Clinical Cohort Study in Canine Patients, to Determine the Average Platelet and White Blood Cell Number and Its Correlation with Patient’s Age, Weight, Breed and Gender: 92 Cases (2019–2020)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Recruitment
2.2. Blood Extraction and PRP Collection
2.3. Statistical Analysis
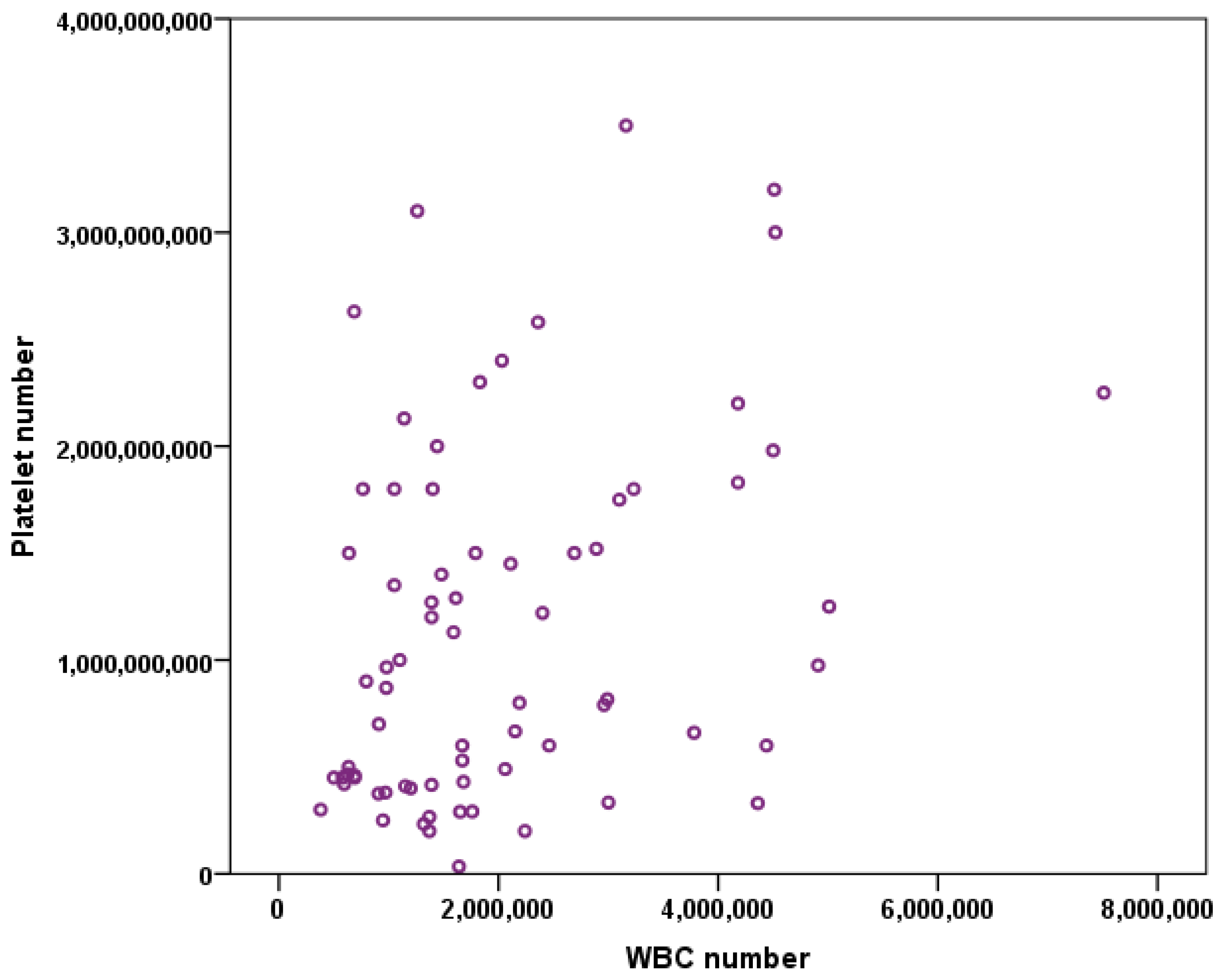
3. Results
3.1. Platelet Number vs. WBC Number
3.2. Platelets and WBC vs. Age
3.3. Platelets and WBC Number vs. Weight
3.4. Platelets and WBC vs. Breed
3.5. Platelets and WBC vs. Sex
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wu, P.I.; Diaz, R.; Borg-Stein, J. Platelet-Rich Plasma. Phys. Med. Rehabil. Clin. N. Am. 2016, 27, 825–853. [Google Scholar] [CrossRef]
- Malanga, G.A.; Goldin, M. PRP: Review of the current evidence for musculoskeletal conditions. Curr. Phys. Med. Rehabil. Rep. 2014, 2, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Marx, R.E. Platelet-rich plasma: Evidence to support its use. J. Oral Maxillofac. Surg. 2004, 62, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Gato-Calvo, L.; Magalhaes, J.; Ruiz-Romero, C.; Blanco, F.J.; Burguera, E.F. Platelet-rich plasma in osteoarthritis treatment: Review of current evidence. Ther. Adv. Chronic Dis. 2019, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anitua, E. Plasma rich in growth factors: Preliminary results of use in the preparation of future sites for implants. Int. J. Oral Maxillofac. Implant 1999, 14, 529–535. [Google Scholar]
- Marx, R.E.; Carlson, E.R.; Eichstaedt, R.M.; Schimmele, S.R.; Strauss, J.E.; Georgeff, K.R. Platelet-rich plasma: Growth factor enhancement for bone grafts. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 1998, 85, 638–646. [Google Scholar] [CrossRef]
- DelRossi, A.J.; Cernaianu, A.C.; Vertrees, R.A.; Wacker, C.J.; Fuller, S.J.; Cilley, J.H., Jr.; Baldino, W.A. Platelet-rich plasma reduces postoperative blood loss after cardiopulmonary bypass. J. Thorac. Cardiovasc. Surg. 1990, 100, 281–286. [Google Scholar] [CrossRef]
- Ferrari, M.; Zia, S.; Valbonesi, M.; Henriquet, F.; Venere, G.; Spagnolo, S.; Grasso, M.A.; Panzani, I. A new technique for hemodilution, preparation of autologous platelet-rich plasma and intraoperative blood salvage in cardiac surgery. Int. J. Artif. Organs 1987, 10, 47–50. [Google Scholar] [CrossRef]
- He, Y.; Chen, J.; Huang, Y.; Pan, Q.; Nie, M. Local Application of Platelet-Rich Fibrin During Lower Third Molar Extraction Improves Treatment Outcomes. J. Oral Maxillofac. Surg. 2017, 75, 2497–2506. [Google Scholar] [CrossRef] [Green Version]
- Sriram, S.; Sankaralingam, R.; Mani, M.; Tamilselvam, T.N. Autologous platelet rich plasma in the management of non-healing vasculitic ulcers. Int. J. Rheum. Dis. 2016, 19, 1331–1336. [Google Scholar] [CrossRef] [PubMed]
