Bilateral Renal Large B Cell Lymphoma in a Dog: A Case Report and Review of the Literature
Abstract
:1. Introduction
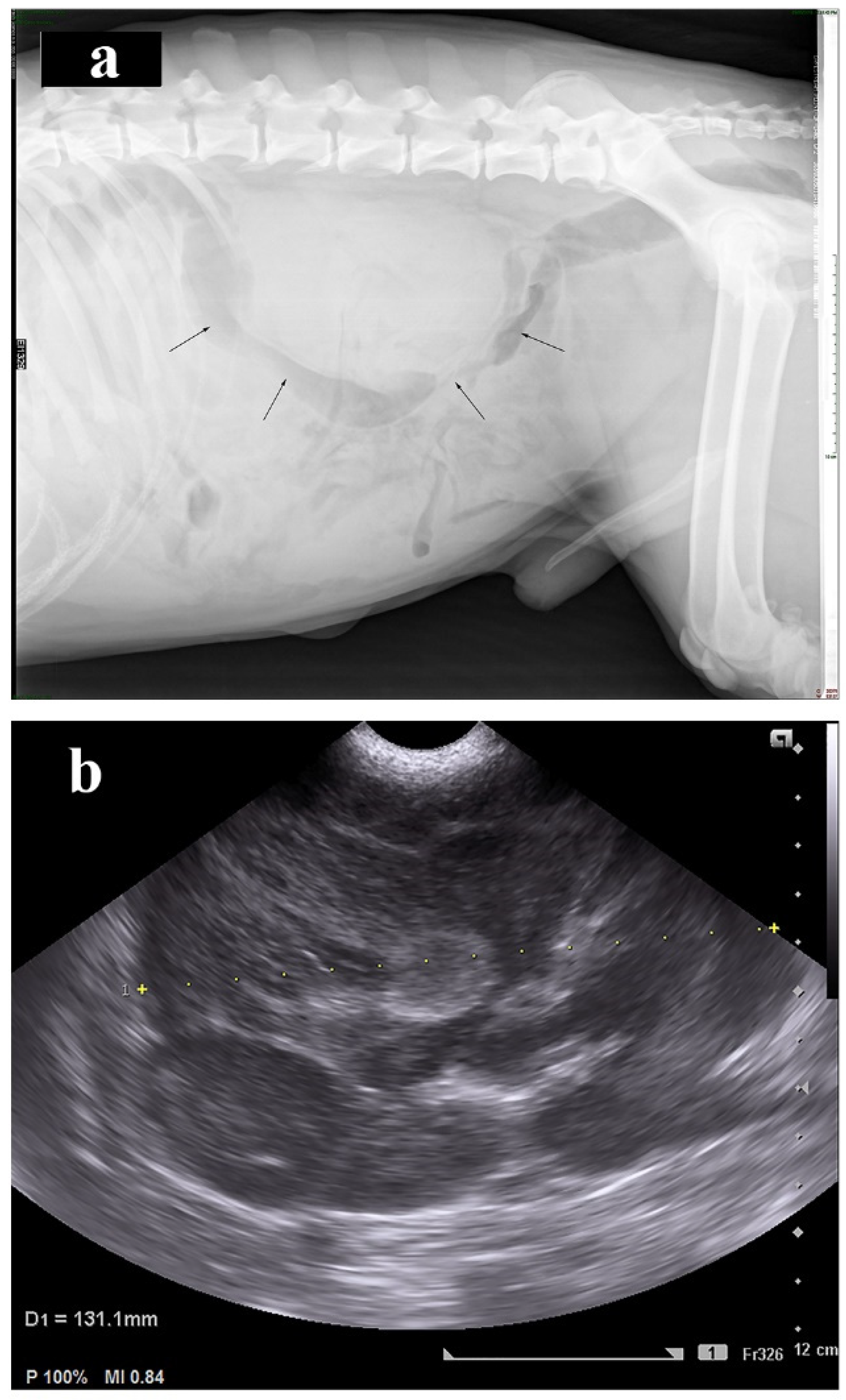
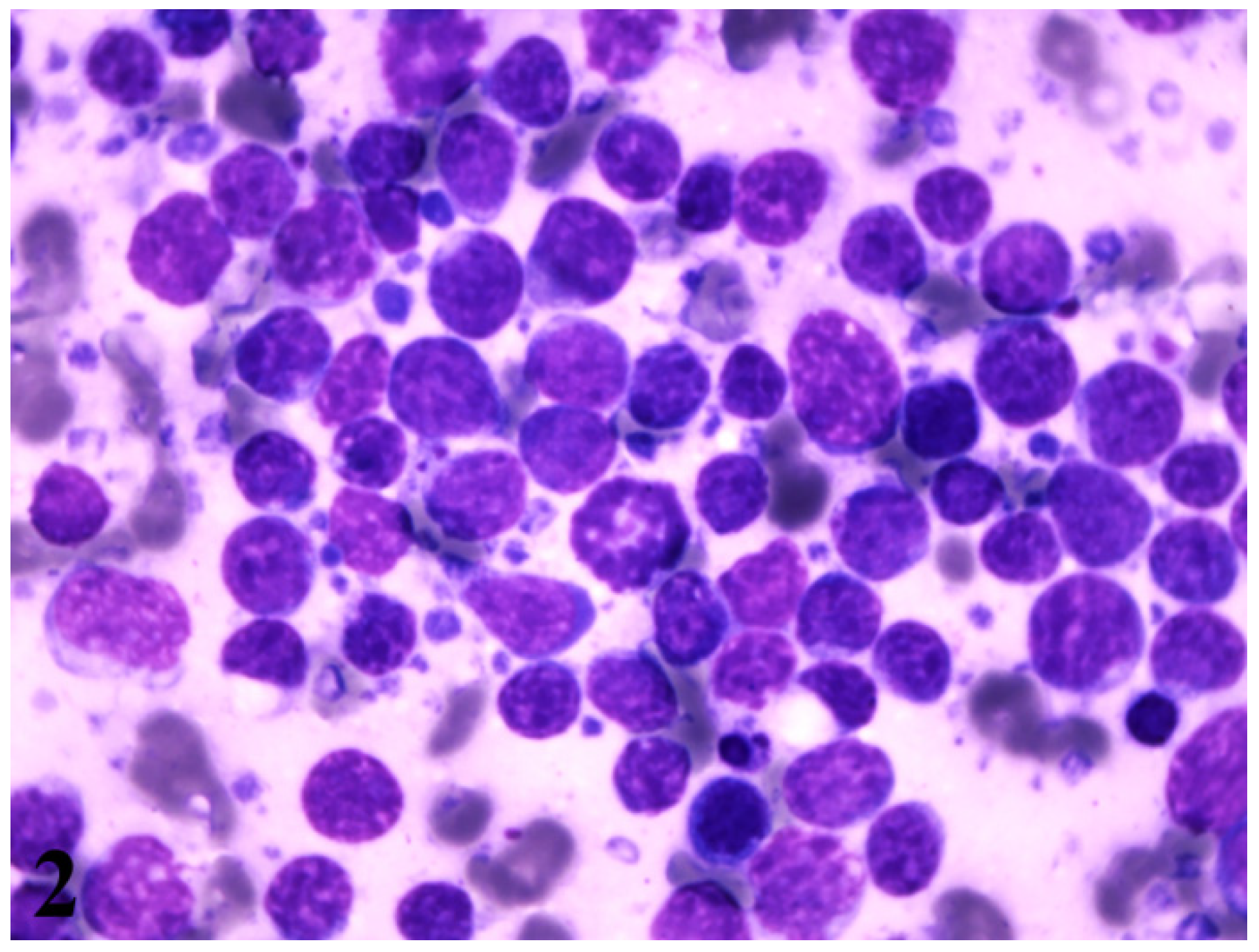
2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Baskin, G.B.; De Paoli, A. Primary renal neoplasms of the dog. Vet. Pathol. 1977, 14, 591–605. [Google Scholar] [CrossRef] [PubMed]
- Valli, V.E.; Bienzle, D.; Meuten, D.J.; Linder, K.E. Tumors of the Hemolymphatic System. In Tumors in Domestic Animals, 5th ed.; Meuten, D.J., Ed.; John Wiley & Sons: Ames, IA, USA, 2017; pp. 203–321. [Google Scholar]
- Cienava, E.A.; Barnhart, K.F.; Brown, R.; Mansell, J.; Dunstan, R.; Credille, K. Morphologic, immunohistochemical, and molecular characterization of hepatosplenic T-cell lymphoma in a dog. Vet. Clin. Pathol. 2004, 33, 105–110. [Google Scholar] [CrossRef]
- Dank, G.; Rassnick, K.M.; Kristal, O.; Rodriguez, C.O.; Clifford, C.A.; Ward, R.; Mallett, C.L.; Gieger, T.; Segev, G. Clinical characteristics, treatment, and outcome of dogs with presumed primary hepatic lymphoma: 18 cases (1992–2008). J. Am. Vet. Med. Assoc. 2011, 239, 966–971. [Google Scholar] [CrossRef] [PubMed]
- Kaldrymidou, E.; Papaioannou, N.; Poutahidis, T.; Karayannopoulou, M.; Gruys, E.; Toliou, T.; Tsangaris, T. Malignant lymphoma in nasal cavity and paranasal sinuses of a dog. J. Vet. Med. A Physiol. Pathol. Clin. Med. 2000, 47, 457–462. [Google Scholar] [CrossRef]
- Keller, S.M.; Vernau, W.; Hodges, J.; Kass, P.H.; Vilches-Moure, J.G.; McElliot, V.; Moore, P.F. Hepatosplenic and hepatocytotropic T-cell lymphoma: Two distinct types of T-cell lymphoma in dogs. Vet. Pathol. 2013, 50, 281–290. [Google Scholar] [CrossRef]
- Long, S.N.; Johnston, P.E.; Anderson, T.J. Primary T-cell lymphoma of the central nervous system in a dog. J. Am. Vet. Med. Assoc. 2001, 218, 719–722. [Google Scholar] [CrossRef]
- Maiolino, P.; Devico, G. Primary Epitheliotropic T-cell Lymphoma of the Urinary Bladder in a Dog. Vet. Pathol. 2000, 37, 184–186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thuilliez, C.; Watrelot-Virieux, D.; Chanut, F.; Fournel-Fleury, C.; Ponce, F.; Marchal, T. Presumed primary muscular lymphoma in a dog. J. Vet. Diagn. Investig. 2008, 20, 824–826. [Google Scholar] [CrossRef] [Green Version]
