Assessment of Changes in the Oral Microbiome That Occur in Dogs with Periodontal Disease
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects and Inclusion Criteria
2.2. Analysis of the Oral Cavity Microbiome
2.3. Bioinformatics Analyses
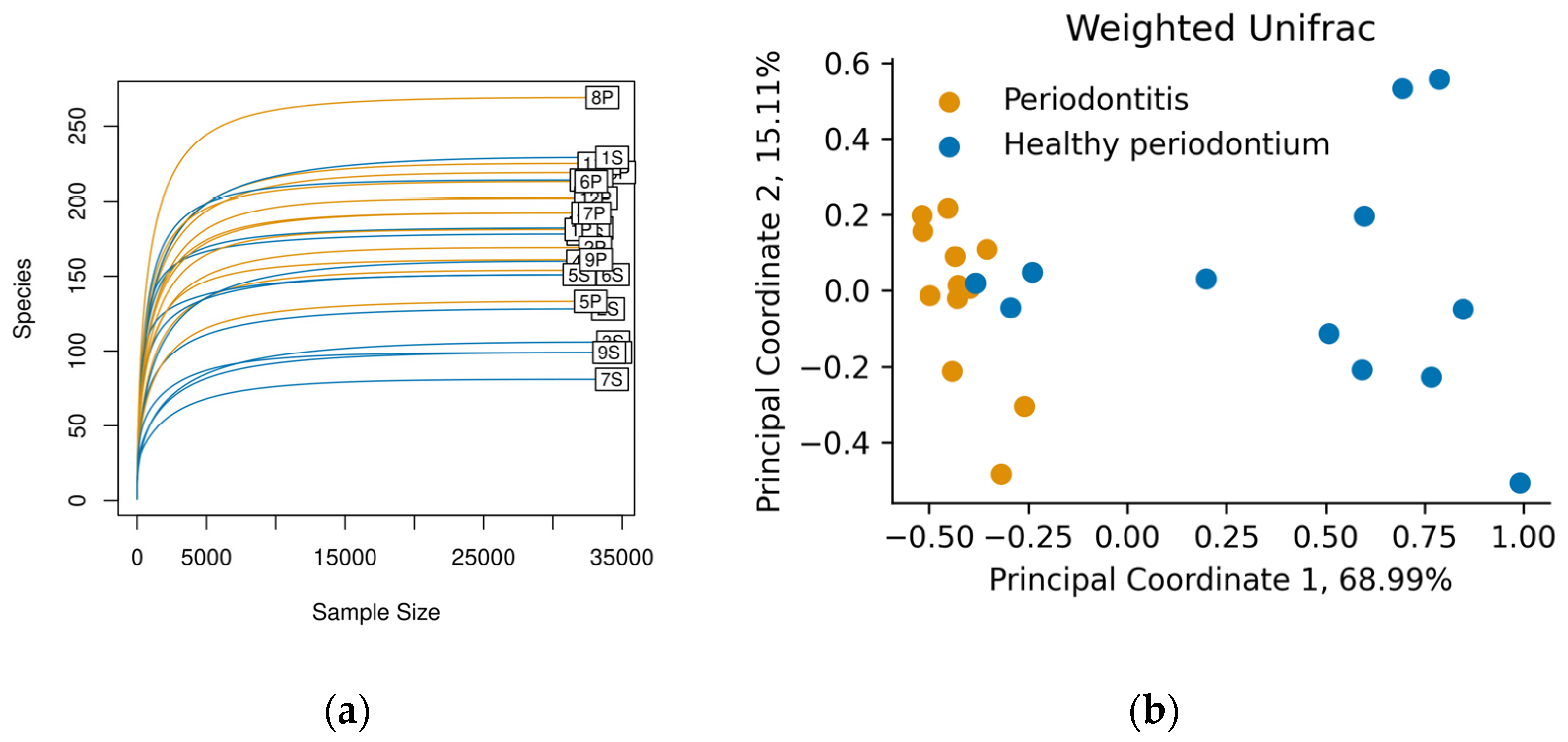
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, W.-P.; Chang, S.-H.; Tang, C.-Y.; Liou, M.-L.; Tsai, S.-J.J.; Lin, Y.-L. Composition analysis and feature selection of the oral microbiota associated with periodontal disease. BioMed Res. Int. 2018, 2018, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Di Bello, A.; Buonavoglia, A.; Franchini, D.; Valastro, C.; Ventrella, G.; Greco, M.F.; Corrente, M. Periodontal disease associated with red complex bacteria in dogs. J. Small Anim. Pr. 2014, 55, 160–163. [Google Scholar] [CrossRef]
- Marshall, M.D.; Wallis, C.V.; Milella, L.; Colyer, A.; Tweedie, A.D.; Harris, S. A longitudinal assessment of periodontal disease in 52 miniature schnauzers. BMC Vet. Res. 2014, 10, 166. [Google Scholar] [CrossRef] [Green Version]
- Stella, J.L.; Bauer, A.E.; Croney, C.C. A cross-sectional study to estimate prevalence of periodontal disease in a population of dogs (Canis familiaris) in commercial breeding facilities in Indiana and Illinois. PLoS ONE 2018, 13, e0191395. [Google Scholar] [CrossRef]
- Wallis, C.; Holcombe, L.J. A review of the frequency and impact of periodontal disease in dogs. J. Small Anim. Pr. 2020, 61, 529–540. [Google Scholar] [CrossRef] [PubMed]
- Ruparell, A.; Inui, T.; Staunton, R.; Wallis, C.; Deusch, O.; Holcombe, L.J. The canine oral microbiome: Variation in bacterial populations across different niches. BMC Microbiol. 2020, 20, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Beikler, T.; Bunte, K.; Chan, Y.; Weiher, B.; Selbach, S.; Peters, U.; Klocke, A.; Watt, R.; Flemmig, T. Oral microbiota transplant in dogs with naturally occurring periodontitis. J. Dent. Res. 2021, 100, 764–770. [Google Scholar] [CrossRef]
- Ruparell, A.; Wallis, C.; Haydock, R.; Cawthrow, A.; Holcombe, L.J. Comparison of subgingival and gingival margin plaque microbiota from dogs with healthy gingiva and early periodontal disease. Res. Vet. Sci. 2021, 136, 396–407. [Google Scholar] [CrossRef]
- Levy, M.; Kolodziejczyk, A.A.; Thaiss, C.A.; Elinav, E. Dysbiosis and the immune system. Nat. Rev. Immunol. 2017, 17, 219–232. [Google Scholar] [CrossRef]