- Alio, J.L.; Rodriguez, A.E.; Ferreira-Oliveira, R.; Wróbel-Dudzińska, D.; Abdelghany, A.A. Treatment of Dry Eye Disease with Autologous Platelet-Rich Plasma: A Prospective, Interventional, Non-Randomized Study. Ophthalmol. Ther. 2017, 6, 285–293. [Google Scholar] [CrossRef] [Green Version]
- Canapp, S.O.; Leasure, C.S.; Cox, C.; Ibrahim, V.; Carr, B.J. Partial Cranial Cruciate Ligament Tears Treated with Stem Cell and Platelet-Rich Plasma Combination Therapy in 36 Dogs: A Retrospective Study. Front. Vet. Sci. 2016, 3, 112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, R.; Gu, Y.; Ran, J.; Hu, Y.; Zheng, Z.; Zeng, M.; Heng, B.C.; Chen, X.; Yin, Z.; Chen, W.; et al. Intratendon Delivery of Leukocyte-Poor Platelet-Rich Plasma Improves Healing Compared with Leukocyte-Rich Platelet-Rich Plasma in a Rabbit Achilles Tendinopathy Model. Am. J. Sports Med. 2017, 45, 1909–1920. [Google Scholar] [CrossRef]
- Reurink, G.; Goudswaard, G.J.; Moen, M.H.; Weir, A.; Verhaar, J.A.; Bierma-Zeinstra, S.M.; Maas, M.; Tol, J.L. Rationale, secondary outcome scores and 1-year follow-up of a randomised trial of platelet-rich plasma injections in acute hamstring muscle injury: The Dutch Hamstring Injection Therapy study. Br. J. Sports Med. 2015, 49, 1206–1212. [Google Scholar] [CrossRef] [PubMed]
- Sengul, A.T.; Buyukkkarabacak, Y.B.; Altunkaynak, B.Z.; Yetim, T.D.; Altun, G.Y.; Sengul, B.; Basoglu, A. Effects of platelet-rich plasma on cartilage regeneration after costal cartilage resection: A stereological and histopathological study. Acta Chir. Belg. 2017, 117, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Namazi, H.; Mehbudi, A. Investigating the effect of intra-articular PRP injection on pain and function improvement in patients with distal radius fracture. Orthop. Traumatol. Surg. Res. OTSR 2016, 102, 47–52. [Google Scholar] [CrossRef]
- Laver, L.; Marom, N.; Dnyanesh, L.; Mei-Dan, O.; Espregueira-Mendes, J.; Gobbi, A. PRP for Degenerative Cartilage Disease: A Systematic Review of Clinical Studies. Cartilage 2017, 8, 341–364. [Google Scholar] [CrossRef]
- Laver, L.; Carmont, M.R.; McConkey, M.O.; Palmanovich, E.; Yaacobi, E.; Mann, G.; Nyska, M.; Kots, E.; Mei-Dan, O. Plasma rich in growth factors (PRGF) as a treatment for high ankle sprain in elite athletes: A randomized control trial. Knee Surg. Sports Traumatol. Arthrosc. 2015, 23, 3383–3392. [Google Scholar] [CrossRef] [PubMed]
- Venator, K.P.; Frye, C.W.; Gamble, L.J.; Wakshlag, J.J. Assessment of a Single Intra-Articular Stifle Injection of Pure Platelet Rich Plasma on Symmetry Indices in Dogs with Unilateral or Bilateral Stifle Osteoarthritis from Long-Term Medically Managed Cranial Cruciate Ligament Disease. Vet. Med. 2020, 11, 31–38. [Google Scholar] [CrossRef] [Green Version]
- Upchurch, D.A.; Renberg, W.C.; Roush, J.K.; Milliken, G.A.; Weiss, M.L. Effects of administration of adipose-derived stromal vascular fraction and platelet-rich plasma to dogs with osteoarthritis of the hip joints. Am. J. Vet. Res. 2016, 77, 940–951. [Google Scholar] [CrossRef] [Green Version]
