Investigation of Citric Acid By-Products from Rice Produced by Microbial Fermentation on Growth Performance and Villi Histology of Thai Broiler Chicken (KKU 1)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Production Techniques of Citric Acid
2.2. Animals and Experimental Design
2.3. Data Collection
2.3.1. Growth Performance
2.3.2. Villi Histology
2.3.3. Microbial Contamination on Fecal Matter
2.4. Statistical Analysis
3. Results
3.1. Growth Performance
3.2. Villi Histology
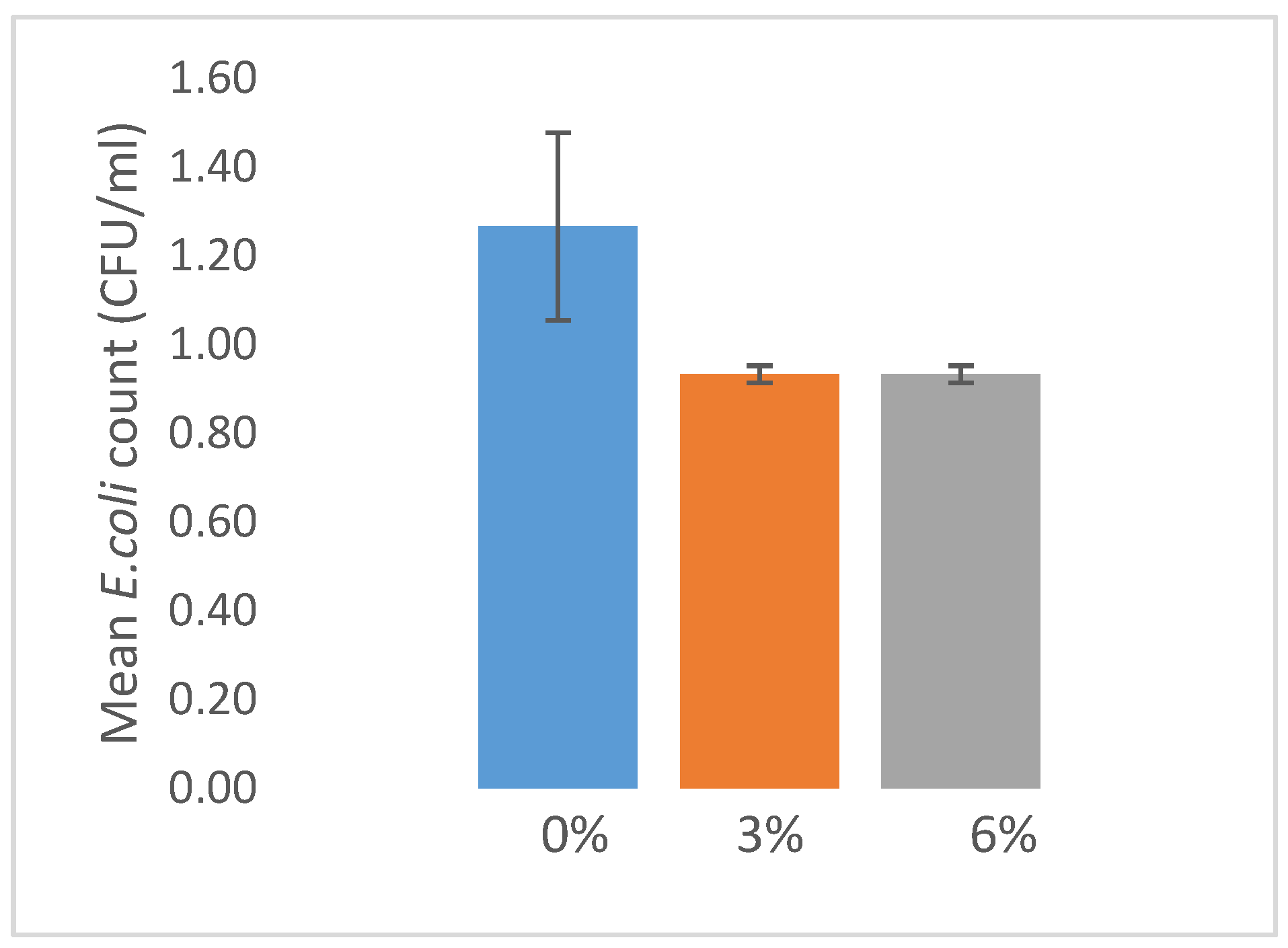
3.3. Microbial Contamination on Fecal Matter
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dhillon, G.; Brar, S.; Dhillon, S.; Verma, M. Bioproduction and extraction optimization of citric acid from Aspergillus niger by rotating drum type solid-state bioreactor. Ind. Crops Prod. 2013, 41, 78–84. [Google Scholar] [CrossRef]
- Kudzai, C.T.; Ajay, K.; Ambika, P. Citric acid production by Aspergillus niger using different substrates. Malays. J. Microbiol. 2018, 12, 199–204. [Google Scholar] [CrossRef]
- Ngammuangtueng, P.; Jakrawatana, N.; Nilsalab, P. Water, Energy and Food Nexus in Rice Production in Thailand. Sustainability 2019, 11, 5852. [Google Scholar] [CrossRef] [Green Version]
- Imandi, S.B.; Bandaru, V.V.R.; Somalanka, S.R.; Bandaru, S.R.; Garapati, H.R. Application of statistical experimental designs for the optimization of medium constituents for the production of citric acid from pineapple waste. Bioresour. Technol. 2008, 99, 4445–4450. [Google Scholar] [CrossRef]
- Ayeni, A.O.; Daramola, M.O.; Taiwo, O.; Olanrewaju, O.I.; Oyekunle, D.T.; Sekoai, P.T.; Elehinafe, F.B. Production of Citric Acid from the Fermentation of Pineapple Waste by Aspergillus niger. Open Chem. Eng. J. 2019, 13, 88–96. [Google Scholar] [CrossRef]
- Femi-Ola, T.O.; Atere, V.A. Citric acid production from brewers spent grain by Aspergillus niger and Saccharomyces cerevisiae. Int. J. Res. Biosci. 2013, 2, 30–36. [Google Scholar]
- Li, X.; Li, G.; Li, J.; Yu, Y.; Feng, Y.; Chen, Q.; Komarneni, S.; Wang, Y. Producing petrochemicals from catalytic fast pyrolysis of corn fermentation residual by-products generated from citric acid production. Renew. Energy 2016, 89, 331–338. [Google Scholar] [CrossRef]
- Tanpong, S.; Cherdthong, A.; Tengjaroenkul, B.; Reungsang, A.; Sutthibak, N.; Wongtangtintharn, S. A study on citric acid by-product as an energy source for Japanese quail. Trop. Anim. Health Prod. 2021, 53, 474. [Google Scholar] [CrossRef]
