Epidemiology, Diagnosis, and Control of Canine Infectious Cyclic Thrombocytopenia and Granulocytic Anaplasmosis: Emerging Diseases of Veterinary and Public Health Significance
Abstract
:1. Introduction
2. History
3. Etiology
4. Taxonomical Position of Anaplasma Bacteria
4.1. Evolution of Taxonomy
4.2. Current Classification
5. Epidemiology
5.1. Anaplasma platys
5.2. Anaplasma phagocytophilum
| Domestic Canid | Countries (Region) | Prevalences (%) | Methods (Target Genes) | References |
|---|---|---|---|---|
| Dog | Thailand | 13.9 | PCRa (groEL) | [62] |
| Thailand | 29.4 | PCRa (16S rRNA) | [63] | |
| Thailand | 7.0 | PCRa (16S rRNA)/mHRMb | [64] | |
| West Indies (Grenada) | 18.7 | PCRa (16S rRNA) | [65] | |
| West Indies (Grenada) | 33.0 | PCRa (16S rRNA)/ELISAc | [66] | |
| West Indies (Grenada) | 16.4 | RT-PCRd (16S rRNA) | [67] | |
| West Indies (Trinidad) | 2.3 | PCRa (16S rRNA)/RBLe | [68] | |
| Pakistan | 11.34 | PCRa (16S rRNA) | [69] | |
| Paraguay | 10.67 | PCRa (16S rRNA) | [70] | |
| Colombia | 20.2 | RT-PCRd (16S rRNA) | [71] | |
| Greece | Case report | Blood smear/ELISAc | [72] | |
| Indonesia | 11.76 | PCRa (groEL) | [73] | |
| Cape Verde | 7.7 | PCRa (16S rRNA) | [74] | |
| Italy | 70.5 | PCRa (groEL) | [75] | |
| Italy (Putignano) | 52.9 | RT-PCRc (16S r RNA) | [76] | |
| Italy (Teramo Kennel) | 33.0 | PCRa (16S rRNA)/RLBe | [48] | |
| Croatia | Case report | RT-PCRd (groEL) | [77] | |
| Australia | 51.3 | RT-PCRd (16S rRNA) | [78] | |
| Australia | 23.7 | ELISAc | [78] | |
| Australia | 32.0 | PCRa (16S/18S rRNA) | [49] | |
| Australia | 3.8 | Blood smear/ELISAc/PCRa | [79] | |
| Romania | Case report | PCRa (16S rRNA) | [80] | |
| Dominican Republic | 11 | RT-PCRd (16S/18S rRNA) | [81] | |
| Nicaragua | 13 | RT-PCRd(16S/18S rRNA) | [82] | |
| Caribbean | 10.3 | ELISAc | [83] | |
| Canada | 1.8 | ELISAc | [83] | |
| USA (South) | 2.0 | ELISAc | [83] | |
| USA (Mid Atlantic) | 1.1 | ELISAc | [83] | |
| USA (Northeast) | 1.5 | ELISAc | [83] | |
| USA (Midwest) | 0.6 | ELISAc | [83] | |
| USA (West) | 1.0 | ELISAc | [83] | |
| Mexico | 31.0 | PCRa (16S rRNA) | [84] | |
| Brazil | 7.19 | PCRa (16S rRNA) | [51] | |
| Turkey | 0.5 | RLBd | [85] | |
| Costa Rica | 1 | PCRa (16S rRNA, groEL) | [86] | |
| Brazil | 16.96 | nPCRf (16S rRNA) | [87] | |
| Brazil | 19.4 | PCRa (16S rRNA) | [88] | |
| Brazil | 14.07 | nPCRf (16S rRNA)/ELISAc | [89] | |
| Colombia | 53.0 | PCRa (16S rRNA)/ELISAc | [90] | |
| Palestine | 53.0 | PCRa (16S rRNA) | [91] | |
| China | 62.1 | RT-LAMPg/nPCRf (16S rRNA) | [92] | |
| Caribbean | 18.7 | PCRa (16S rRNA, gltA, groEl) | [65] | |
| Argentina | 37.5 | PCRa (16S rRNA, groESL) | [93] | |
| Costa Rica | 6.25 | nPCRf (16S rRNA)/ELISAc | [94] | |
| Myanmar | 0.25 | PCRa (16S rRNA) | [95] | |
| Malawi | 2.4 | PCRa (16S rRNA) | [96] | |
| Galápagos | 6.9 | PCRa (16S rRNA)/ELISAc | [97] | |
| Saudi Arabia | 57.1 | RT-PCRc (16S rRNA) | [98] | |
| Greek islands | 18.0 | PCRa (16S rRNA)/IFATh | [99] | |
| Malta | 22.7 | PCRa (16S rRNA, cox1) | [100] | |
| Haiti | 6.3 | PCRa (16S/18S rRNA) | [101] | |
| Cambodia | 32.0 | NGSi based metabarcoding | [102] | |
| Uganda | 18.9 | RT-PCRd (16S rRNA)/IFATh | [103] | |
| Albania | 3.3 | PCRa (16S rRNA)/ELISAb | [104] | |
| Nigeria | 6.6 | RT-PCRd (16S rRNA) | [105] | |