- Cortese, L.; Christopherson, P.W.; Pelagalli, A. Platelet Function and Therapeutic Applications in Dogs: Current Status and Future Prospects. Animals 2020, 10, 201. [Google Scholar] [CrossRef] [Green Version]
- Carr, B.J.; Canapp, S.O., Jr.; Mason, D.R.; Cox, C.; Hess, T. Canine Platelet-Rich Plasma Systems: A Prospective Analysis. Front. Vet. Sci. 2015, 2, 73. [Google Scholar] [CrossRef]
- Davis, V.L.; Abukabda, A.B.; Radio, N.M.; Witt-Enderby, P.A.; Clafshenkel, W.P.; Cairone, J.V.; Rutkowski, J.L. Platelet-rich preparations to improve healing. Part I: Workable options for every size practice. J. Oral Implantol. 2014, 40, 500–510. [Google Scholar] [CrossRef] [PubMed]
- Boswell, S.G.; Cole, B.J.; Sundman, E.A.; Karas, V.; Fortier, L.A. Platelet-rich plasma: A milieu of bioactive factors. Arthroscopy 2012, 28, 429–439. [Google Scholar] [CrossRef] [PubMed]
- Blair, P.; Flaumenhaft, R. Platelet alpha-granules: Basic biology and clinical correlates. Blood Rev. 2009, 23, 177–189. [Google Scholar] [CrossRef] [Green Version]
- DeLong, J.M.; Russell, R.P.; Mazzocca, A.D. Platelet-rich plasma: The PAW classification system. Arthroscopy 2012, 28, 998–1009. [Google Scholar] [CrossRef]
- Magalon, J.; Chateau, A.L.; Bertrand, B.; Louis, M.L.; Silvestre, A.; Giraudo, L.; Veran, J.; Sabatier, F. DEPA classification: A proposal for standardising PRP use and a retrospective application of available devices. BMJ Open Sport Exerc. Med. 2016, 2, e000060. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fadadu, P.P.; Mazzola, A.J.; Hunter, C.W.; Davis, T.T. Review of concentration yields in commercially available platelet-rich plasma (PRP) systems: A call for PRP standardization. Reg. Anesth. Pain Med. 2019, 44, 652–659. [Google Scholar] [CrossRef]
- Xu, Z.; Yin, W.; Zhang, Y.; Qi, X.; Chen, Y.; Xie, X.; Zhang, C. Comparative evaluation of leukocyte- and platelet-rich plasma and pure platelet-rich plasma for cartilage regeneration. Sci. Rep. 2017, 7, 43301. [Google Scholar] [CrossRef]
- Weibrich, G.; Hansen, T.; Kleis, W.; Buch, R.; Hitzler, W.E. Effect of platelet concentration in platelet-rich plasma on peri-implant bone regeneration. Bone 2004, 34, 665–671. [Google Scholar] [CrossRef]
- Bendinelli, P.; Matteucci, E.; Dogliotti, G.; Corsi, M.M.; Banfi, G.; Maroni, P.; Desiderio, M.A. Molecular basis of anti-inflammatory action of platelet-rich plasma on human chondrocytes: Mechanisms of NF-κB inhibition via HGF. J. Cell. Physiol. 2010, 225, 757–766. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Braun, H.J.; Kim, H.J.; Chu, C.R.; Dragoo, J.L. The effect of platelet-rich plasma formulations and blood products on human synoviocytes: Implications for intra-articular injury and therapy. Am. J. Sports Med. 2014, 42, 1204–1210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dragoo, J.L.; Braun, H.J.; Durham, J.L.; Ridley, B.A.; Odegaard, J.I.; Luong, R.; Arnoczky, S.P. Comparison of the acute inflammatory response of two commercial platelet-rich plasma systems in healthy rabbit tendons. Am. J. Sports Med. 2012, 40, 1274–1281. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, R.; Arnold, D.; Strasser, E.; Ringwald, J.; Schlegel, A.; Wiltfang, J.; Eckstein, R. Sample preparation technique and white cell content influence the detectable levels of growth factors in platelet concentrates. Vox Sang. 2003, 85, 283–289. [Google Scholar] [CrossRef]
- Fujiwara, M.; Yonezawa, T.; Arai, T.; Yamamoto, I.; Ohtsuka, H. Alterations with age in peripheral blood lymphocyte subpopulations and cytokine synthesis in beagles. Vet. Med. 2012, 3, 79–84. [Google Scholar] [CrossRef] [Green Version]
- Bourgès-Abella, N.H.; Gury, T.D.; Geffré, A.; Concordet, D.; Thibault-Duprey, K.C.; Dauchy, A.; Trumel, C. Reference intervals, intraindividual and interindividual variability, and reference change values for hematologic variables in laboratory beagles. J. Am. Assoc. Lab. Anim. Sci. JAALAS 2015, 54, 17–24. [Google Scholar]
- Harper, E.J.; Hackett, R.M.; Wilkinson, J.; Heaton, P.R. Age-related variations in hematologic and plasma biochemical test results in Beagles and Labrador Retrievers. J. Am. Vet. Med. Assoc. 2003, 223, 1436–1442. [Google Scholar] [CrossRef]
- Xiong, G.; Lingampalli, N.; Koltsov, J.C.B.; Leung, L.L.; Bhutani, N.; Robinson, W.H.; Chu, C.R. Men and Women Differ in the Biochemical Composition of Platelet-Rich Plasma. Am. J. Sports Med. 2018, 46, 409–419. [Google Scholar] [CrossRef] [PubMed]
- Evanson, J.R.; Guyton, M.K.; Oliver, D.L.; Hire, J.M.; Topolski, R.L.; Zumbrun, S.D.; McPherson, J.C.; Bojescul, J.A. Gender and age differences in growth factor concentrations from platelet-rich plasma in adults. Mil. Med. 2014, 179, 799–805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taşoğlu, Ö.; Şahin, A.; Karataş, G.; Koyuncu, E.; Taşoğlu, İ.; Tecimel, O.; Özgirgin, N. Blood mean platelet volume and platelet lymphocyte ratio as new predictors of hip osteoarthritis severity. Medicine 2017, 96, e6073. [Google Scholar] [CrossRef]
- Nemeth, N.; Kiss, F.; Furka, I.; Miko, I. Gender differences of blood rheological parameters in laboratory animals. Clin. Hemorheol. Microcirc. 2010, 45, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Furuncuoğlu, Y.; Tulgar, S.; Dogan, A.N.; Cakar, S.; Tulgar, Y.K.; Cakiroglu, B. How obesity affects the neutrophil/lymphocyte and platelet/lymphocyte ratio, systemic immune-inflammatory index and platelet indices: A retrospective study. Eur. Rev. Med Pharmacol. Sci. 2016, 20, 1300–1306. [Google Scholar]