- Rehman, Z.; Mirza, M.; Mukhtar, N. Poultry Research Use of Organic Acids as Potential Feed Additives in Poultry Production. J. World’s Poult. Res. 2016, 6, 105–116. [Google Scholar]
- Tanpong, S.; Cherdthong, A.; Tengjaroenkul, B.; Tengjaroenkul, U.; Wongtangtintharn, S. Evaluation of physical and chemical properties of citric acid industrial waste. Trop. Anim. Health Prod. 2019, 51, 2167–2174. [Google Scholar] [CrossRef]
- Mehdikhany, S.; Zarei, A.; Lotfollahian, H.; Hoseini, S.A. Determination of nutritive value and the effect of citric acid production by-product on broiler performance. Indian J. Anim. Res. 2012, 46, 143–147. [Google Scholar]
- Oryza.S, M.; Wongtangtintharn, S.; Tengjaroenkul, B.; Cherdthong, A.; Tanpong, S.; Bunchalee, P.; Pootthachaya, P.; Reungsang, A.; Polyorach, S. Physico-Chemical Characteristics and Amino Acid Content Evaluation of Citric Acid by-Product Produced by Microbial Fermentation as a Potential Use in Animal Feed. Fermentation 2021, 7, 149. [Google Scholar] [CrossRef]
- Xu, J.; Su, X.F.; Bao, J.W.; Chen, Y.Q.; Zhang, H.J.; Tang, L.; Wang, K.; Zhang, J.H.; Chen, X.S.; Mao, Z.G. Cleaner production of citric acid by recycling its extraction wastewater treated with anaerobic digestion and electrodialysis in an integrated citric acid-methane production process. Bioresour. Technol. 2015, 189, 186–194. [Google Scholar] [CrossRef]
- Maliwan, P.; Khempaka, S.; Molee, W. Evaluation of various feeding programmes on growth performance, carcass and meat qualities of Thai indigenous crossbred chickens. S. Afr. J. Anim. Sci. 2017, 47, 16–25. [Google Scholar] [CrossRef]
- Singh, A.; Rashid, M. Impact of Livestock Waste on Environment and Strategies to Reduce Environmental Contamination—A Review. Vet. Sci. J. 2018, 8, 76–86. [Google Scholar] [CrossRef]
- De Verdal, H.; Mignon-Grasteau, S.; Jeulin, C.; le Bihan-Duval, E.; Leconte, M.; Mallet, S.; Martin, C.; Narcy, A. Digestive tract measurements and histological adaptation in broiler lines divergently selected for digestive efficiency. Poult. Sci. 2010, 89, 1955–1961. [Google Scholar] [CrossRef]
- Bird, P.; Bastin, B.; Klass, N.; Crowley, E.; Agin, J.; Goins, D.; Bakken, H.; Lingle, C.; Schumacher, A. Evaluation of the 3MTM PetrifilmTM Rapid E. coli/ Coliform Count Plate for the Enumeration of E. coli and Coliforms: Collaborative Study, First Action: 2018.13. J. AOAC Int. 2021, 103, 513–522. [Google Scholar] [CrossRef] [Green Version]
- SAS University Edition: Statistic, 6th ed.; SAS Inst. Inc.: Cary, NC, USA, 2015; Volume 9.