| Qatar | 1.6 | PCRa (16S rRNA) | [106] | |
| Texas | 0.17 | RT-PCRd (16S rRNA) | [107] | |
| India | 22.6 | PCRa (16S rRNA) | [108] | |
| Japan | 32.0 | PCRa (16S rRNA) | [109] |
6. Transmission
7. Life Cycle
8. Clinical Findings
9. Diagnosis
9.1. Direct Detection
9.2. Serology
9.3. Molecular Detection
9.4. Isolation and In Vitro Cultivation
10. Control
10.1. Vector Control
10.2. Vaccination against A. phagocytophilum and A. platys
10.3. Chemotherapeutic Use
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Atif, F.A. Alpha proteobacteria of genus Anaplasma (Rickettsiales: Anaplasmataceae): Epidemiology and characteristics of Anaplasma species related to veterinary and public health importance. Parasitology 2016, 143, 659–685. [Google Scholar] [CrossRef] [PubMed]
- Ben Said, M.; Belkahia, H.; Messadi, L. Anaplasma spp. in North Africa: A review on molecular epidemiology, associated risk factors and genetic characteristics. Ticks Tick Borne Dis. 2018, 9, 543–555. [Google Scholar] [CrossRef]
- Stiller, D.; Crosbie, P.R.; Boyce, W.M.; Goff, W.L. Dermacentor hunteri (Acari: Ixodidae): An experimental vector of Anaplasma marginale and A. ovis (Rickettsiales: Anaplasmataceae) to calves and sheep. J. Med. Entomol. 1999, 36, 321–324. [Google Scholar] [CrossRef]
- Harvey, J.W.; Simpson, C.F.; Gaskin, J.M. Cyclic thrombocytopenia induced by a Rickettsia-like agent in dogs. J. Infect. Dis. 1978, 137, 182–188. [Google Scholar] [CrossRef]
- Sainz, A.; Roura, X.; Miro, G.; Estrada-Pena, A.; Kohn, B.; Harrus, S.; Solano-Gallego, L. Guideline for veterinary practitioners on canine ehrlichiosis and anaplasmosis in Europe. Parasit. Vectors 2015, 8, 2–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Battilani, M.; De Arcangeli, S.; Balboni, A.; Dondi, F. Genetic diversity and molecular epidemiology of Anaplasma. Infect. Genet. Evol. 2017, 49, 195–211. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.M.; Dumler, J.S.; Bakken, J.S.; Walker, D.H. Identification of a granulocytotropic Ehrlichia species as the etiologic agent of human disease. J. Clin. Microbiol. 1994, 32, 589–595. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arraga-Alvarado, C.; Palmar, M.; Parra, O.; Salas, P. Fine structural characterisation of a Rickettsia-like organism in human platelets from patients with symptoms of ehrlichiosis. J. Med. Microbiol. 1999, 48, 991–997. [Google Scholar] [CrossRef] [PubMed]
- Maggi, R.G.; Mascarelli, P.E.; Havenga, L.N.; Naidoo, V.; Breitschwerdt, E.B. Co-infection with Anaplasma platys, Bartonella henselae and Candidatus Mycoplasma haematoparvum in a veterinarian. Parasit. Vectors 2013, 6, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Breitschwerdt, E.B.; Hegarty, B.C.; Qurollo, B.A.; Saito, T.B.; Maggi, R.G.; Blanton, L.S.; Bouyer, D.H. Intravascular persistence of Anaplasma platys, Ehrlichia chaffeensis, and Ehrlichia ewingii DNA in the blood of a dog and two family members. Parasit. Vectors 2014, 7, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- CDC. Centers for Disease Control and Prevention, National Center for Emerging and Zoonotic Infectious Diseases (NCEZID), Division of Vector-Borne Diseases (DVBD). Available online: https://www.cdc.gov/anaplasmosis/stats/index.html (assessed on 23 April 2019).
