; van Gelderen, B.; Dinkla, A.; Frangoulidis, D.; van Zijderveld, F.; Rebel, J.; van Keulen, L. Q fever in pregnant goats: Pathogenesis and excretion of Coxiella burnetii. PLoS ONE 2012, 7, e48949. [Google Scholar] [CrossRef]
- Sánchez, J.; Souriau, A.; Buendía, A.J.; Arricau-Bouvery, N.; Martínez, C.M.; Salinas, J.; Rodolakis, A.; Navarro, J.A. Experimental Coxiella burnetii infection in pregnant goats: A histopathological and immunohistochemical study. J. Comp. Pathol. 2006, 135, 108–115. [Google Scholar] [CrossRef]
- Bauer, B.U.; Runge, M.; Campe, A.; Henning, K.; Mertens-Scholz, K.; Boden, K.; Sobotta, K.; Frangoulidis, D.; Knittler, M.R.; Matthiesen, S.; et al. Coxiella burnetii: A review focusing on infections in German sheep and goat flocks. Berl. Munch. Tierarztl. Wochenschr. 2020, 133, 184–200. [Google Scholar] [CrossRef]
- EFSA. Scientific opinion on Q fever. EFSA J. 2010, 8, 1595. [Google Scholar] [CrossRef]
- Agerholm, J.S. Coxiella burnetii associated reproductive disorders in domestic animals—A critical review. Acta Vet. Scand. 2013, 55, 13. [Google Scholar] [CrossRef] [PubMed]
- van den Brom, R.; van Engelen, E.; Roest, H.I.J.; van der Hoek, W.; Vellema, P. Coxiella burnetii infections in sheep or goats: An opinionated review. Vet. Microbiol. 2015, 181, 119–129. [Google Scholar] [CrossRef]
- Eldin, C.; Mélenotte, C.; Mediannikov, O.; Ghigo, E.; Million, M.; Edouard, S.; Mege, J.-L.; Maurin, M.; Raoult, D. From Q Fever to Coxiella burnetii infection: A paradigm change. Clin. Microbiol. Rev. 2017, 30, 115–190. [Google Scholar] [CrossRef]
- Georgiev, M.; Afonso, A.; Neubauer, H.; Needham, H.; Thiery, R.; Rodolakis, A.; Roest, H.J.; Stark, K.D.; Stegeman, J.A.; Vellema, P. Q fever in humans and farm animals in four European countries, 1982 to 2010. Eurosurveillance 2013, 18, 20407. [Google Scholar] [CrossRef]
- Vellema, P.; van den Brom, R. The rise and control of the 2007-2012 human Q fever outbreaks in the Netherlands. Small Rumin. Res. 2014, 118, 69–78. [Google Scholar] [CrossRef]
- Buhariwalla, F.; Cann, B.; Marrie, T.J. A dog-related outbreak of Q fever. Clin. Infect. Dis. 1996, 23, 753–755. [Google Scholar] [CrossRef]
- Malo, J.A.; Colbran, C.; Young, M.; Vasant, B.; Jarvinen, K.; Viney, K.; Lambert, S.B. An outbreak of Q fever associated with parturient cat exposure at an animal refuge and veterinary clinic in southeast Queensland. Aust. N. Z. J. Public Health 2018, 42, 451–455. [Google Scholar] [CrossRef]
- Pinsky, R.L.; Fishbein, D.B.; Greene, C.R.; Gensheimer, K.F. An outbreak of cat-associated Q fever in the United States. J. Infect. Dis. 1991, 164, 202–204. [Google Scholar] [CrossRef] [PubMed]
- Meadows, S.; Jones-Bitton, A.; McEwen, S.; Jansen, J.; Menzies, P. Coxiella burnetii seropositivity and associated risk factors in goats in Ontario, Canada. Prev. Vet. Med. 2015, 121, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Schimmer, B.; Luttikholt, S.; Hautvast, J.L.A.; Graat, E.A.M.; Vellema, P.; Duynhoven, Y.T.H.P.v. Seroprevalence and risk factors of Q fever in goats on commercial dairy goat farms in the Netherlands, 2009–2010. BMC Vet. Res. 2011, 7, 81. [Google Scholar] [CrossRef]