- Nourmohammadi, R.; Hosseini, S.M.; Farhangfar, H. Influence of Citric Acid and Microbial Phytase on Growth Performance and Carcass Characteristics of Broiler Chickens. Am. J. Anim. Vet. Sci. 2010, 5, 282–288. [Google Scholar] [CrossRef]
- Mroz, Z. Organic Acids as potential Alternatives to antibiotic growth promoters for pigs. Adv. Pork Prod. 2005, 16, 169–182. [Google Scholar]
- Thompson, J.L.; Hinton, M. Antibacterial activity of formic and propionic acids in the diet of hens on salmonellas in the crop. Br. Poult. Sci. 1997, 38, 59–65. [Google Scholar] [CrossRef]
- Langhout, P. New additives for broiler chickens. World Poult. 2000, 16, 22–27. [Google Scholar]
- Pelicano, E.; Souza, P.; Souza, H.; Figueiredo, D.; Boiago, M.; Carvalho, S.; Bordon, V. Intestinal mucosa development in broiler chickens fed natural growth promoters. Rev. Bras. Ciência Avícola 2005, 7, 221–229. [Google Scholar] [CrossRef]
- Xu, Z.R.; Hu, C.H.; Xia, M.S.; Zhan, X.A.; Wang, M.Q. Effects of dietary fructooligosaccharide on digestive enzyme activities, intestinal microflora and morphology of male broilers. Poult. Sci. 2003, 82, 1030–1036. [Google Scholar] [CrossRef] [PubMed]
- Mohammadagheri, N.; Najafi, R.; Najafi, G. Effects of dietary supplementation of organic acids and phytase on performance and intestinal histomorphology of broilers. Vet. Res. Forum Int. Q. J. 2016, 7, 189–195. [Google Scholar]
- Yason, C.V.; Summer, B.A.; Schat, K.A. Pathogenesis of rotavirus infection in various age groups of chickens and turkeys. Am. J. Vet. Res. 1987, 48, 38. [Google Scholar] [CrossRef]
- Aptekmann, K.P.; Baraldi Artoni, S.M.; Stefanini, M.A.; Orsi, M.A. Morphometric analysis of the intestine of domestic quails (Coturnix coturnix japonica) treated with different levels of dietary calcium. Anat. Histol. Embryol. 2001, 30, 277–280. [Google Scholar] [CrossRef]
- Adil, S.; Banday, T.; Bhat, G.A.; Mir, M.S.; Rehman, M. Effect of dietary supplementation of organic acids on performance, intestinal histomorphology, and serum biochemistry of broiler chicken. Vet. Med. Int. 2010, 2010, 479485. [Google Scholar] [CrossRef] [Green Version]
- Montagne, L.; Pluske, J.R.; Hampson, D.J. A review of interactions between dietary fibre and the intestinal mucosa, and their consequences on digestive health in young non-ruminant animals. Anim. Feed Sci. Technol. 2003, 108, 95–117. [Google Scholar] [CrossRef]
