Baculovirus Vectors Induce the Production of Interferons in Swine: Their Potential in the Development of Antiviral Strategies
Abstract
1. Introduction
2. Materials and Methods
2.1. Cells and Viruses
2.2. Pigs
2.3. Ethics Statement
2.4. Peripheral Blood Mononuclear Cells
2.5. IFN Measurement
2.6. Antiviral Activity Assays
2.7. Recombinant AcMNPV Construction
2.8. Western Blot
2.9. Statistics
3. Results
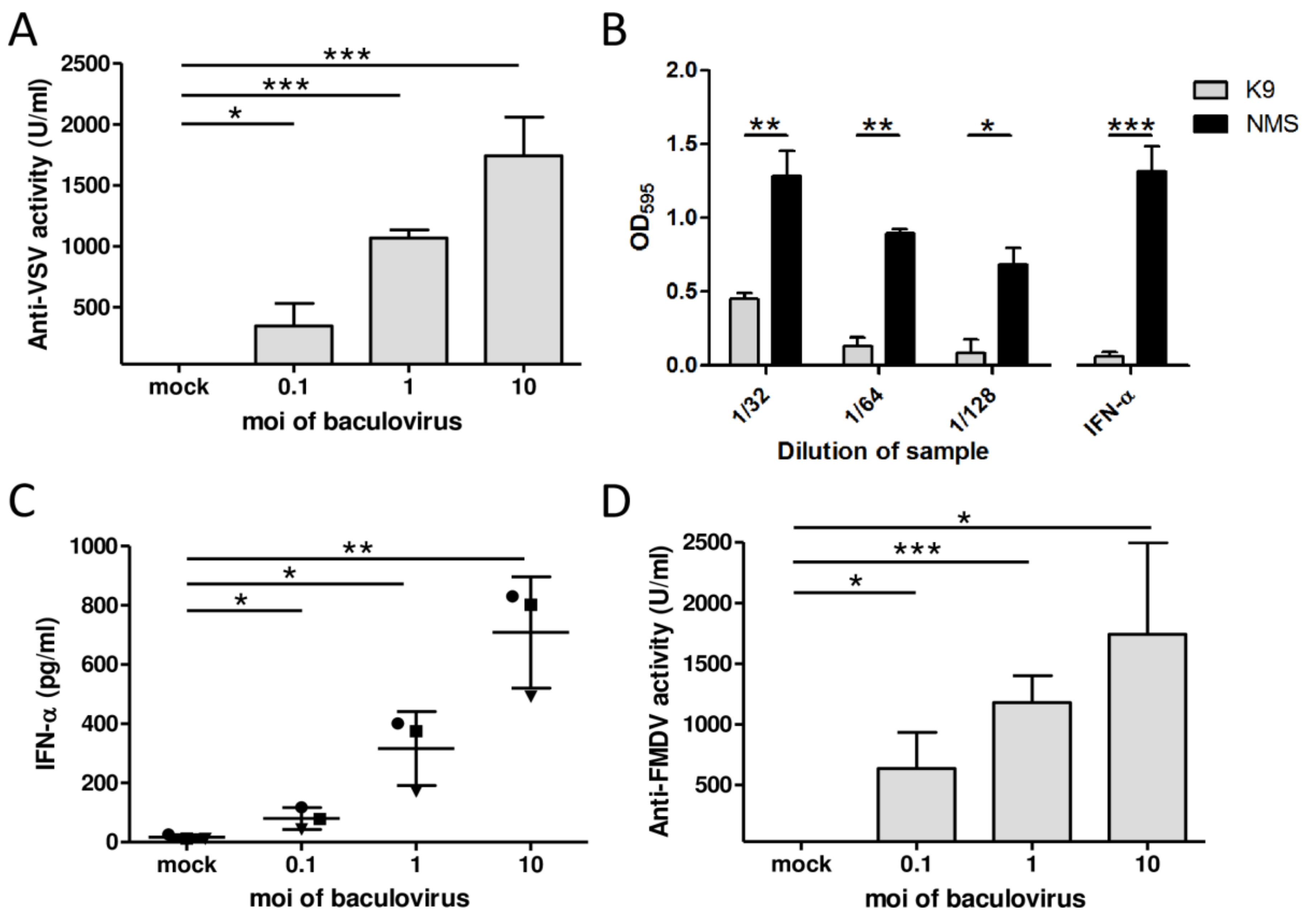
3.1. AcMNPV Elicits IFN-α-Mediated Antiviral Activity in Porcine PBMCs In Vitro
3.2. AcMNPV Induces Type I and II IFNs and Antiviral Activity in Swine
3.3. Pseudotyping with G-VSV but Not the Enrichment with pCpG Motifs Improves the Ability of AcMNPV to Induce IFN-α In Vitro
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Perfumo, C.J.; Pereda, A.; Jongkaewwattana, A.; Chen, Z.; Perez, D.R.; Ma, J. Editorial: Emerging Swine Viruses. Front. Vet. Sci. 2020, 7, 132. [Google Scholar] [CrossRef]
- Wilkinson, K.; Grant, W.P.; Green, L.E.; Hunter, S.; Jeger, M.J.; Lowe, P.; Medley, G.F.; Mills, P.; Phillipson, J.; Poppy, G.M.; et al. Infectious diseases of animals and plants: An interdisciplinary approach. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2011, 366, 1933–1942. [Google Scholar] [CrossRef]
- Gibbert, K.; Schlaak, J.; Yang, D.; Dittmer, U. IFN-α subtypes: Distinct biological activities in anti-viral therapy. Br. J. Pharmacol. 2013, 168, 1048–1058. [Google Scholar] [CrossRef]
- Leal, R.O.; Gil, S. The Use of Recombinant Feline Interferon Omega Therapy as an Immune-Modulator in Cats Naturally Infected with Feline Immunodeficiency Virus: New Perspectives. Vet. Sci. 2016, 3, 32. [Google Scholar] [CrossRef]
- Woo, A.S.J.; Kwok, R.; Ahmed, T. Alpha-interferon treatment in hepatitis B. Ann. Transl. Med. 2017, 5, 159. [Google Scholar] [CrossRef] [PubMed]
- Puchades Renau, L.; Berenguer, M. Introduction to hepatitis C virus infection: Overview and history of hepatitis C virus therapies. Hemodial. Int. 2018, 22 (Suppl. 1), S8–S21. [Google Scholar] [CrossRef] [PubMed]
- Du, T.; Nan, Y.; Xiao, S.; Zhao, Q.; Zhou, E.-M. Antiviral Strategies against PRRSV Infection. Trends Microbiol. 2017, 25, 968–979. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Luo, J.; Yu, J.; Mao, X.; Luo, Y.; Zheng, P.; He, J.; Yu, B.; Chen, D. Manipulation of Intestinal Antiviral Innate Immunity and Immune Evasion Strategies of Porcine Epidemic Diarrhea Virus. BioMed Res. Int. 2019, 2019, 1862531. [Google Scholar] [CrossRef]
- Fan, W.; Jiao, P.; Zhang, H.; Chen, T.; Zhou, X.; Qi, Y.; Sun, L.; Shang, Y.; Zhu, H.; Hu, R.; et al. Inhibition of African Swine Fever Virus Replication by Porcine Type I and Type II Interferons. Front. Microbiol. 2020, 11, 1203. [Google Scholar] [CrossRef] [PubMed]