- Arricau-Bouvery, N.; Hauck, Y.; Bejaoui, A.; Frangoulidis, D.; Bodier, C.C.; Souriau, A. Molecular characterization of Coxiella burnetii isolates by infrequent restriction site-PCR and MLVA typing. BMC Microbiol. 2006, 6, 38. [Google Scholar] [CrossRef] [PubMed]
- Svraka, S.; Toman, R.; Skultety, L.; Slaba, K.; Homan, W.L. Establishment of a genotyping scheme for Coxiella burnetii. FEMS Microbiol. Lett. 2006, 254, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Astobiza, I.; Tilburg, J.J.; Piñero, A.; Hurtado, A.; García-Pérez, A.L.; Nabuurs-Franssen, M.H.; Klaassen, C.H. Genotyping of Coxiella burnetii from domestic ruminants in northern Spain. BMC Vet. Res. 2012, 8, 241. [Google Scholar] [CrossRef]
- De Bruin, A.; Van Alphen, P.T.; Van der Plaats, R.Q.; De Heer, L.N.; Reusken, C.B.; Van Rotterdam, B.J.; Janse, I. Molecular typing of Coxiella burnetii from animal and environmental matrices during Q fever epidemics in the Netherlands. BMC Vet. Res. 2012, 8, 165. [Google Scholar] [CrossRef]
- Frangoulidis, D.; Walter, M.C.; Antwerpben, M.; Zimmermann, P.; Janowetz, B.; Alex, M.; Böttcher, J.; Henning, K.; Hilbert, A.; Ganter, M.; et al. Molecular analysis of Coxiella burnetii in Germany reveals evolution of unique clonal clusters. Int. J. Med. Microbiol. 2014, 304, 868–876. [Google Scholar] [CrossRef]
- Prigent, M.; Rousset, E.; Yang, E.; Thiery, R.; Sidi-Boumedine, K. Validation study for using lab-on-chip technology for Coxiella burnetii multi-locus-VNTR-analysis (MLVA) typing: Application for studying genotypic diversity of strains from domestic ruminants in France. Microb. Infect. 2015, 17, 782–788. [Google Scholar] [CrossRef]
- Roest, H.I.; Ruuls, R.C.; Tilburg, J.J.; Nabuurs-Franssen, M.H.; Klaassen, C.H.; Vellema, P. Molecular epidemiology of Coxiella burnetii from ruminants in Q fever outbreak, the Netherlands. Emerg. Infect. Dis. 2011, 17, 668–675. [Google Scholar] [CrossRef] [PubMed]
- Bauer, B.; Prüfer, L.; Walter, M.; Ganter, I.; Frangoulidis, D.; Runge, M.; Ganter, M. Comparison of Coxiella burnetii excretion between sheep and goats naturally infected with one cattle-associated genotype. Pathogens 2020, 9, 652. [Google Scholar] [CrossRef]
- Joulié, A.; Sidi-Boumedine, K.; Bailly, X.; Gasqui, P.; Barry, S.; Jaffrelo, L.; Poncet, C.; Abrial, D.; Yang, E.; Leblond, A.; et al. Molecular epidemiology of Coxiella burnetii in French livestock reveals the existence of three main genotype clusters and suggests species-specific associations as well as regional stability. Infect. Genet. Evol. 2017, 48, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Stoker, M.; Fiset, P. Phase variation of the Nine Mile and other strains of Rickettsia burneti. Can. J. Microbiol. 1956, 2, 310–321. [Google Scholar] [CrossRef]
- Williams, J.; Johnston, M.; Peacock, M.; Thomas, L.; Stewart, S.; Portis, J. Monoclonal antibodies distinguish phase variants of Coxiella burnetii. Infect. Immun. 1984, 43, 421–428. [Google Scholar] [CrossRef]