- Garriga, M.; Pascual, M.; Monfort, J.M.; Hugas, M. Selection of lactobacilli for chicken probiotic adjuncts. J. Appl. Microbiol. 1998, 84, 125–132. [Google Scholar] [CrossRef]
- Silva, M.; Pessotti, B.; Zanini, S.; Colnago, G.; Rodrigues, M.R.; Nunes, L.; Zanini, M.; Martins, I. Intestinal mucosa structure of broiler chickens infected experimentally with Eimeriatenella and treated with essential oil of oregano. Ciência Rural 2009, 30, 1471–1477. [Google Scholar] [CrossRef] [Green Version]
- Islam, K.M.S. Use of citric acid in broiler diets. Worlds. Poult. Sci. J. 2012, 68, 104–118. [Google Scholar] [CrossRef]
- Lückstädts, C. Use of organic acids as feed additives- sutainable aquaculture production the non-antibiotic way. Int. Aquafeed 2006, 9, 21–26. [Google Scholar] [CrossRef]
- Kermanshahi, H.; Nassiri Moghaddam, H.; Gilani, A. Alteration of gut microflora through citric acid treated drinking water in preslaughter male broilers. Afr. J. Microbiol. Res. 2013, 7, 564–567. [Google Scholar] [CrossRef]
- Pluske, J.R.; Thompson, M.J.; Atwood, C.S.; Bird, P.H.; Williams, I.H.; Hartmann, P.E. Maintenance of villus height and crypt depth, and enhancement of disaccharide digestion and monosaccharide absorption, in piglets fed on cows’ whole milk after weaning. Br. J. Nutr. 1996, 76, 409–422. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Ingredient (%) | Citric Acid By-Product, % Dry Matter | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 3 | 6 | |||||||
| Period | Period | Period | |||||||
| 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 3 | |
| Corn meal | 50.00 | 55.57 | 59.47 | 48.00 | 53.47 | 57.37 | 46.00 | 51.37 | 55.27 |
| Soybean meal | 26.90 | 19.30 | 12.46 | 25.80 | 18.30 | 11.46 | 24.70 | 17.30 | 10.46 |
| Full fat soybean | 17.00 | 19.00 | 22.00 | 17.00 | 19.00 | 22.00 | 17.00 | 19.00 | 22.00 |
| Dicalcium phosphate % P21 | 1.80 | 1.60 | 1.50 | 1.80 | 1.60 | 1.50 | 1.80 | 1.60 | 1.50 |
| Limestone | 1.60 | 1.40 | 1.30 | 1.60 | 1.40 | 1.30 | 1.60 | 1.40 | 1.30 |
| DL-Met | 0.25 | 0.20 | 0.17 | 0.25 | 0.20 | 0.17 | 0.25 | 0.20 | 0.17 |
| Lysine | 0.20 | 0.18 | 0.15 | 0.20 | 0.18 | 0.15 | 0.20 | 0.18 | 0.15 |
| Rice crude bran oil | 1.50 | 2.00 | 2.20 | 1.60 | 2.10 | 2.30 | 1.70 | 2.20 | 2.40 |
| Salt | 0.30 | 0.30 | 0.30 | 0.30 | 0.30 | 0.30 | 0.30 | 0.30 | 0.30 |
| Choline chloride 60% | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 | 0.1 | 0.10 | 0.10 | 0.10 |
| Premix | 0.35 | 0.35 | 0.35 | 0.35 | 0.35 | 0.35 | 0.35 | 0.35 | 0.35 |