- de Freitas, C.O.T.; Gomes-Filho, I.S.; Naves, R.C.; da Rocha Nogueira Filho, G.; da Cruz, S.S.; de Souza Teles Santos, C.A.; Dunningham, L.; de Miranda, L.F.; da Silva Barbosa, M.D. Influence of periodontal therapy on C-reactive protein level: A systematic review and meta-analysis. J. Appl. Oral Sci. 2012, 20, 1–8. [Google Scholar] [CrossRef]
- Kačírová, J. Dental Biofilm as Etiological Agent of Canine Periodontal Disease; Maďar, M., Ed.; IntechOpen: Rijeka, Croatia, 2020; p. Ch. 15. ISBN 978-1-78985-900-3. [Google Scholar]
- Booij-Vrieling, H.E.; van der Reijden, W.A.; Houwers, D.J.; de Wit, W.E.A.J.; Bosch-Tijhof, C.J.; Penning, L.C.; van Winkelhoff, A.J.; Hazewinkel, H.A.W. Comparison of periodontal pathogens between cats and their owners. Vet. Microbiol. 2010, 144, 147–152. [Google Scholar] [CrossRef]
- Pereira dos Santos, J.D.; Cunha, E.; Nunes, T.; Tavares, L.; Oliveira, M. Relation between periodontal disease and systemic diseases in dogs. Res. Vet. Sci. 2019, 125, 136–140. [Google Scholar] [CrossRef]
- Nomura, R.; Shirai, M.; Kato, Y.; Murakami, M.; Nakano, K.; Hirai, N.; Mizusawa, T.; Naka, S.; Yamasaki, Y.; Matsumoto-Nakano, M.; et al. Diversity of fimbrillin among Porphyromonas gulae clinical isolates from Japanese dogs. J. Vet. Med. Sci. 2012, 74, 885–891. [Google Scholar] [CrossRef] [Green Version]
- Fiorillo, L.; Cervino, G.; Laino, L.; D’Amico, C.; Mauceri, R.; Tozum, T.F.; Gaeta, M.; Cicciù, M. Porphyromonas gingivalis, periodontal and systemic implications: A systematic review. Dent. J. 2019, 7, 114. [Google Scholar] [CrossRef] [Green Version]
- Gawron, K.; Wojtowicz, W.; Łazarz-Bartyzel, K.; Łamasz, A.; Qasem, B.; Mydel, P.; Chomyszyn-Gajewska, M.; Potempa, J.; Mlynarz, P. Metabolomic status of the oral cavity in chronic periodontitis. In Vivo 2019, 33, 1165–1174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iwashita, N.; Nomura, R.; Shirai, M.; Kato, Y.; Murakami, M.; Matayoshi, S.; Kadota, T.; Shirahata, S.; Ohzeki, L.; Arai, N.; et al. Identification and molecular characterization of Porphyromonas gulae fimA types among cat isolates. Vet. Microbiol. 2019, 229, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, N.; Mori, A.; Shono, S.; Oda, H.; Sako, T. Evaluation of changes in periodontal bacteria in healthy dogs over 6 months using quantitative real-time PCR. Pol. J. Vet. Sci. 2018, 21, 127–132. [Google Scholar]