- Reppert, E.; Galindo, R.C.; Breshears, M.A.; Kocan, K.M.; Blouin, E.F.; de la Fuente, J. Demonstration of transplacental transmission of a human isolate of Anaplasma phagocytophilum in an experimentally infected sheep. Transbound. Emerg. Dis. 2013, 60, 93–96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stuen, S.; Granquist, E.G.; Silaghi, C. Anaplasma phagocytophilum-a widespread multi-host pathogen with highly adaptive strategies. Front. Cell. Infect. Microbiol. 2013, 3, 31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gordon, W.S.; Brownlee, A.; Wilson, D.R.; MacLeod, J. Tick-Borne Fever (A hitherto undescribed disease of sheep). J. Comp. Path. 1932, 45, 106. [Google Scholar] [CrossRef]
- MacLeod, J.; Gordon, W.S. Studies on tick borne fever in sheep I. Transmission by the tick Ixodes ricinus and description of the disease produced. Parasitology 1933, 25, 273–283. [Google Scholar] [CrossRef]
- MacLeod, J. Studies on tick-borne fever of sheep. 2. Experiment on transmission and distribution of the disease. Parasitology. 1936, 28, 320–329. [Google Scholar] [CrossRef]
- Foggie, A. Studies on tick-borne fever in sheep. J. Gen. Microbiol. 1949, 3, 5–6. [Google Scholar]
- Dumler, J.; Choi, K.; Garcia, J.; Barat, N.; Scorpio, D.; Garyu, J.; Grab, D.; Bakken, J. Human Granulocytic Anaplasmosis and Anaplasma phagocytophilum. Emerg. Infect. Dis. 2005, 11, 1828–1839. [Google Scholar] [CrossRef] [PubMed]
- Gribble, D.H. Equine ehrlichiosis. J. Am. Vet. Med. Assoc. 1969, 155, 462–469. [Google Scholar] [PubMed]
- Lewis, J.E.; Huxsoll, D.L.; Ristic, M.; Johnson, A.J. Experimentally induced infection of dogs, cats, and nonhuman primates with Ehrlichia equi, etiologic agent of equine ehrlichiosis. Am. J. Vet. Res. 1975, 36, 85–88. [Google Scholar]
- Bjöersdorff, A.; Bagert, B.; Massung, R.F.; Gusa, A.; Eliasson, I. Isolation and characterization of two European strains of Ehrlichia phagocytophila of equine origin. Clin. Diagn. Lab. Immunol. 2002, 9, 341–343. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Zheng, Y.; Ma, L.; Jia, N.; Jiang, B.; Jiang, R.; Huo, Q.; Wang, Y.; Liu, H.; Chu, Y.L.; et al. Human infection with a novel tick-borne Anaplasma species in China: A surveillance study. Lancet Infect. Dis. 2015, 15, 663–670. [Google Scholar] [CrossRef]
- Chandra, S.; Smith, K.; Alanazi, A.D.; Alyousif, M.S.; Emery, D.; Šlapeta, J. Rhipicephalus sanguineus sensu lato from dogs and dromedary camels in Riyadh, Saudi Arabia: Low prevalence of vector-borne pathogens in dogs detected using multiplexed tandem PCR panel. Folia Parasitol. 2019, 66, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atif, F.A. Anaplasma marginale and Anaplasma phagocytophilum: Rickettsiales pathogens of veterinary and public health significance. Parasitol. Res. 2015, 114, 3941–3957. [Google Scholar] [CrossRef] [PubMed]