- Fournier, P.E.; Marrie, T.; Didier, R. Diagnosis of Q fever. J. Clin. Microbiol. 1998, 36, 1823–1834. [Google Scholar] [CrossRef] [PubMed]
- Schimmer, B.; Lenferink, A.; Schneeberger, P.; Aangenend, H.; Vellema, P.; Hautvast, J.; van Duynhoven, Y. Seroprevalence and risk factors for Coxiella burnetii (Q fever) seropositivity in dairy goat farmers’ households in The Netherlands, 2009–2010. PLoS ONE 2012, 7, e42364. [Google Scholar] [CrossRef]
- Rousset, E.; Durand, B.; Berri, M.; Dufour, P.; Prigent, M.; Russo, P.; Delcroix, T.; Touratier, A.; Rodolakis, A.; Aubert, M. Comparative diagnostic potential of three serological tests for abortive Q fever in goat herds. Vet. Microbiol. 2007, 124, 286–297. [Google Scholar] [CrossRef] [PubMed]
- Rousset, E.; Sidi-Boumedine, K.; Kadra, B.; Kupcsulik, B. Q fever. In Manual of Diagnostic Tests and Vaccines for Terrestrial Animals 2018, 8th ed.; Office International des Epizooties: Paris, France, 2018; pp. 560–577. [Google Scholar]
- Böttcher, J.; Vossen, A.; Janowetz, B.; Alex, M.; Gangl, A.; Randt, A.; Meier, N. Insights into the dynamics of endemic Coxiella burnetii infection in cattle by application of phase-specific ELISAs in an infected dairy herd. Vet. Microbiol. 2011, 151, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Hatchette, T.; Campbell, N.; Hudson, R.; Raoult, D.; Marrie, T.J. Natural history of Q fever in goats. Vector Borne Zoonotic Dis. 2003, 3, 11–15. [Google Scholar] [CrossRef]
- Muleme, M.; Campbell, A.; Stenos, J.; Devlin, J.M.; Vincent, G.; Cameron, A.; Graves, S.; Wilks, C.R.; Firestone, S. A longitudinal study of serological responses to Coxiella burnetii and shedding at kidding among intensively-managed goats supports early use of vaccines. Vet. Res. 2017, 48, 50. [Google Scholar] [CrossRef]
- Roest, H.; Post, J.; van Gelderen, B.; van Zijderveld, F.G.; Rebel, J.M. Q fever in pregnant goats: Humoral and cellular immune responses. Vet. Res. 2013, 44, 67. [Google Scholar] [CrossRef] [PubMed]
- Sting, R.; Molz, K.; Philipp, W.; Bothe, F.; Runge, M.; Ganter, M. Quantitative real-time PCR and phase specific serology are mutually supportive in Q fever diagnostics in goats. Vet. Microbiol. 2013, 167, 600–608. [Google Scholar] [CrossRef]
- Shapiro, A.J.; Bosward, K.L.; Heller, J.; Norris, J.M. Seroprevalence of Coxiella burnetii in domesticated and feral cats in eastern Australia. Vet. Microbiol. 2015, 177, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, A.J.; Norris, J.M.; Heller, J.; Brown, G.; Malik, R.; Bosward, K.L. Seroprevalence of Coxiella burnetii in Australian dogs. Zoonoses Public Health 2016, 63, 458–466. [Google Scholar] [CrossRef] [PubMed]
- García-Gómez, E.; González-Pedrajo, B.; Camacho-Arroyo, I. Role of sex steroid hormones in bacterial-host interactions. BioMed Res. Int. 2013, 2013, 928290. [Google Scholar] [CrossRef]
- Robinson, D.P.; Klein, S.L. Pregnancy and pregnancy-associated hormones alter immune responses and disease pathogenesis. Horm. Behav. 2012, 62, 263–271. [Google Scholar] [CrossRef]