- Özavci, V.; Erbas, G.; Parin, U.; Yüksel, H.T.; Kirkan, Ş. Molecular detection of feline and canine periodontal pathogens. Vet. Anim. Sci. 2019, 8, 100069. [Google Scholar] [CrossRef]
- Löe, H. The Gingival Index, the Plaque Index and the Retention Index Systems. J. Periodontol. 1967, 38, 610–616. [Google Scholar] [CrossRef]
- Chun, J.L.; Bang, H.T.; Ji, S.Y.; Jeong, J.Y.; Kim, M.; Kim, B.; Lee, S.D.; Lee, Y.K.; Reddy, K.E.; Kim, K.H. A simple method to evaluate body condition score to maintain the optimal body weight in dogs. J Anim Sci Technol. 2019, 61, 366–370. [Google Scholar] [CrossRef] [Green Version]
- Kato, Y.; Shirai, M.; Murakami, M.; Mizusawa, T.; Hagimoto, A.; Wada, K.; Nomura, R.; Nakano, K.; Ooshima, T.; Asai, F. Molecular detection of human periodontal pathogens in oral swab specimens from dogs in Japan. J. Vet. Dent. 2011, 28, 84–89. [Google Scholar] [CrossRef]
- Thomson, P.; Santibañez, R.; Aguirre, C.; Galgani, J.E.; Garrido, D. Short-term impact of sucralose consumption on the metabolic response and gut microbiome of healthy adults. Br. J. Nutr. 2019, 122, 856–862. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [Green Version]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, P.; Parfrey, L.W.; Yarza, P.; Gerken, J.; Pruesse, E.; Quast, C.; Schweer, T.; Peplies, J.; Ludwig, W.; Glöckner, F.O. The SILVA and “All-species Living Tree Project (LTP)” taxonomic frameworks. Nucleic Acids Res. 2014, 42, D643–D648. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naïve Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Douglas, G.M.; Maffei, V.J.; Zaneveld, J.R.; Yurgel, S.N.; Brown, J.R.; Taylor, C.M.; Huttenhower, C.; Langille, M.G.I. PICRUSt2 for prediction of metagenome functions. Nat. Biotechnol. 2020, 38, 685–688. [Google Scholar] [CrossRef]
- Caspi, R.; Billington, R.; Ferrer, L.; Foerster, H.; Fulcher, C.A.; Keseler, I.M.; Kothari, A.; Krummenacker, M.; Latendresse, M.; Mueller, L.A.; et al. The MetaCyc database of metabolic pathways and enzymes and the BioCyc collection of pathway/genome databases. Nucleic Acids Res. 2016, 44, D471–D480. [Google Scholar] [CrossRef]
- Mann, H.B.; Whitney, D.R. On a test of whether one of two random variables is stochastically larger than the other. Ann. Math. Stat. 1947, 18, 50–60. [Google Scholar] [CrossRef]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef] [Green Version]