- Kawahara, M.; Rikihisa, Y.; Lin, Q.; Isogai, E.; Tahara, K.; Itagaki, A.; Hiramitsu, Y.; Tajima, T. Novel genetic variants of Anaplasma phagocytophilum, Anaplasma bovis, Anaplasma centrale, and a novel Ehrlichia sp. in wild deer and ticks on two major islands in Japan. Appl. Environ. Microbiol. 2006, 72, 1102–1109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, J.; Liu, Z.; Niu, Q.; Liu, J.; Han, R.; Liu, G.; Shi, Y.; Luo, J.; Yin, H. Molecular survey and characterization of a novel Anaplasma species closely related to Anaplasma capra in ticks, northwestern China. Parasit. Vectors 2016, 9, 603–605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, Y.; Lu, C.; Yan, Y.; Shi, K.; Chen, Q.; Zhao, C.; Wang, R.; Zhang, L.; Jian, F.; Ning, C. The first detection of Anaplasma capra, an emerging zoonotic Anaplasma sp., in erythrocytes. Emerg. Microbes. Infect. 2021, 10, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Tate, C.M.; Howerth, E.W.; Mead, D.G.; Dugan, V.G.; Luttrell, M.P.; Sahora, A.I.; Munderloh, U.G.; Davidson, W.R.; Yabsley, M.J. Anaplasma odocoilei sp. nov. (family Anaplasmataceae) from white-tailed deer (Odocoileus virginianus). Ticks Tick Borne Dis. 2013, 4, 110–119. [Google Scholar] [CrossRef] [Green Version]
- Lbacha, A.H.; Zouagui, Z.; Alali, S.; Rhalem, A.; Petit, E.; Ducrotoy, M.J.; Boulouis, H.-J.; Maillard, R. “Candidatus anaplasma camelii” in one-humped camels (Camelus dromedarius) in Morocco: A novel and emerging Anaplasma species? Infect. Dis. Poverty 2017, 6, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Gofton, A.W.; Hair, S.; Ryan, U.; Irwin, P. Initial detection of Ehrlichia mineirensis and ‘ Candidatus Anaplasma boleense’ in an Australian steer. Mol. Genet. Genom. 2018, 5, 119. [Google Scholar]
- Dahmani, M.; Davoust, B.; Sambou, M.; Bassene, H.; Scandola, P.; Ameur, T.; Raoult, D.; Fenollar, F.; Mediannikov, O. Molecular investigation and phylogeny of species of the Anaplasmataceae infecting animals and ticks in Senegal. Parasit. Vectors 2019, 12, 495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vanstreels, R.E.T.; Yabsley, M.J.; Parsons, N.J.; Swanepoel, L.; Pistorius, P.A. A novel candidate species of Anaplasma that infects avian erythrocytes. Parasit. Vectors 2018, 11, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.P.; Tian, J.H.; Lin, X.D.; Ni, X.B.; Chen, X.P.; Liao, Y.; Yang, S.Y.; Dumler, J.S.; Holmes, E.C.; Zhang, Y.Z. Extensive genetic diversity of Rickettsiales bacteria in multiple mosquito species. Sci. Rep. 2016, 6, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Diaz-Sanchez, S.; Hernández-Jarguín, A.; de Mera, I.G.F.; Alberdi, P.; Zweygarth, E.; Gortazar, C.; de la Fuente, J. Draft genome sequences of Anaplasma phagocytophilum, A. marginale, and A. ovis Isolates from different hosts. Genome Announc. 2018, 6, e01503–e01517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Llanes, A.; Rajeev, S. First whole genome sequence of Anaplasma platys, an obligate intracellular rickettsial pathogen of dogs. Pathogens 2020, 9, 277. [Google Scholar] [CrossRef] [PubMed]
- Chochlakis, D.; Ioannou, I.; Tselentis, Y.; Psaroulaki, A. Human anaplasmosis and Anaplasma ovis variant. Emerg. Infect. Dis. 2010, 16, 1031–1032. [Google Scholar] [CrossRef]
- Rar, V.; Golovljova, I. Anaplasma, Ehrlichia, and “Candidatus Neoehrlichia” bacteria, pathogenicity, biodiversity, and molecular genetic characteristics, a review. Infect. Genet. Evol. 2011, 11, 1842–1861. [Google Scholar] [CrossRef] [PubMed]