- Ilyushina, N.A.; Donnelly, R. In vitro anti-influenza A activity of interferon (IFN)-λ1 combined with IFN-β or oseltamivir carboxylate. Antivir. Res. 2014, 111, 112–120. [Google Scholar] [CrossRef]
- Derbyshire, J.B. The interferon sensitivity of selected porcine viruses. Can. J. Vet. Res. 1989, 53, 52–55. [Google Scholar]
- Golde, W.T.; Pacheco, J.M.; Duque, H.; Doel, T.; Penfold, B.; Ferman, G.S.; Gregg, D.R.; Rodriguez, L.L. Vaccination against foot-and-mouth disease virus confers complete clinical protection in 7 days and partial protection in 4 days: Use in emergency outbreak response. Vaccine 2005, 23, 5775–5782. [Google Scholar] [CrossRef]
- Grubman, M.; Delossantos, T. Rapid control of foot-and-mouth disease outbreaks: Is RNAi a possible solution? Trends Immunol. 2005, 26, 65–68. [Google Scholar] [CrossRef] [PubMed]
- Moraes, M.P.; de Los Santos, T.; Koster, M.; Turecek, T.; Wang, H.; Andreyev, V.G.; Grubman, M.J. Enhanced Antiviral Activity against Foot-and-Mouth Disease Virus by a Combination of Type I and II Porcine Interferons. J. Virol. 2007, 81, 7124–7135. [Google Scholar] [CrossRef]
- Dias, C.C.; Moraes, M.P.; Segundo, F.D.; de Los Santos, T.; Grubman, M.J. Porcine Type I Interferon Rapidly Protects Swine Against Challenge with Multiple Serotypes of Foot-and-Mouth Disease Virus. J. Interf. Cytokine Res. 2011, 31, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Perez-Martin, E.; Diaz-San Segundo, F.; Weiss, M.; Sturza, D.F.; Dias, C.C.; Ramirez-Medina, E.; Grubman, M.J.; Santos, T.D.L. Type III Interferon Protects Swine Against Foot-and-Mouth Disease. J. Interf. Cytokine Res. 2014, 34, 810–821. [Google Scholar] [CrossRef]
- Fernandez-Sainz, I.; Ramanathan, P.; O’Donnell, V.; Diaz-San Segundo, F.; Velazquez-Salinas, L.; Sturza, D.F.; Zhu, J.; Santos, T.D.L.; Borca, M. Treatment with interferon-alpha delays disease in swine infected with a highly virulent CSFV strain. Virology 2015, 483, 284–290. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Keating, S.E.; Baran, M.; Bowie, A.G. Cytosolic DNA sensors regulating type I interferon induction. Trends Immunol. 2011, 32, 574–581. [Google Scholar] [CrossRef]
- Ablasser, A.; Hur, S. Regulation of cGAS- and RLR-mediated immunity to nucleic acids. Nat. Immunol. 2020, 21, 17–29. [Google Scholar] [CrossRef]
- Auray, G.; Keller, I.; Python, S.; Gerber, M.; Bruggmann, R.; Ruggli, N.; Summerfield, A. Characterization and Transcriptomic Analysis of Porcine Blood Conventional and Plasmacytoid Dendritic Cells Reveals Striking Species-Specific Differences. J. Immunol. 2016, 197, 4791–4806. [Google Scholar] [CrossRef] [PubMed]
- Miyake, K.; Shibata, T.; Ohto, U.; Shimizu, T.; Saitoh, S.-I.; Fukui, R.; Murakami, Y. Mechanisms controlling nucleic acid-sensing Toll-like receptors. Int. Immunol. 2018, 30, 43–51. [Google Scholar] [CrossRef]