- Monteiro, C.; Kasahara, T.; Sacramento, P.M.; Dias, A.; Leite, S.; Silva, V.G.; Gupta, S.; Agrawal, A.; Bento, C.A.M. Human pregnancy levels of estrogen and progesterone contribute to humoral immunity by activating TFH/B cell axis. Eur. J. Immunol. 2021, 51, 167–179. [Google Scholar] [CrossRef] [PubMed]
- Howard, Z.P.; Omsland, A. Selective inhibition of Coxiella burnetii replication by the steroid hormone progesterone. Infect. Immun. 2020, 88, e00894-19. [Google Scholar] [CrossRef] [PubMed]
- Leone, M.; Honstettre, A.; Lepidi, H.; Capo, C.; Bayard, F.; Raoult, D.; Mege, J.-L. Effect of sex on Coxiella burnetii infection: Protective role of 17β-estradiol. J. Infect. Dis. 2004, 189, 339–345. [Google Scholar] [CrossRef]
- González, F.; Cabrera, F.; Batista, M.; Rodrίguez, N.; Álamo, D.; Sulon, J.; Beckers, J.-F.; Gracia, A. A comparison of diagnosis of pregnancy in the goat via transrectal ultrasound scanning, progesterone, and pregnancy-associated glycoprotein assays. Theriogenology 2004, 62, 1108–1115. [Google Scholar] [CrossRef]
- Bauer, B.U.; Knittler, M.R.; Prüfer, T.L.; Wolf, A.; Matthiesen, S.; Runge, M.; Ganter, M. Humoral immune response to Q fever vaccination of three sheep flocks naturally pre-infected with Coxiella burnetii. Vaccine 2021, 39, 1499–1507. [Google Scholar] [CrossRef]
- Fasemore, A.M.; Helbich, A.; Walter, M.C.; Dandekar, T.; Vergnaud, G.; Förstner, K.U.; Frangoulidis, D. CoxBase: An online platform for epidemiological surveillance, visualization, analysis and typing of Coxiella burnetii genomic sequence. bioRxiv 2020. [Google Scholar] [CrossRef]
- Roest, H.I.J.; Dinkla, A.; Koets, A.P.; Post, J.; van Keulen, L. Experimental Coxiella burnetii infection in non-pregnant goats and the effect of breeding. Vet. Res. 2020, 51, 74. [Google Scholar] [CrossRef] [PubMed]
- Lucchese, L.; Capello, K.; Barberio, A.; Zuliani, F.; Stegeman, A.; Ceglie, L.; Guerrini, E.; Marangon, S.; Natale, A. IFAT and ELISA phase I/phase II as tools for the identification of Q fever chronic milk shedders in cattle. Vet. Microbiol. 2015, 179, 102–108. [Google Scholar] [CrossRef]
- Álvarez-Alonso, R.; Basterretxea, M.; Barandika, J.F.; Hurtado, A.; Idiazabal, J.; Jado, I.; Beraza, X.; Montes, M.; Liendo, P.; García-Pérez, A.L. A Q fever outbreak with a high rate of abortions in a dairy goat farm: Coxiella burnetii shedding, environmental contamination and viability. Appl. Environ. Microbiol. 2018, 84, e01650-18. [Google Scholar] [CrossRef] [PubMed]
- Rodolakis, A. Q fever in dairy animals. Ann. N. Y. Acad. Sci. 2009, 1166, 90–93. [Google Scholar] [CrossRef]
- Hellenbrand, W.; Breuer, T.; Petersen, L. Changing epidemiology of Q fever in Germany, 1947–1999. Emerg. Infect. Dis. 2001, 7, 789–796. [Google Scholar] [CrossRef]
- Muzzio, D.; Zygmunt, M.; Jensen, F. The role of pregnancy-associated hormones in the development and function of regulatory B cells. Front. Endocrinol. 2014, 5, 39. [Google Scholar] [CrossRef]
- Recalde, G.; Moreno-Sosa, T.; Yúdica, F.; Quintero, C.A.; Sánchez, M.B.; Jahn, G.A.; Kalergis, A.M.; Mackern-Oberti, J.P. Contribution of sex steroids and prolactin to the modulation of T and B cells during autoimmunity. Autoimmun. Rev. 2018, 17, 504–512. [Google Scholar] [CrossRef]