- Takeuchi, Y.; Fujino, Y.; Goto-Koshino, Y.; Ohno, K.; Uchida, K.; Nakayama, H.; Tsujimoto, H. Long Term Survival of Primary Skeletal Muscle Lymphoma in a Miniature Dachshund. J. Vet. Med. Sci. 2010, 72, 673–677. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bryan, J.N.; Henry, C.J.; Turnquist, S.E.; Tyler, J.W.; Liptak, J.M.; Rizzo, S.A.; Sfiligoi, G.; Steinberg, S.J.; Smith, A.N.; Jackson, T. Primary renal neoplasia of dogs. J. Vet. Intern. Med. 2006, 20, 1155–1160. [Google Scholar] [CrossRef]
- Batchelor, D.J.; Bright, S.R.; Ibarrola, P.; Tzannes, S.; Blackwood, L. Long-term survival after combination chemotherapy for bilateral renal malignant lymphoma in a dog. N. Z. Vet. J. 2006, 54, 147–150. [Google Scholar] [CrossRef]
- Breshears, M.A.; Meinkoth, J.H.; Stern, A.W.; Buoncompagni, S.; Thomason, J.D. Pathology in practice. Renal lymphoma. J. Am. Vet. Med. Assoc. 2011, 238, 167–169. [Google Scholar] [CrossRef]
- Cook, S.M.; Lothrop, C.D.J. Serum erythropoietin concentrations measured by radioimmunoassay in normal, polycythemic, and anemic dogs and cats. J. Vet. Intern. Med. 1994, 8, 18–25. [Google Scholar] [CrossRef]
- Cotchin, E. Further observations on neoplasms in dogs, with particular reference to site of origin and malignancy. Part II: Male genital, skeletal, lymphatic and other systems. Br. Vet. J. 1954, 110, 274–286. [Google Scholar] [CrossRef]
- Durno, A.S.; Webb, J.A.; Gauthier, M.J.; Bienzle, D. Polycythemia and inappropriate erythropoietin concentrations in two dogs with renal T-cell lymphoma. J. Am. Anim. Hosp. Assoc. 2011, 47, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Froment, R.; Gara-Boivin, C. Bilateral renal T-cell lymphoma with hepatic infiltration and secondary polycythemia in a dog: Utility of cytology slides. Can. Vet. J. 2015, 56, 1287–1291. [Google Scholar]