- Dewhirst, F.E.; Klein, E.A.; Thompson, E.C.; Blanton, J.M.; Chen, T.; Milella, L.; Buckley, C.M.F.; Davis, I.J.; Bennett, M.-L.; Marshall-Jones, Z.V. The canine oral microbiome. PLoS ONE 2012, 7, e36067. [Google Scholar] [CrossRef]
- Oba, P.M.; Carroll, M.Q.; Alexander, C.; Valentine, H.; Somrak, A.J.; Keating, S.C.J.; Sage, A.M.; Swanson, K.S. Microbiota populations in supragingival plaque, subgingival plaque, and saliva habitats of adult dogs. Anim. Microbiome 2021, 3, 38. [Google Scholar] [CrossRef] [PubMed]
- Niemiec, B.A.; Gawor, J.; Tang, S.; Prem, A.; Krumbeck, J.A. The bacteriome of the oral cavity in healthy dogs and dogs with periodontal disease. Am. J. Vet. Res. 2022, 1–9. [Google Scholar] [CrossRef]
- Lira-Junior, R.; Åkerman, S.; Klinge, B.; Boström, E.A.; Gustafsson, A. Salivary microbial profiles in relation to age, periodontal, and systemic diseases. PLoS ONE 2018, 13, e0189374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- An, J.Y.; Darveau, R.; Kaeberlein, M. Oral health in geroscience: Animal models and the aging oral cavity. GeroScience 2017, 40, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Davis, I.J.; Wallis, C.; Deusch, O.; Colyer, A.; Milella, L.; Loman, N.; Harris, S.A. Cross-sectional survey of bacterial species in plaque from client owned dogs with healthy gingiva, gingivitis, or mild periodontitis. PLoS ONE 2013, 8, e83158. [Google Scholar] [CrossRef] [PubMed]
- Gadê-Neto, C.R.; Rodrigues, R.R.; Louzada, L.M.; Arruda-Vasconcelos, R.; Teixeira, F.B.; Viana Casarin, R.C.; Gomes, B.P. Microbiota of periodontal pockets and root canals in induced experimental periodontal disease in dogs. J. Investig. Clin. Dent. 2019, 10, e12439. [Google Scholar] [CrossRef]
- Holcombe, L.J.; Patel, N.; Colyer, A.; Deusch, O.; O’Flynn, C.; Harris, S. Early canine plaque biofilms: Characterization of key bacterial interactions involved in initial colonization of enamel. PLoS ONE 2014, 9, e113744. [Google Scholar] [CrossRef] [Green Version]
- Valm, A.M. The structure of dental plaque microbial communities in the transition from health to dental caries and periodontal disease. J. Mol. Biol. 2019, 431, 2957–2969. [Google Scholar] [CrossRef]
- Lyu, X.; Wang, L.; Shui, Y.; Jiang, Q.; Chen, L.; Yang, W.; He, X.; Zeng, J.; Li, Y. Ursolic acid inhibits multi-species biofilms developed by Streptococcus mutans, Streptococcus sanguinis, and Streptococcus gordonii. Arch. Oral Biol. 2021, 125, 105107. [Google Scholar] [CrossRef]