- Werling, D.; Jann, O.C.; Offord, V.; Glass, E.J.; Coffey, T. Variation matters: TLR structure and species-specific pathogen recognition. Trends Immunol. 2009, 30, 124–130. [Google Scholar] [CrossRef]
- Leifer, C.A.; Kennedy, M.N.; Mazzoni, A.; Lee, C.; Kruhlak, M.J.; Segal, D.M. TLR9 Is Localized in the Endoplasmic Reticulum Prior to Stimulation. J. Immunol. 2004, 173, 1179–1183. [Google Scholar] [CrossRef]
- Guiducci, C.; Ott, G.; Chan, J.H.; Damon, E.; Calacsan, C.; Matray, T.; Lee, K.-D.; Coffman, R.L.; Barrat, F.J. Properties regulating the nature of the plasmacytoid dendritic cell response to Toll-like receptor 9 activation. J. Exp. Med. 2006, 203, 1999–2008. [Google Scholar] [CrossRef]
- Moseman, A.P.; Moseman, E.A.; Schworer, S.; Smirnova, I.; Volkova, T.; Von Andrian, U.; Poltorak, A. Mannose Receptor 1 Mediates Cellular Uptake and Endosomal Delivery of CpG-Motif Containing Oligodeoxynucleotides. J. Immunol. 2013, 191, 5615–5624. [Google Scholar] [CrossRef]
- Ayres, M.D.; Howard, S.C.; Kuzio, J.; Lopez-Ferber, M.; Possee, R.D. The Complete DNA Sequence of Autographa californica Nuclear Polyhedrosis Virus. Virology 1994, 202, 586–605. [Google Scholar] [CrossRef]
- Lin, S.-Y.; Chung, Y.-C.; Hu, Y.-C. Update on baculovirus as an expression and/or delivery vehicle for vaccine antigens. Expert Rev. Vaccines 2014, 13, 1501–1521. [Google Scholar] [CrossRef]
- Kolangath, S.M.; Basagoudanavar, S.H.; Hosamani, M.; Saravanan, P.; Tamil Selvan, R.P. Baculovirus mediated transduction: Analysis of vesicular stomatitis virus glycoprotein pseudotyping. Virusdisease 2014, 25, 441–446. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ono, C.; Okamoto, T.; Abe, T.; Matsuura, Y. Baculovirus as a Tool for Gene Delivery and Gene Therapy. Viruses 2018, 10, 510. [Google Scholar] [CrossRef] [PubMed]
- Tjia, S.T.; zu Altenschildesche, G.M.; Doerfler, W. Autographa californica nuclear polyhedrosis virus (AcNPV) DNA does not persist in mass cultures of mammalian cells. Virology 1983, 125, 107–117. [Google Scholar] [PubMed]
- Volkman, L.E.; Goldsmith, P.A. In Vitro Survey of Autographa californica Nuclear Polyhedrosis Virus Interaction with Nontarget Vertebrate Host Cells. Appl. Environ. Microbiol. 1983, 45, 1085–1093. [Google Scholar] [CrossRef]
- Kukkonen, S.P.; Airenne, K.J.; Marjomäki, V.; Laitinen, O.; Lehtolainen, P.; Kankaanpää, P.; Mähönen, A.J.; Räty, J.K.; Nordlund, H.R.; Oker-Blom, C.; et al. Baculovirus capsid display: A novel tool for transduction imaging. Mol. Ther. 2003, 8, 853–862. [Google Scholar] [CrossRef]