- Klein, M.K.; Cockerell, G.L.; Harris, C.K.; Withrow, S.J.; Lulich, J.P.; Ogilvie, G.K.; Norris, A.M.; Harvey, H.J.; Richardson, R.F.; Fowler, J.D.; et al. Canine primary renal neoplasms: A retrospective review of 54 cases. J. Am. Anim. Hosp. Assoc. 1988, 24, 443–452. [Google Scholar]
- Lane, E.P.; Lobetti, R.G. Renal T-cell lymphoma with cerebral metastasis in a dog with chronic canine ehrlichiosis. J. S. Afr. Vet. Assoc. 2002, 73, 83–85. [Google Scholar] [CrossRef] [Green Version]
- Lascelles, B.D.; Monnet, E.; Liptak, J.; Johnson, J.; Dernell, W.S. Surgical treatment of right-sided renal lymphoma with invasion of the caudal vena cava. J. Small Anim. Pract. 2003, 44, 135–138. [Google Scholar] [CrossRef] [PubMed]
- Nelson, R.W.; Hager, D.; Zanjani, E.D. Renal lymphosarcoma with inappropriate erythropoietin production in a dog. J. Am. Vet. Med. Assoc. 1983, 182, 1396–1397. [Google Scholar]
- Osborne, C.A.; Johnson, K.H.; Kurtz, H.J.; Hanlon, G.F. Renal lymphoma in the dog and cat. J. Am. Vet. Med. Assoc. 1971, 158, 2058–2070. [Google Scholar]
- Snead, E.C. A case of bilateral renal lymphosarcoma with secondary polycythaemia and paraneoplastic syndromes of hypoglycaemia and uveitis in an English springer spaniel. Vet. Comp. Oncol. 2005, 3, 139–144. [Google Scholar] [CrossRef]
- Walter, P.A.; Feeney, D.A.; Johnston, G.R.; O’Leary, T.P. Ultrasonographic evaluation of renal parenchymal diseases in dogs: 32 cases. J. Am. Vet. Med. Assoc. 1986, 191, 999–1007. [Google Scholar]
- Zhao, D.; Yamaguchi, R.; Tateyama, S.; Yamazaki, Y.; Ogawa, H. Bilateral renal lymphosarcoma in a dog. J. Vet. Med. Sci. 1993, 55, 657–659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zandvliet, M. Canine lymphoma: A review. Vet. Q. 2016, 36, 76–104. [Google Scholar] [CrossRef]
- Chen, X.; Hu, D.; Fang, L.; Chen, Y.; Che, X.; Tao, J.; Weng, G.; Ye, X. Primary renal lymphoma: A case report and literature review. Oncol. Lett. 2016, 12, 4001–4008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Comazzi, S.; Marelli, S.; Cozzi, M.; Rizzi, R.; Finotello, R.; Henriques, J.; Pastor, J.; Ponce, F.; Rohrer-Bley, C.; Rütgen, B.C.; et al. Breed-associated risks for developing canine lymphoma differ among countries: An European canine lymphoma network study. BMC Vet. Res. 2018, 14, 232. [Google Scholar] [CrossRef] [Green Version]