- Zhao, W.-W.; Zan, K.; Wu, J.-Y.; Gao, W.; Yang, J.; Ba, Y.-Y.; Wu, X.; Chen, X.-Q. Antibacterial triterpenoids from the leaves of Ilex hainanensis Merr. Nat. Prod. Res. 2019, 33, 2435–2439. [Google Scholar] [CrossRef]
- Takahashi, N.; Nyvad, B. The Role of Bacteria in the Caries Process: Ecological Perspectives. J. Dent. Res. 2010, 90, 294–303. [Google Scholar] [CrossRef] [PubMed]
- de Araújo, A.A.; de Morais, H.B.; de Medeiros, C.A.C.X.; de Castro Brito, G.A.; Guedes, P.M.M.; Hiyari, S.; Pirih, F.Q.; de Araújo Júnior, R.F. Gliclazide reduced oxidative stress, inflammation, and bone loss in an experimental periodontal disease model. J. Appl. Oral Sci. 2019, 27, e20180211. [Google Scholar] [CrossRef]
- Nomura, R.; Inaba, H.; Yasuda, H.; Shirai, M.; Kato, Y.; Murakami, M.; Iwashita, N.; Shirahata, S.; Yoshida, S.; Matayoshi, S.; et al. Inhibition of Porphyromonas gulae and periodontal disease in dogs by a combination of clindamycin and interferon alpha. Sci. Rep. 2020, 10, 3113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Andrade, K.Q.; Almeida-Da-Silva, C.L.C.; Coutinho-Silva, R. Immunological pathways triggered by Porphyromonas gingivalis and Fusobacterium nucleatum: Therapeutic possibilities? Mediat. Inflamm. 2019, 2019, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Aziz, J.; Rahman, M.T.; Vaithilingam, R.D. Dysregulation of metallothionein and zinc aggravates periodontal diseases. J. Trace Elem. Med. Biol. 2021, 66, 126754. [Google Scholar] [CrossRef]
- Guimaraes-Stabili, M.R.; de Medeiros, M.C.; Rossi, D.; Camilli, A.C.; Zanelli, C.F.; Valentini, S.R.; Spolidorio, L.C.; Kirkwood, K.L.; Rossa, C. Silencing matrix metalloproteinase-13 (Mmp-13) reduces inflammatory bone resorption associated with LPS-induced periodontal disease in vivo. Clin. Oral Investig. 2021, 25, 3161–3172. [Google Scholar] [CrossRef]
- Wallis, C.; Marshall, M.; Colyer, A.; O’Flynn, C.; Deusch, O.; Harris, S. A longitudinal assessment of changes in bacterial community composition associated with the development of periodontal disease in dogs. Vet. Microbiol. 2015, 181, 271–282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Flynn, C.; Deusch, O.; Darling, A.E.; Eisen, J.A.; Wallis, C.; Davis, I.J.; Harris, S.J. Comparative genomics of the genus Porphyromonas identifies adaptations for heme synthesis within the prevalent canine oral species Porphyromonas cangingivalis. Genome Biol. Evol. 2015, 7, 3397–3413. [Google Scholar] [CrossRef]