- Weisburg, W.G.; Barns, S.M.; Pelletier, D.A.; Lane, D.J. 16S ribosomal DNA amplification for phylogenetic study. J. Bacteriol. 1991, 173, 697–703. [Google Scholar] [CrossRef] [Green Version]
- Sumner, J.W.; Nicholson, W.L.; Massung, R.F. PCR amplification and comparison of nucleotide sequences from the groESL heat shock operon of Ehrlichia species. J. Clin. Microbiol. 1997, 35, 2087–2092. [Google Scholar] [CrossRef] [Green Version]
- Dumler, J.S.; Barbet, A.F.; Bekker, C.P.; Dasch, G.A.; Palmer, G.H.; Ray, S.C.; Rikihisa, Y.; Rurangirwa, F.R. Reorganization of genera in the families Rickettsiaceae and Anaplasmataceae in the order Rickettsiales: Unification of some species of Ehrlichia with Anaplasma, Cowdria with Ehrlichia and Ehrlichia with Neorickettsia, descriptions of six new species combinations and designation of Ehrlichia equi and ‘HGE agent’ as subjective synonyms of Ehrlichia phagocytophila. Int. J. Syst. Evol. Microbiol. 2001, 51, 2145–2165. [Google Scholar] [PubMed] [Green Version]
- Carrade, D.D.; Foley, J.E.; Borjesson, D.L.; Sykes, J.E. Canine granulocytic anaplasmosis: A review. J. Vet. Intern. Med. 2009, 23, 1129–1141. [Google Scholar] [CrossRef] [PubMed]
- Berger, S. Anaplasmosis, Global Status; Gideon Informatics. Inc.: Los Angeles, CA, USA, 2021; Available online: https://www.gideononline.com/ebooks/disease/anaplasmosis-global-status/ (assessed on 11 August 2021).
- Lima, M.L.; Soares, P.T.; Ramos, C.A.; Araújo, F.R.; Ramos, R.A.; Souza, I.I.; Faustino, M.A.; Alves, L.C. Molecular detection of Anaplasma platys in a naturally-infected cat in Brazil. Braz. J. Microbiol. 2010, 41, 381–385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kocan, K.M.; de la Fuente, J.; Guglielmone, A.A.; Melendez, R.D. Antigens and Alternatives for Control of Anaplasma marginale Infection in Cattle. Clin. Microbiol. Rev. 2003, 16, 698–712. [Google Scholar] [CrossRef] [Green Version]
- Arraga-Alvarado, C.M.; Qurollo, B.A.; Parra, O.C.; Berrueta, M.A.; Hegarty, B.C.; Breitschwerdt, E.B. Case report: Molecular evidence of Anaplasma platys infection in two women from Venezuela. Am. J. Trop. Med. Hyg. 2014, 91, 1161–1165. [Google Scholar] [CrossRef] [PubMed]
- Sainz, A.; Amusategui, I.; Tesouro, M.A. Ehrlichia platys infection and disease in dogs in Spain. J. Vet. Diagn. Investig. 1999, 11, 382–384. [Google Scholar] [CrossRef] [Green Version]
- Suksawat, J.; Xuejie, Y.; Hancock, S.I.; Hegarty, B.C.; Nilkumhang, P.; Breitschwerdt, E.B. Serologic and molecular evidence of coinfection with multiple vector-borne pathogens in dogs from Thailand. J. Vet. Intern. Med. 2001, 15, 453–462. [Google Scholar] [CrossRef]
- Sparagano, O.A.E.; Vos, A.P.d.; Paoletti, B.; Camma, C.; Santis, P.d.; Otranto, D.; Giangaspero, A. Molecular detection of Anaplasma platys in dogs using polymerase chain reaction and reverse line blot hybridization. J. Vet. Diagn. Investig. 2003, 15, 527–534. [Google Scholar] [CrossRef] [Green Version]
- Brown, R.N.; Lane, R.; Dennis, D.T. Geographic distributions of tick-borne diseases and their Vectors. Tick Borne Dis. Humans 2005, 21, 363–391. [Google Scholar]