- Grindem, C.B.; Breitschwerdt, E.B.; Corbett, W.T.; Page, R.L.; Jans, H.E. Thrombocytopenia Associated with Neoplasia in Dogs. J. Vet. Intern. Med. 1994, 8, 400–405. [Google Scholar] [CrossRef] [PubMed]
- Kooistra, H.S. Diseases and Disorders. In Clinical Veterinary Advisor: Dogs and Cats, 3rd ed.; Cote, E., Ed.; Elsevier: St Louis, MI, USA, 2017; pp. 4968–4979. [Google Scholar]
- Nelson, R.W. Endocrine, Metabolic, and Lipid Disorders. In Small Animal Clinical Diagnosis by Laboratory Methods, 5th ed.; Willard, M.D., Tvedten, H., Eds.; Elsevier: St Louis, MI, USA, 2012; pp. 156–190. [Google Scholar]
- Weiss, D.J.; Tvedten, H. Erythrocyte Disorders. In Small Animal Clinical Diagnosis by Laboratory Methods, 5th ed.; Willard, M.D., Tvedten, H., Eds.; Elsevier: St Louis, MI, USA, 2012; pp. 38–62. [Google Scholar]
- Meuten, D.J.; Meuten, T.L.K. Tumors of the Urinary System. In Tumors in Domestic Animals, 5th ed.; Meuten, D.J., Ed.; John Wiley & Sons: Ames, IA, USA, 2017; pp. 632–688. [Google Scholar]
- Rissman, C.M.; Dagrosa, L.M.; Pettus, J.R.; Dillon, J.L.; Sverrisson, E.F. Primary renal lymphoma: An unusual finding following radical nephrectomy. Clin. Nephrol. Case Stud. 2017, 5, 1–4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ingle, S.B.; Ingle, C.R.H. Primary splenic lymphoma: Current diagnostic trends. World J. Clin. Cases 2016, 4, 385–389. [Google Scholar] [CrossRef] [PubMed]
- Dasgupta, T.; Coombes, B.; Brasfield, R.D. Primary malignant neoplasms of the spleen. Surg. Gynecol. Obstet. 1965, 120, 947–960. [Google Scholar]
- Skarin, A.T.; Davey, F.R.; Moloney, W.C. Lymphosarcoma of the spleen. Arch. Intern. Med. 1971, 127, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Kraemer, B.B.; Osborne, B.M.; Butler, J.J. Primary splenic presentation of malignant lymphoma and related disorders—A study of 49 cases. Cancer 1984, 54, 1606–1619. [Google Scholar] [CrossRef]
- Kehoe, J.; Straus, D.J. Primary lymphoma of the spleen: Clinical features and outcome after splenectomy. Cancer 1988, 62, 1434–1438. [Google Scholar] [CrossRef]