- Aquino-Martinez, R.; Rowsey, J.L.; Fraser, D.G.; Eckhardt, B.A.; Khosla, S.; Farr, J.N.; Monroe, D.G. LPS-induced premature osteocyte senescence: Implications in inflammatory alveolar bone loss and periodontal disease pathogenesis. Bone 2020, 132, 115220. [Google Scholar] [CrossRef]
- Ramenzoni, L.L.; Annasohn, L.; Miron, R.J.; Attin, T.; Schmidlin, P.R. Combination of enamel matrix derivative and hyaluronic acid inhibits lipopolysaccharide-induced inflammatory response on human epithelial and bone cells. Clin. Oral Investig. 2021. [Google Scholar] [CrossRef] [PubMed]





| Classification | Code | Sex | Age (y) | Race | Weight (kg) | BS | Diet Type | Gingivitis or Periodontitis | Gingival Index | Dental Calculus | Halitosis | Tooth Exfoliation |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Healthy Periodontium (H) | 1H | M | 2 | Mutt | 9.8 | 8 | CD&HM | 0 | 0 | 0 | 0 | 0 |
| 2H | F | 4 | Mutt | 29 | 5 | CD&HM | 0 | 0 | 0 | 0 | 0 | |
| 3H | M | 1 | Poodle | 7 | 5 | CD | 0 | 0 | 0 | 0 | 0 | |
| 4H | M | 2 | Mutt | 10 | 5 | CD&HM | 0 | 0 | 0 | 0 | 0 | |
| 5H | F | 1 | Mutt | 20 | 5 | CD&HM | 0 | 0 | 0 | 0 | 0 | |
| 6S | F | 2.5 | Labrador | 25 | 5 | CD | 0 | 0 | 0 | 0 | 0 | |
| 7H | F | 1 | German Shepherd | 33 | 5 | CD | 0 | 0 | 0 | 0 | 0 | |
| 8H | M | 7 | Teckel | 5.8 | 5 | CD | 0 | 0 | 0 | 0 | 0 | |
| 9H | M | 3 | Mutt | 17 | 5 | CD | 0 | 0 | 0 | 0 | 0 | |
| 10H | M | 5 | Mutt | 21 | 5 | CD&HM | 0 | 0 | 0 | 0 | 0 | |
| 11H | F | 2 | Mutt | 18 | 5 | CD&HM | 0 | 0 | 0 | 0 | 0 | |
| 12H | M | 3 | Maltese | 5 | 5 | CD | 0 | 0 | 0 | 0 | 0 | |
| Average H | 2.79 | 16.72 | 5.25 | |||||||||
| Periodontitis (P) | 1P | F | 8 | Mutt | 35 | 5 | CD | 1 | 1 | 1 | 1 | 0 |
| 2P | M | 7 | Poodle | 6.2 | 5 | CD | 1 | 1 | 1 | 1 | 1 | |
| 3P | M | 9 | Poodle | 4.9 | 5 | CD&HM | 1 | 1 | 1 | 1 | 0 | |
| 4P | F | 3 | Mutt | 20 | 5 | CD&HM | 1 | 1 | 1 | 1 | 0 | |
| 5P | M | 4 | Mutt | 17 | 5 | CD&HM | 1 | 0 | 1 | 1 | 0 | |
| 6P | F | 3 | Cocker Spaniel | 12.5 | 5 | CD&HM | 1 | 1 | 1 | 1 | 0 | |
| 7P | F | 6 | Teckel | 7 | 7 | CD | 1 | 1 | 1 | 1 | 0 | |
| 8P | F | 8 | Mutt | 6 | 5 | CD&HM | 1 | 1 | 1 | 1 | 1 | |
| 9P | F | 13 | Poodle | 8.8 | 7 | CD | 1 | 1 | 1 | 1 | 0 | |
| 10P | M | 8 | Maltese | 5 | 5 | CD&HM | 1 | 1 | 1 | 1 | 0 | |
| 11P | M | 6 | Chihuahua | 4.6 | 7 | CD&HM | 1 | 1 | 1 | 1 | 0 | |
| 12P | F | 2 | Yorkshire | 3.5 | 5 | CD&HM | 1 | 1 | 1 | 1 | 0 | |
| Average P | 6.42 | 10.88 | 5.50 | 1.00 | 0.92 | 1.00 | 1.00 |