- Lambert, K.C.; Curran, E.M.; Judy, B.M.; Milligan, G.N.; Lubahn, D.B.; Estes, D.M. Estrogen receptor α (ERα) deficiency in macrophages results in increased stimulation of CD4+ T cells while 17ß-estradiol acts through ERα to increase IL-4 and GATA-3 expression in CD4+ T cells independent of antigen presentation. J. Immunol. 2005, 175, 5716–5723. [Google Scholar] [CrossRef]
- Baumgärtner, W.; Bachmann, S. Histological and immunocytochemical characterization of Coxiella burnetii-associated lesions in the murine uterus and placenta. Infect. Immun. 1992, 60, 5232–5241. [Google Scholar] [CrossRef] [PubMed]
- Fischer, B.; Bavister, B.D. Oxygen tension in the oviduct and uterus of rhesus monkeys, hamsters and rabbits. J. Reprod. Fertil. 1993, 99, 673–679. [Google Scholar] [CrossRef]
- Jauniaux, E.; Watson, A.; Burton, G. Evaluation of respiratory gases and acid-base gradients in human fetal fluids and uteroplacental tissue between 7 and 16 weeks’ gestation. Am. J. Obstet. Gynecol. 2001, 184, 998–1003. [Google Scholar] [CrossRef]
- Rodesch, F.; Simon, P.; Donner, C.; Jauniaux, E. Oxygen measurements in endometrial and trophoblastic tissues during early pregnancy. Obstet. Gynecol. 1992, 80, 283–285. [Google Scholar] [PubMed]
- Soares, M.J.; Iqbal, K.; Kozai, K. Hypoxia and placental development. Birth Defects Res. 2017, 109, 1309–1329. [Google Scholar] [CrossRef]
- Hayek, I.; Fischer, F.; Schulze-Luehrmann, J.; Dettmer, K.; Sobotta, K.; Schatz, V.; Kohl, L.; Boden, K.; Lang, R.; Oefner, P.J.; et al. Limitation of TCA cycle intermediates represents an oxygen-independent nutritional antibacterial effector mechanism of macrophages. Cell Rep. 2019, 26, 3502–3510.e3506. [Google Scholar] [CrossRef] [PubMed]
- Kanellopoulos-Langevin, C.; Caucheteux, S.M.; Verbeke, P.; Ojcius, D.M. Tolerance of the fetus by the maternal immune system: Role of inflammatory mediators at the feto-maternal interface. Reprod. Biol. Endocrinol. 2003, 1, 121. [Google Scholar] [CrossRef][Green Version]
- Guleria, I.; Pollard, J.W. The trophoblast is a component of the innate immune system during pregnancy. Nat. Med. 2000, 6, 589–593. [Google Scholar] [CrossRef]
- Hönig, A.; Rieger, L.; Kapp, M.; Sütterlin, M.; Dietl, J.; Kämmerer, U. Indoleamine 2,3-dioxygenase (IDO) expression in invasive extravillous trophoblast supports role of the enzyme for materno-fetal tolerance. J. Reprod. Immunol. 2004, 61, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Uckan, D.; Steele, A.; Cherry; Wang, B.Y.; Chamizo, W.; Koutsonikolis, A.; Gilbert-Barness, E.; Good, R.A. Trophoblasts express Fas ligand: A proposed mechanism for immune privilege in placenta and maternal invasion. Mol. Hum. Reprod. 1997, 3, 655–662. [Google Scholar] [CrossRef]
- Yang, F.; Zheng, Q.; Jin, L. Dynamic function and composition changes of immune cells during normal and pathological pregnancy at the maternal-fetal interface. Front. Immunol. 2019, 10, 2317. [Google Scholar] [CrossRef]