| Breed | Age | Sex | Laboratory Tests | Imaging | FNA | Necropsy | Histopathology | IHC/Cell Type |
|---|---|---|---|---|---|---|---|---|
| Scottish Terrier | 5 | ♂ | NA | NA | NA | (+) | NA | NA |
| Basenji | 2 | ♀ | (+) | (+) | NA | (+) | (+) | NA |
| Collie | 5.5 | ♂ | NA | NA | NA | (+) | (+) | NA |
| Labrador | 9 | ♂ | (+) | (+) | NA | NA | (+) | NA |
| Unknown | NA | NA | NA | (+) | NA | NA | (+) | NA |
| Medium or large breed | NA | NA | (+) | (+) | NA | (+) | (+) | NA |
| ShibaInu | 3 | ♀ | (+) | (+) | NA | (+) | (+) | NA |
| Doberman | 5 | ♀ | (+) | NA | NA | NA | (+) | NA |
| Staffordshire | 5 | ♀ | (+) | NA | NA | (+) | (+) | (+)/T |
| Basset Hound | 8 | ♂ | (+) | (+) | NA | NA | (+) | (+)/T |
| Springer Spaniel | 8 | ♂ | (+) | (+) | (+) | (+) | (+) | NA |
| Flat coated Retriever | 6 | ♂ | (+) | (+) | (+) | NA | NA | NA |
| Mixed breed | 2 | ♂ | (+) | (+) | NA | (+) | (+) | (+)/T |
| Cocker Spaniel | 3 | ♂ | (+) | (+) | NA | (+) | (+) | (+)/T |
| Border Collie | 9 | ♀ | (+) | (+) | NA | (+) | (+) | (+)/T |
| Bernese mountain | 8 | ♂ | (+) | (+) | (+) | NA | NA | (+)/T |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Apostolopoulou, E.P.; Vlemmas, I.; Pardali, D.; Adamama-Moraitou, K.K.; Poutahidis, T.; Papadopoulou, P.L.; Brellou, G.D. Bilateral Renal Large B Cell Lymphoma in a Dog: A Case Report and Review of the Literature. Vet. Sci. 2021, 8, 258. https://doi.org/10.3390/vetsci8110258
Apostolopoulou EP, Vlemmas I, Pardali D, Adamama-Moraitou KK, Poutahidis T, Papadopoulou PL, Brellou GD. Bilateral Renal Large B Cell Lymphoma in a Dog: A Case Report and Review of the Literature. Veterinary Sciences. 2021; 8(11):258. https://doi.org/10.3390/vetsci8110258
Chicago/Turabian StyleApostolopoulou, Emmanouela P., Ioannis Vlemmas, Dimitra Pardali, Katerina K. Adamama-Moraitou, Theofilos Poutahidis, Paraskevi L. Papadopoulou, and Georgia D. Brellou. 2021. "Bilateral Renal Large B Cell Lymphoma in a Dog: A Case Report and Review of the Literature" Veterinary Sciences 8, no. 11: 258. https://doi.org/10.3390/vetsci8110258