| Genus | Healthy Periodontium (Mean%) | Periodontitis (Mean%) | Ratio S/P | Ratio P/S | p-Value |
|---|---|---|---|---|---|
| Acholeplasma | 0.055 ± 0.19 | 0.34 ± 0.21 | 0.16 | 6.06 | 0.00116 |
| Acinetobacter | 0.27 ± 0.44 | 0.0015 ± 0.0038 | 177.32 | 0.01 | 0.00015 |
| Alloprevotella | 0.46 ± 0.58 | 1.7 ± 3.1 | 0.27 | 3.74 | 0.04040 |
| Bacteroides | 0.62 ± 1.1 | 1.6 ± 1.7 | 0.38 | 2.62 | 0.01414 |
| Bosea | 0.1 ± 0.31 | 0.0031 ± 0.0062 | 32.16 | 0.03 | 0.01828 |
| Bradyrhizobium | 3.9 ± 6.6 | 0.007 ± 0.0099 | 552.06 | 0.000795 | 0.00042 |
| Brevundimonas | 0.079 ± 0.18 | 0.015 ± 0.015 | 5.3 | 0.19 | 0.03289 |
| Campylobacter | 0.24 ± 0.41 | 0.33 ± 0.2 | 0.73 | 1.38 | 0.04374 |
| Candidatus Tammella | 0.00049 ± 0.0017 | 0.13 ± 0.14 | 0.0038 | 259.03 | 0.00080 |
| Catonella | 0.0076 ± 0.024 | 0.17 ± 0.17 | 0.05 | 21.77 | 0.00024 |
| ChristensenellaceaeR-7 group | 0.76 ± 1.1 | 2 ± 2.3 | 0.38 | 2.62 | 0.02258 |
| Cutibacterium | 2 ± 3.3 | 0.0021 ± 0.0051 | 950.79 | 0.0011 | 0.00039 |
| DefluviitaleaceaeUCG-011 | 0.16 ± 0.21 | 0.72 ± 0.46 | 0.23 | 4.4 | 0.00134 |
| Desulfobulbus | 0.00079 ± 0.0027 | 0.35 ± 0.52 | 0.0023 | 447.73 | 0.00101 |
| Desulfoplanes | 0.002 ± 0.0052 | 0.14 ± 0.19 | 0.01 | 69.55 | 0.04242 |
| Desulfovibrio | 0.31 ± 0.57 | 1.3 ± 1.4 | 0.24 | 4.12 | 0.00426 |
| Ezakiella | 0.021 ± 0.058 | 0.35 ± 0.51 | 0.06 | 16.39 | 0.00081 |
| Fastidiosipila | 0.00051 ± 0.0018 | 0.11 ± 0.12 | 0.0046 | 216.92 | 0.00080 |
| Filifactor | 0.57 ± 0.75 | 1.8 ± 1.4 | 0.32 | 3.1 | 0.01018 |
| Finegoldia | 0.93 ± 1.6 | 0.00076 ± 0.0026 | 1233.58 | 0.00082 | 0.00253 |
| Flexilinea | 0.22 ± 0.48 | 1.8 ± 1.7 | 0.12 | 8.01 | 0.00072 |
| Fusibacter | 0.39 ± 0.71 | 0.89 ± 0.64 | 0.43 | 2.31 | 0.01003 |
| Gemella | 0.39 ± 1 | 0.0062 ± 0.015 | 62.64 | 0.02 | 0.03913 |
| H1 | 0.027 ± 0.074 | 0.16 ± 0.12 | 0.17 | 5.95 | 0.00121 |
| Helcococcus | 0.0051 ± 0.0055 | 0.47 ± 0.64 | 0.01 | 92.4 | 0.00165 |
| Luteibacter | 0.1 ± 0.17 | 0.022 ± 0.0098 | 4.45 | 0.22 | 0.00610 |
| Massilia | 1.1 ± 3.5 | 0.055 ± 0.015 | 19.7 | 0.05 | 0.00244 |
| Odoribacter | 0.0024 ± 0.0062 | 0.29 ± 0.54 | 0.01 | 118.57 | 0.00012 |
| Pelomonas | 1.7 ± 3 | 0.0015 ± 0.0051 | 1127.24 | 0.00088 | 0.00253 |
| Peptoanaerobacter | 0.19 ± 0.42 | 0.37 ± 0.35 | 0.52 | 1.92 | 0.03371 |
| Peptococcus | 0.019 ± 0.043 | 0.61 ± 0.56 | 0.03 | 32.56 | 0.00008 |
| Peptoniphilus | 0.27 ± 0.46 | 0.092 ± 0.22 | 2.94 | 0.34 | 0.04230 |