| Citric acid by-product rice | 0.00 | 0.00 | 0.00 | 3.00 | 3.00 | 3.00 | 6.00 | 6.00 | 6.00 |
| Total | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 |
| Calculated | |||||||||
| CP, % | 22.39 | 20.05 | 18.29 | 22.33 | 20.02 | 18.26 | 22.26 | 19.99 | 18.23 |
| ME, Kcal/kg | 3013 | 3129 | 3209 | 3004 | 3118 | 3198 | 2994 | 3108 | 3188 |
| Parameter | Citric Acid By-Product Rice, % Dry Matter | p-Value | ||
|---|---|---|---|---|
| 0 | 3 | 6 | ||
| Days 1–21 | ||||
| Initial weight (g/b) | 32.77 ± 0.13 | 32.73 ± 0.22 | 32.80 ± 0.42 | 0.9496 |
| Body weight (g/b) | 384.16 ± 30.49 | 392.22 ± 11.60 | 371.96 ± 26.91 | 0.5225 |
| BWG (g/b) | 351.39 ± 30.59 | 359.49 ± 11.71 | 339.16 ± 22.21 | 0.5249 |
| FI (g/b) | 526.36 ± 12.39 | 514.52 ± 27.59 | 541.26 ± 30.79 | 0.3572 |
| FCR | 1.51 ± 0.13 | 1.43 ± 0.07 | 1.60 ± 0.12 | 0.1305 |
| SR (%) | 100.00 ± 0.00 | 100.00 ± 0.00 | 96.88 ± 3.61 | 0.1004 |
| PI | 122.65 ± 18.77 | 130.72 ± 7.79 | 107.89 ± 14.82 | 0.1540 |
| Days 22–49 | ||||
| Initial weight (g/b) | 384.16 ± 30.49 | 392.22 ± 11.60 | 371.96 ± 26.91 | 0.5225 |
| Body weight (g/b) | 1461.67 ± 119.71 | 1475.26 ± 23.39 | 1423.53 ± 74.98 | 0.6687 |
| BWG (g/b) | 1077.51 ± 100.86 | 1083.04 ± 20.84 | 1051.58 ± 52.33 | 0.7813 |
| FI (g/b) | 2171.01 ± 154.87 | 2158.61 ± 120.24 | 2091.71 ± 46.42 | 0.6017 |
| FCR | 2.02 ± 0.17 | 1.99 ± 0.08 | 1.99 ± 0.13 | 0.9291 |
| SR (%) | 93.75 ± 12.50 | 95.31 ± 5.98 | 96.88 ± 6.25 | 0.8825 |
| PI | 140.48 ± 34.44 | 144.27 ± 11.76 | 142.14 ± 21.02 | 0.9759 |
| Days 50–56 | ||||
| Initial weight (g/b) | 1461.67 ± 119.71 | 1475.26 ± 23.39 | 1423.53 ± 74.98 | 0.6687 |
| Body weight (g/b) | 1774.09 ± 84.51 | 1770.13 ± 41.44 | 1713.49 ± 116.86 | 0.5631 |
| BWG (g/b) | 312.42 ± 46.64 | 294.88 ± 36.02 | 289.96 ± 44.29 | 0.2137 |
| FI (g/b) | 875.72 ± 35.63 | 866.17 ± 57.67 | 946.18 ± 88.63 | 0.7649 |
| FCR | 2.87 ± 0.53 | 2.97 ± 0.38 | 3.30 ± 0.42 | 0.3240 |
| SR (%) | 100.00 ± 0.00 | 100.00 ± 0.00 | 100.00 ± 0.00 | NA |
| PI | 112.97 ± 19.00 | 107.92 ± 14.42 | 94.54 ± 20.16 | 0.3692 |
| Overall (days 1–56) | ||||
| Initial weight (g/b) | 32.77 ± 0.13 | 32.73 ± 0.22 | 32.80 ± 0.42 | 0.9496 |
| Body weight (g/b) | 1774.09 ± 84.51 | 1770.13 ± 41.44 | 1713.49 ± 116.86 | 0.5631 |
| BWG (g/b) | 1741.32 ± 84.64 | 1737.40 ± 41.58 | 1680.69 ± 117.07 | 0.5637 |
| FI (g/b) | 3616.28 ± 255.13 | 3566.13 ± 210.28 | 3597.24 ± 83.50 | 0.9364 |
| FCR | 2.08 ± 0.18 | 2.05 ± 0.09 | 2.15 ± 0.15 | 0.6254 |
| SR (%) | 93.75 ± 12.50 | 95.31 ± 5.98 | 93.75 ± 5.10 | 0.9564 |