- Salminen, M.; Airenne, K.J.; Rinnankoski, R.; Reimari, J.; Välilehto, O.; Rinne, J.; Suikkanen, S.; Kukkonen, S.; Ylä-Herttuala, S.; Kulomaa, M.S.; et al. Improvement in Nuclear Entry and Transgene Expression of Baculoviruses by Disintegration of Microtubules in Human Hepatocytes. J. Virol. 2005, 79, 2720–2728. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, C.; Kaname, Y.; Taguwa, S.; Abe, T.; Fukuhara, T.; Tani, H.; Moriishi, K.; Matsuura, Y. Baculovirus GP64-Mediated Entry into Mammalian Cells. J. Virol. 2011, 86, 2610–2620. [Google Scholar] [CrossRef]
- Molina, G.N.; Tavarone, E.; Taboga, O.; Molinari, P. Two Distinctive Phenotypes of AcMNPV Display Different Immune Abilities and Intracellular Destiny. PLoS ONE 2016, 11, e0168939. [Google Scholar] [CrossRef] [PubMed]
- Amalfi, S.; Molina, G.N.; Bevacqua, R.; Lopez, M.G.; Taboga, O.; Alfonso, V. Baculovirus transduction in mammalian cells is affected by the production of type I and III IFNs, which is mainly mediated by the cGAS-STING pathway. J. Virol. 2020, 94, e01555-20. [Google Scholar] [CrossRef]
- Abe, T.; Kaname, Y.; Wen, X.; Tani, H.; Moriishi, K.; Uematsu, S.; Takeuchi, O.; Ishii, K.; Kawai, T.; Akira, S.; et al. Baculovirus Induces Type I Interferon Production through Toll-Like Receptor-Dependent and -Independent Pathways in a Cell-Type-Specific Manner. J. Virol. 2009, 83, 7629–7640. [Google Scholar] [CrossRef] [PubMed]
- Abe, T.; Matsuura, Y. Host innate immune responses induced by baculovirus in mammals. Curr. Gene Ther. 2010, 10, 226–231. [Google Scholar] [CrossRef] [PubMed]
- Hervas-Stubbs, S.; Riezu-Boj, J.-I.; Mancheño, U.; Rueda, P.; Lopez, L.; Alignani, D.; Rodríguez-García, E.; Thieblemont, N.; Leclerc, C. Conventional but Not Plasmacytoid Dendritic Cells Foster the Systemic Virus–Induced Type I IFN Response Needed for Efficient CD8 T Cell Priming. J. Immunol. 2014, 193, 1151–1161. [Google Scholar] [CrossRef] [PubMed]
- Moriyama, T.; Suzuki, T.; Chang, M.O.; Kitajima, M.; Takaku, H. Baculovirus directly activates murine NK cells via TLR9. Cancer Gene Ther. 2017, 24, 175–179. [Google Scholar] [CrossRef]
- Hervas-Stubbs, S.; Olivier, A.; Boisgerault, F.; Thieblemont, N.; Leclerc, C. TLR3 ligand stimulates fully functional memory CD8+ T cells in the absence of CD4+ T-cell help. Blood 2007, 109, 5318–5326. [Google Scholar] [CrossRef]
- Molinari, P.; Crespo, M.I.; Gravisaco, M.J.; Taboga, O.; Morón, G. Baculovirus Capsid Display Potentiates OVA Cytotoxic and Innate Immune Responses. PLoS ONE 2011, 6, e24108. [Google Scholar] [CrossRef] [PubMed]
- Abe, T.; Takahashi, H.; Hamazaki, H.; Miyano-Kurosaki, N.; Matsuura, Y.; Takaku, H. Baculovirus Induces an Innate Immune Response and Confers Protection from Lethal Influenza Virus Infection in Mice. J. Immunol. 2003, 171, 1133–1139. [Google Scholar] [CrossRef]