| Peptostreptococcus | 0.014 ± 0.018 | 0.87 ± 1.1 | 0.02 | 61.97 | 0.00086 |
| Porphyromonas | 13 ± 15 | 39 ± 11 | 0.32 | 3.1 | 0.00048 |
| Prevotella7 | 0.0022 ± 0.0076 | 0.24 ± 0.36 | 0.01 | 110.08 | 0.00038 |
| Propionivibrio | 0.035 ± 0.072 | 0.092 ± 0.082 | 0.38 | 2.63 | 0.00997 |
| Proteiniphilum | 0.0022 ± 0.0076 | 0.22 ± 0.37 | 0.01 | 100.8 | 0.00314 |
| Proteocatella | 0.2 ± 0.45 | 0.45 ± 0.56 | 0.45 | 2.22 | 0.01885 |
| Pseudarthrobacter | 0.39 ± 1.1 | 0.0075 ± 0.021 | 51.86 | 0.02 | 0.01563 |
| Pseudomonas | 0.94 ± 2.8 | 0.073 ± 0.018 | 12.85 | 0.08 | 0.00244 |
| RikenellaceaeRC9 gut group | 0.0025 ± 0.0059 | 0.2 ± 0.25 | 0.01 | 79.88 | 0.00015 |
| Roseburia | 0.0084 ± 0.026 | 0.22 ± 0.23 | 0.04 | 26.23 | 0.00004 |
| Ruminiclostridium9 | 0.0011 ± 0.0037 | 0.47 ± 0.93 | 0.0023 | 442.97 | 0.00101 |
| RuminococcaceaeUCG-004 | 0.08 ± 0.21 | 0.35 ± 0.47 | 0.23 | 4.41 | 0.04291 |
| Salinisphaera | 0.99 ± 1.5 | 0.0015 ± 0.0036 | 647.56 | 0.0015 | 0.00280 |
| Sediminispirochaeta | 0.0049 ± 0.017 | 0.045 ± 0.045 | 0.11 | 9.16 | 0.00587 |
| Sphaerochaeta | 0.0081 ± 0.028 | 0.034 ± 0.071 | 0.24 | 4.21 | 0.04809 |
| Sphingomonas | 0.13 ± 0.13 | 0.074 ± 0.017 | 1.8 | 0.56 | 0.01202 |
| Staphylococcus | 2.4 ± 3.3 | 0.0027 ± 0.0067 | 873.18 | 0.0011 | 0.00009 |
| Streptococcus | 1.7 ± 1.9 | 0.079 ± 0.24 | 21.19 | 0.05 | 0.00203 |
| Suttonella | 0.16 ± 0.15 | 0.078 ± 0.028 | 2.02 | 0.49 | 0.04639 |
| Treponema2 | 0.35 ± 0.57 | 3 ± 1.8 | 0.12 | 8.48 | 0.00019 |
| Variovorax | 2.2 ± 3.1 | 0.026 ± 0.024 | 84.26 | 0.01 | 0.00006 |
| Verticia | 0.12 ± 0.026 | 0.072 ± 0.028 | 1.73 | 0.58 | 0.00016 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santibáñez, R.; Rodríguez-Salas, C.; Flores-Yáñez, C.; Garrido, D.; Thomson, P. Assessment of Changes in the Oral Microbiome That Occur in Dogs with Periodontal Disease. Vet. Sci. 2021, 8, 291. https://doi.org/10.3390/vetsci8120291
Santibáñez R, Rodríguez-Salas C, Flores-Yáñez C, Garrido D, Thomson P. Assessment of Changes in the Oral Microbiome That Occur in Dogs with Periodontal Disease. Veterinary Sciences. 2021; 8(12):291. https://doi.org/10.3390/vetsci8120291
Chicago/Turabian StyleSantibáñez, Rodrigo, Camila Rodríguez-Salas, Carla Flores-Yáñez, Daniel Garrido, and Pamela Thomson. 2021. "Assessment of Changes in the Oral Microbiome That Occur in Dogs with Periodontal Disease" Veterinary Sciences 8, no. 12: 291. https://doi.org/10.3390/vetsci8120291