- Aguirre, E.; Tesouro, M.A.; Ruiz, L.; Amusategui, I.; Sainz, A. Genetic characterization of Anaplasma (Ehrlichia) platys in dogs in Spain. J. Vet. Med. Ser. B. 2006, 53, 197–200. [Google Scholar] [CrossRef]
- Melo, A.L.T.; Witter, R.; Martins, T.F.; Pacheco, T.A.; Alves, A.S.; Chitarra, C.S.; Aguiar, D.M. A survey of tick-borne pathogens in dogs and their ticks in the Pantanal biome, Brazil. Med. Vet. Entomol. 2016, 30, 112–116. [Google Scholar] [CrossRef] [PubMed]
- Sudan, V.; Sharma, R.L.; Borah, M.K. Subclinical anaplasmosis in camel (Camelus dromedarius) and its successful therapeutic management. J. Parasit. Dis. 2014, 38, 163–165. [Google Scholar] [CrossRef] [Green Version]
- Lorusso, V.; Wijnveld, M.; Majekodunmi, A.O.; Dongkum, C.; Fajinmi, A.; Dogo, A.G.; Thrusfield, M.; Mugenyi, A.; Vaumourin, E.; Igweh, A.C.; et al. Tick-borne pathogens of zoonotic and veterinary importance in Nigerian cattle. Parasit. Vectors 2016, 9, 217–226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bahrami, S.; Hamidinejat, H.; Tafreshi, A.R.G. First molecular detection of Anaplasma Phagocytophilum in Dromedaries ( Camelus Dromedarius). J. Zoo Wildl. Med. 2018, 49, 844–848. [Google Scholar] [PubMed]
- Woldehiwet, Z. Anaplasma phagocytophilum in ruminants in Europe. Ann. N. Y. Acad. Sci. 2006, 1078, 446–460. [Google Scholar] [CrossRef]
- Dahlgren, F.S.; Mandel, E.J.; Krebs, J.W.; Massung, R.F.; McQuiston, J.H. Increasing incidence of Ehrlichia chaffeensis and Anaplasma phagocytophilum in the United States, 2000–2007. Am. J. Trop. Med. Hyg. 2011, 85, 124–131. [Google Scholar] [CrossRef]
- Bakken, J.S.; Dumler, J.S. Human granulocytic anaplasmosis. Infect. Dis. Clin. 2015, 29, 341–355. [Google Scholar] [CrossRef] [Green Version]
- Fishbein, D.B.; Raoult, D. A cluster of Coxiella burnetii infections associated with exposure to vaccinated goats and their unpasteurized dairy products. Am. J. Trop. Med. Hyg. 1992, 47, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Dumler, J.S.; Bakken, J.S. Human ehrlichioses: Newly recognized infections transmitted by ticks. Annu. Rev. Med. 1998, 49, 201–213. [Google Scholar] [CrossRef]
- Blanco, J.R.; Oteo, J.A. Human granulocytic ehrlichiosis in Europe. Clin. Microbiol. Infect. 2002, 8, 763–772. [Google Scholar] [CrossRef] [Green Version]
- Woldehiwet, Z. The natural history of Anaplasma phagocytophilum. Vet. Parasitol. 2010, 167, 108–122. [Google Scholar] [CrossRef]
- Do, T.; Phoosangwalthong, P.; Kamyingkird, K.; Kengradomkij, C.; Chimnoi, W.; Inpankaew, T. Molecular detection of tick-borne pathogens in stray dogs and Rhipicephalus sanguineus sensu lato ticks from Bangkok, Thailand. Pathogens 2021, 10, 561. [Google Scholar] [CrossRef]
- Piratae, S.; Senawong, P.; Chalermchat, P.; Harnarsa, W.; Sae-Chue, B. Molecular evidence of Ehrlichia canis and Anaplasma platys and responses in naturally infected dogs in Kalasin, Thailand. Vet. World. 2019, 12, 131–135. [Google Scholar] [CrossRef] [Green Version]
- Buddhachat, K.; Meerod, T.; Pradit, W.P.S.; Chomdej, S.; Nganvongpanit, K. Simultaneous differential detection of canine blood parasites: Multiplex high-resolution melting analysis (mHRM). Ticks Tick Borne Dis. 2020, 11, 101370. [Google Scholar] [CrossRef] [PubMed]