- Tami, C.; Peralta, A.; Barbieri, R.; Berinstein, A.; Carrillo, E.; Taboga, O. Immunological properties of FMDV-gP64 fusion proteins expressed on SF9 cell and baculovirus surfaces. Vaccine 2004, 23, 840–845. [Google Scholar] [CrossRef]
- Tavarone, E.; Molina, G.N.; Amalfi, S.; Peralta, A.; Molinari, P.; Taboga, O. The localization of a heterologous displayed antigen in the baculovirus-budded virion determines the type and strength of induced adaptive immune response. Appl. Microbiol. Biotechnol. 2017, 101, 4175–4184. [Google Scholar] [CrossRef]
- Premanand, B.; Zhong Wee, P.; Prabakaran, M. Baculovirus Surface Display of Immunogenic Proteins for Vaccine Development. Viruses 2018, 10, 298. [Google Scholar] [CrossRef]
- Gronowski, A.M.; Hilbert, D.M.; Sheehan, K.C.F.; Garotta, G.; Schreiber, R.D. Baculovirus Stimulates Antiviral Effects in Mammalian Cells. J. Virol. 1999, 73, 9944–9951. [Google Scholar] [CrossRef]
- Molinari, P.; García-Nuñez, S.; Gravisaco, M.J.; Carrillo, E.; Berinstein, A.; Taboga, O. Baculovirus treatment fully protects mice against a lethal challenge of FMDV. Antivir. Res. 2010, 87, 276–279. [Google Scholar] [CrossRef] [PubMed]
- Quattrocchi, V.; Molinari, P.; Langellotti, C.; Gnazzo, V.; Taboga, O.; Zamorano, P. Co-inoculation of baculovirus and FMDV vaccine in mice, elicits very early protection against foot and mouth disease virus without interfering with long lasting immunity. Vaccine 2013, 31, 2713–2718. [Google Scholar] [CrossRef] [PubMed]
- Molina, G.N.; Cacciabue, M.; Gismondi, M.I.; Taboga, O.; Molinari, P. Baculovirus AcMNPV induces type I interferons and NK/NKT cells-mediated protection against foot-and-mouth disease virus in mice. Antivir. Res. 2020, 180, 104850. [Google Scholar] [CrossRef]
- Xu, X.-G.; Wang, Z.-S.; Zhang, Q.; Li, Z.-C.; Ding, L.; Li, W.; Wu, H.-Y.; Chang, C.-D.; Lee, L.-H.; Tong, D.-W.; et al. Baculovirus as a PRRSV and PCV2 bivalent vaccine vector: Baculovirus virions displaying simultaneously GP5 glycoprotein of PRRSV and capsid protein of PCV2. J. Virol. Methods 2012, 179, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Argilaguet, J.; Perez-Martin, E.; López, S.; Goethe, M.; Escribano, J.; Giesow, K.; Keil, G.M.; Rodríguez, F. BacMam immunization partially protects pigs against sublethal challenge with African swine fever virus. Antivir. Res. 2013, 98, 61–65. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, Y.; Zhang, Y.; Yang, Y.; Ren, J.; Zhang, X.; Du, E. Surface displaying of swine IgG1 Fc enhances baculovirus-vectored vaccine efficacy by facilitating viral complement escape and mammalian cell transduction. Vet. Res. 2017, 48, 29. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-Y.; Hsu, W.-T.; Chao, Y.-C.; Chang, H.-W. Display of Porcine Epidemic Diarrhea Virus Spike Protein on Baculovirus to Improve Immunogenicity and Protective Efficacy. Viruses 2018, 10, 346. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Li, G.; Zheng, W.; Shu, J.; Chen, J.; Yang, F.; Wu, Y.; He, Y. Development of a Combined Genetic Engineering Vaccine for Porcine Circovirus Type 2 and Mycoplasma Hyopneumoniae by a Baculovirus Expression System. Int. J. Mol. Sci. 2019, 20, 4425. [Google Scholar] [CrossRef]