| PI | 145.08 ± 32.35 | 147.19 ± 13.96 | 134.72 ± 21.75 | 0.7397 |
| Parameter | Citric Acid By-Product Rice % Dry Matter | p-Value | ||
|---|---|---|---|---|
| 0 | 3 | 6 | ||
| Villi height (µm) | ||||
| Duodenum | 870.47 ± 81.71 | 906.87 ± 66.01 | 913.21 ± 211.12 | 0.7111 |
| Jejunum | 768.45 ± 72.05 a | 769.87 ± 70.65 a | 659.87 ± 111.45 b | 0.0047 |
| Ileum | 508.45 ± 60.63 | 502.48 ± 73.23 | 482.84 ± 22.94 | 0.5155 |
| Crypt depth (µm) | ||||
| Duodenum | 185.59 ± 9.06 a | 173.95 ± 18.82 a | 158.04 ± 23.91 b | 0.0033 |
| Jejunum | 257.66 ± 24.73 a | 175.70 ± 14.49 b | 156.11 ± 28.56 c | 0.0001 |
| Ileum | 172.00 ± 39.09 a | 108.98 ± 20.24 c | 142.99 ± 18.02 b | 0.0001 |
| Villi: Crypt | ||||
| Duodenum | 4.68 ± 0.29 b | 5.28 ± 0.78 ab | 5.81 ± 1.12 a | 0.0067 |
| Jejunum | 2.99 ± 0.23 b | 4.42 ± 0.63 a | 4.38 ± 1.26 a | 0.0001 |
| Ileum | 3.23 ± 1.32 b | 4.81 ± 1.30 a | 3.41 ± 0.34 b | 0.0020 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oryza.S, M.; Wongtangtintharn, S.; Tengjaroenkul, B.; Cherdthong, A.; Tanpong, S.; Pootthachaya, P.; Boonkum, W.; Pintaphrom, N. Investigation of Citric Acid By-Products from Rice Produced by Microbial Fermentation on Growth Performance and Villi Histology of Thai Broiler Chicken (KKU 1). Vet. Sci. 2021, 8, 284. https://doi.org/10.3390/vetsci8110284
Oryza.S M, Wongtangtintharn S, Tengjaroenkul B, Cherdthong A, Tanpong S, Pootthachaya P, Boonkum W, Pintaphrom N. Investigation of Citric Acid By-Products from Rice Produced by Microbial Fermentation on Growth Performance and Villi Histology of Thai Broiler Chicken (KKU 1). Veterinary Sciences. 2021; 8(11):284. https://doi.org/10.3390/vetsci8110284
Chicago/Turabian StyleOryza.S, Mutyarsih, Sawitree Wongtangtintharn, Bundit Tengjaroenkul, Anusorn Cherdthong, Sirisak Tanpong, Padsakorn Pootthachaya, Wuttigrai Boonkum, and Nisakon Pintaphrom. 2021. "Investigation of Citric Acid By-Products from Rice Produced by Microbial Fermentation on Growth Performance and Villi Histology of Thai Broiler Chicken (KKU 1)" Veterinary Sciences 8, no. 11: 284. https://doi.org/10.3390/vetsci8110284
APA StyleOryza.S, M., Wongtangtintharn, S., Tengjaroenkul, B., Cherdthong, A., Tanpong, S., Pootthachaya, P., Boonkum, W., & Pintaphrom, N. (2021). Investigation of Citric Acid By-Products from Rice Produced by Microbial Fermentation on Growth Performance and Villi Histology of Thai Broiler Chicken (KKU 1). Veterinary Sciences, 8(11), 284. https://doi.org/10.3390/vetsci8110284