- Sojka, D.K.; Yang, L.; Yokoyama, W.M. Uterine natural killer cells. Front. Immunol. 2019, 10, 960. [Google Scholar] [CrossRef] [PubMed]
- Blois, S.M.; Kammerer, U.; Soto, C.A.; Tometten, M.C.; Shaikly, V.; Barrientos, G.; Jurd, R.; Rukavina, D.; Thomson, A.W.; Klapp, B.F.; et al. Dendritic Cells: Key to fetal tolerance? Biol. Reprod. 2007, 77, 590–598. [Google Scholar] [CrossRef]
- Tagliani, E.; Erlebacher, A. Dendritic cell function at the maternal-fetal interface. Expert Rev. Clin. Immunol. 2011, 7, 593–602. [Google Scholar] [CrossRef]
- von Andrian, U.H.; Mempel, T.R. Homing and cellular traffic in lymph nodes. Nat. Rev. Immunol. 2003, 3, 867–878. [Google Scholar] [CrossRef]
- Kammerer, U.; Kruse, A.; Barrientos, G.; Arck, P.C.; Blois, S.M. Role of dendritic cells in the regulation of maternal immune responses to the fetus during mammalian gestation. Immunol. Investig. 2008, 37, 499–533. [Google Scholar] [CrossRef]
- Zenclussen, M.; Bertoja, A.; Gerlof, K.; Ritschel, S.; Sollwedel, A.; Volk, H.; Zenclussen, A. During pregnancy, treg cells induce a privileged tolerant microenvironment at the fetal-maternal interface by up-regulating HO-1, TGF-β and LIF expression. Am. J. Reprod. Immunol. 2005, 54. [Google Scholar] [CrossRef]
- Karsten, C.M.; Behrends, J.; Wagner, A.K.; Fuchs, F.; Figge, J.; Schmudde, I.; Hellberg, L.; Kruse, A. DC within the pregnant mouse uterus influence growth and functional properties of uterine NK cells. Eur. J. Immunol. 2009, 39, 2203–2214. [Google Scholar] [CrossRef]
- Schwede, S.; Alfer, J.; von Rango, U. Differences in regulatory T-cell and dendritic cell pattern in decidual tissue of placenta accreta/increta cases. Placenta 2014, 35, 378–385. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bauer, B.U.; Knittler, M.R.; Herms, T.L.; Frangoulidis, D.; Matthiesen, S.; Tappe, D.; Runge, M.; Ganter, M. Multispecies Q Fever Outbreak in a Mixed Dairy Goat and Cattle Farm Based on a New Bovine-Associated Genotype of Coxiella burnetii. Vet. Sci. 2021, 8, 252. https://doi.org/10.3390/vetsci8110252
Bauer BU, Knittler MR, Herms TL, Frangoulidis D, Matthiesen S, Tappe D, Runge M, Ganter M. Multispecies Q Fever Outbreak in a Mixed Dairy Goat and Cattle Farm Based on a New Bovine-Associated Genotype of Coxiella burnetii. Veterinary Sciences. 2021; 8(11):252. https://doi.org/10.3390/vetsci8110252
Chicago/Turabian StyleBauer, Benjamin U., Michael R. Knittler, T. Louise Herms, Dimitrios Frangoulidis, Svea Matthiesen, Dennis Tappe, Martin Runge, and Martin Ganter. 2021. "Multispecies Q Fever Outbreak in a Mixed Dairy Goat and Cattle Farm Based on a New Bovine-Associated Genotype of Coxiella burnetii" Veterinary Sciences 8, no. 11: 252. https://doi.org/10.3390/vetsci8110252
APA StyleBauer, B. U., Knittler, M. R., Herms, T. L., Frangoulidis, D., Matthiesen, S., Tappe, D., Runge, M., & Ganter, M. (2021). Multispecies Q Fever Outbreak in a Mixed Dairy Goat and Cattle Farm Based on a New Bovine-Associated Genotype of Coxiella burnetii. Veterinary Sciences, 8(11), 252. https://doi.org/10.3390/vetsci8110252