- LaRocco, M.; Krug, P.W.; Kramer, E.; Ahmed, Z.; Pacheco, J.M.; Duque, H.; Baxt, B.; Rodriguez, L.L. A Continuous Bovine Kidney Cell Line Constitutively Expressing Bovine α V β 6 Integrin Has Increased Susceptibility to Foot-and-Mouth Disease Virus. J. Clin. Microbiol. 2013, 51, 1714–1720, Erratum in 2015, 53, 755. [Google Scholar] [CrossRef]
- Vogel, S.N.; Friedman, R.M.; Hogan, M.M. Measurement of antiviral activity induced by interferons alpha, beta, and gamma. Curr. Protoc. Immunol. 1991, 6.9, 1–8. [Google Scholar] [CrossRef]
- Guzylack-Piriou, L.; Balmelli, C.; McCullough, K.C.; Summerfield, A. Type-A CpG oligonucleotides activate exclusively porcine natural interferon-producing cells to secrete interferon-alpha, tumour necrosis factor-alpha and interleukin-12. Immunology 2004, 112, 28–37. [Google Scholar] [CrossRef]
- Wikström, F.H.; Meehan, B.M.; Berg, M.; Timmusk, S.; Elving, J.; Fuxler, L.; Magnusson, M.; Allan, G.M.; McNeilly, F.; Fossum, C. Structure-Dependent Modulation of Alpha Interferon Production by Porcine Circovirus 2 Oligodeoxyribonucleotide and CpG DNAs in Porcine Peripheral Blood Mononuclear Cells. J. Virol. 2007, 81, 4919–4927. [Google Scholar] [CrossRef]
- Kamstrup, S.; Verthelyi, D.; Klinman, D.M. Response of porcine peripheral blood mononuclear cells to CpG-containing oligodeoxynucleotides. Vet. Microbiol. 2001, 78, 353–362. [Google Scholar] [CrossRef]
- Van der Stede, Y.; Verdonck, F.; Vancaeneghem, S.; Cox, E.; Goddeeris, B.M. CpG-oligodinucleotides as an effective adjuvant in pigs for intramuscular immunizations. Vet. Immunol. Immunopathol. 2002, 86, 31–41. [Google Scholar] [CrossRef]
- Grubman, M.J. Development of novel strategies to control foot-and-mouth disease: Marker vaccines and antivirals. Biologicals 2005, 33, 227–234. [Google Scholar] [CrossRef]
- Brockmeier, S.L.; Loving, C.L.; Eberle, K.C.; Hau, S.J.; Buckley, A.; Van Geelen, A.; Montiel, N.A.; Nicholson, T.; Lager, K.M. Interferon alpha inhibits replication of a live-attenuated porcine reproductive and respiratory syndrome virus vaccine preventing development of an adaptive immune response in swine. Vet. Microbiol. 2017, 212, 48–51. [Google Scholar] [CrossRef] [PubMed]
- Dias, C.C.; Moraes, M.P.; Weiss, M.; Diaz-San Segundo, F.; Perez-Martin, E.; Salazar, A.M.; Santos, T.D.L.; Grubman, M.J. Novel antiviral therapeutics to control foot-and-mouth disease. J. Interferon Cytokine Res. 2012, 32, 462–473. [Google Scholar] [CrossRef] [PubMed]