- Fitzpatrick, J.; Bulsara, M.K.; McCrory, P.R.; Richardson, M.D.; Zheng, M.H. Analysis of Platelet-Rich Plasma Extraction: Variations in Platelet and Blood Components Between 4 Common Commercial Kits. Orthop. J. Sports Med. 2017, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaux, J.F.; Le Goff, C.; Seidel, L.; Péters, P.; Gothot, A.; Albert, A.; Crielaard, J.M. [Comparative study of five techniques of preparation of platelet-rich plasma]. Pathol. Biol. 2011, 59, 157–160. [Google Scholar] [CrossRef] [PubMed]
- Campora, C.; Freeman, K.P.; Lewis, F.I.; Gibson, G.; Sacchini, F.; Sanchez-Vazquez, M.J. Determination of haematological reference intervals in healthy adult greyhounds. J. Small Anim. Pract. 2011, 52, 301–309. [Google Scholar] [CrossRef]
- Sullivan, P.S.; Evans, H.L.; McDonald, T.P. Platelet concentration and hemoglobin function in greyhounds. J. Am. Vet. Med. Assoc. 1994, 205, 838–841. [Google Scholar] [PubMed]
- Campora, C.; Freeman, K.P.; Serra, M.; Sacchini, F. Reference intervals for Greyhounds and Lurchers using the Sysmex XT-2000iV hematology analyzer. Vet. Clin. Pathol. 2011, 40, 467–474. [Google Scholar] [CrossRef]
- Heuser, W. Canine rheumatoid arthritis. Can. Vet. J. 1980, 21, 314–316. [Google Scholar]
- Mikkola, L.; Holopainen, S.; Pessa-Morikawa, T.; Lappalainen, A.K.; Hytönen, M.K.; Lohi, H.; Iivanainen, A. Genetic dissection of canine hip dysplasia phenotypes and osteoarthritis reveals three novel loci. BMC Genom. 2019, 20, 1027. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kirberger, R.M.; Fourie, S.L. Elbow dysplasia in the dog: Pathophysiology, diagnosis and control. J. S. Afr. Vet. Assoc. 1998, 69, 43–54. [Google Scholar] [CrossRef] [Green Version]
- Marx, R.E. Platelet-rich plasma (PRP): What is PRP and what is not PRP? Implant. Dent. 2001, 10, 225–228. [Google Scholar] [CrossRef] [PubMed] [Green Version]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Marcos Carpio, I.; Sanghani-Kerai, A.; Solano, M.A.; Blunn, G.; Jifcovici, A.; Fitzpatrick, N. Clinical Cohort Study in Canine Patients, to Determine the Average Platelet and White Blood Cell Number and Its Correlation with Patient’s Age, Weight, Breed and Gender: 92 Cases (2019–2020). Vet. Sci. 2021, 8, 262. https://doi.org/10.3390/vetsci8110262
de Marcos Carpio I, Sanghani-Kerai A, Solano MA, Blunn G, Jifcovici A, Fitzpatrick N. Clinical Cohort Study in Canine Patients, to Determine the Average Platelet and White Blood Cell Number and Its Correlation with Patient’s Age, Weight, Breed and Gender: 92 Cases (2019–2020). Veterinary Sciences. 2021; 8(11):262. https://doi.org/10.3390/vetsci8110262
Chicago/Turabian Stylede Marcos Carpio, Isabel, Anita Sanghani-Kerai, Miguel A. Solano, Gordon Blunn, Alexandra Jifcovici, and Noel Fitzpatrick. 2021. "Clinical Cohort Study in Canine Patients, to Determine the Average Platelet and White Blood Cell Number and Its Correlation with Patient’s Age, Weight, Breed and Gender: 92 Cases (2019–2020)" Veterinary Sciences 8, no. 11: 262. https://doi.org/10.3390/vetsci8110262