- Alves, M.P.; Guzylack-Piriou, L.; Juillard, V.; Audonnet, J.-C.; Doel, T.; Dawson, H.; Golde, W.T.; Gerber, H.; Peduto, N.; McCullough, K.C.; et al. Innate Immune Defenses Induced by CpG Do Not Promote Vaccine-Induced Protection against Foot-and-Mouth Disease Virus in Pigs. Clin. Vaccine Immunol. 2009, 16, 1151–1157. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-Y.; Lin, C.-Y.; Chen, G.-Y.; Hu, Y.-C. Baculovirus as a gene delivery vector: Recent understandings of molecular alterations in transduced cells and latest applications. Biotechnol. Adv. 2011, 29, 618–631. [Google Scholar] [CrossRef] [PubMed]
- Abe, T.; Hemmi, H.; Miyamoto, H.; Moriishi, K.; Tamura, S.; Takaku, H.; Akira, S.; Matsuura, Y. Involvement of the Toll-Like Receptor 9 Signaling Pathway in the Induction of Innate Immunity by Baculovirus. J. Virol. 2005, 79, 2847–2858. [Google Scholar] [CrossRef] [PubMed]
- Hervas-Stubbs, S.; Rueda, P.; Lopez, L.; Leclerc, C. Insect Baculoviruses Strongly Potentiate Adaptive Immune Responses by Inducing Type I IFN. J. Immunol. 2007, 178, 2361–2369. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Chang, M.O.; Kitajima, M.; Takaku, H. Baculovirus activates murine dendritic cells and induces non-specific NK cell and T cell immune responses. Cell. Immunol. 2010, 262, 35–43. [Google Scholar] [CrossRef] [PubMed]



| ODN | Sequence | References |
|---|---|---|
| 2216 | GGG GGA CGA TCG TCGGGGGG | [58,59] |
| D19 | GGT GCA TCG ATG CAGGGGGG | [58,60] |
| H1a | GGTATTTCGAAATAGGGGGG | [59] |
| ODN 3 | GCT AGA CGT TAG CGT | [61] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Molina, G.N.; Amalfi, S.; Otero, I.; Taboga, O.; Molinari, M.P. Baculovirus Vectors Induce the Production of Interferons in Swine: Their Potential in the Development of Antiviral Strategies. Vet. Sci. 2021, 8, 278. https://doi.org/10.3390/vetsci8110278
Molina GN, Amalfi S, Otero I, Taboga O, Molinari MP. Baculovirus Vectors Induce the Production of Interferons in Swine: Their Potential in the Development of Antiviral Strategies. Veterinary Sciences. 2021; 8(11):278. https://doi.org/10.3390/vetsci8110278
Chicago/Turabian StyleMolina, Guido Nicolás, Sabrina Amalfi, Ignacio Otero, Oscar Taboga, and María Paula Molinari. 2021. "Baculovirus Vectors Induce the Production of Interferons in Swine: Their Potential in the Development of Antiviral Strategies" Veterinary Sciences 8, no. 11: 278. https://doi.org/10.3390/vetsci8110278
APA StyleMolina, G. N., Amalfi, S., Otero, I., Taboga, O., & Molinari, M. P. (2021). Baculovirus Vectors Induce the Production of Interferons in Swine: Their Potential in the Development of Antiviral Strategies. Veterinary Sciences, 8(11), 278. https://doi.org/10.3390/vetsci8110278