- Alhassan, A.; Hove, P.; Sharma, B.; Matthew-Belmar, V.; Karasek, I.; Lanza-Perea, M.; Werners, A.H.; Wilkerson, M.J.; Ganta, R.R. Molecular detection and characterization of Anaplasma platys and Ehrlichia canis in dogs from the Caribbean. Ticks Tick Borne Dis. 2021, 12, 101727. [Google Scholar] [CrossRef] [PubMed]
- Wilkerson, M.J.; Black, K.E.; Lanza-Perea, M.; Sharma, B.; Gibson, K.; Stone, D.M.; George, A.; Nair, A.D.; Ganta, R.R. Initial development and preliminary evaluation of a multiplex bead assay to detect antibodies to Ehrlichia canis, Anaplasma platys, and Ehrlichia chaffeensis outer membrane peptides in naturally infected dogs from Grenada, West Indies. J. Vet. Diagn. Investig. 2017, 29, 109–114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, B.; Ganta, R.; Stone, D.; Alhassan, A.; Lanza-Perea, M.; Matthew Belmar, V.; Karasek, I.; Cooksey, E.M.; Butler, C.; Gibson, K.; et al. Development of a multiplex pcr and magnetic dna capture assay for detecting six species pathogens of the genera Anaplasma and Ehrlichia in canine, bovine, caprine and ovine blood samples from Grenada, West Indies. Pathogens 2021, 10, 192. [Google Scholar] [CrossRef]
- Georges, K.; Ezeokoli, C.D.; Newaj-Fyzul, A.; Campbell, M.; Mootoo, N.; Mutani, A.; Sparagano, O.A. The application of PCR and reverse line blot hybridization to detect arthropod-borne hemopathogens of dogs and cats in Trinidad. Ann. N. Y. Acad. Sci. 2008, 1149, 196–199. [Google Scholar] [CrossRef]
- Ghauri, H.N.; Ijaz, M.; Ahmed, A.; Muhammad Naveed, M.U.A.; Nawab, Y.; Javed, M.U.; Ghaffar, A. Molecular investigation and phylogenetic analysis of anaplasmosis in dogs. J. Parasitol. 2021, 107, 295–303. [Google Scholar] [CrossRef]
- Pérez-Macchi, S.; Pedrozo, R.; Bittencourt, P.; Müller, A. Prevalence, molecular characterization and risk factor analysis of Ehrlichia canis and Anaplasma platys in domestic dogs from Paraguay. Comp. Immunol. Microbiol. Infect. Dis. 2019, 62, 31–39. [Google Scholar] [CrossRef]
- Pesapane, R.; Foley, J.; Thomas, R.; Castro, L.R. Molecular detection and characterization of Anaplasma platys and Ehrlichia canis in dogs from northern Colombia. Vet. Microbiol. 2019, 233, 184–189. [Google Scholar] [CrossRef]
- Kontos, V.I.; Papadopoulos, O.; French, T.W. Natural and experimental canine infections with a Greek strain of Ehrlichia platys. Vet. Clin. Pathol. 1991, 20, 101–105. [Google Scholar] [CrossRef]
- Faizal, M.D.; Haryanto, A.; Tjahajati, I. Diagnosis and molecular characterization of Anaplasma platys in dog patients in Yogyakarta area, Indonesia. Indones. J. Biotechnol. 2019, 24, 43–50. [Google Scholar] [CrossRef] [Green Version]
- Götsch, S.; Leschnik, M.; Duscher, G.; Burgstaller, J.P.; Wille-Piazzai, W.; Joachim, A. Ticks and haemoparasites of dogs from Praia, Cape Verde. Vet. Parasitol. 2009, 166, 171–174. [Google Scholar] [CrossRef] [PubMed]
- de Caprariis, D.; Dantas-Torres, F.; Capelli, G.; Mencke, N.; Stanneck, D.; Breitschwerdt, E.B.; Otranto, D. Evolution of clinical, haematological and biochemical findings in young dogs naturally infected by vector-borne pathogens. Vet. Microbiol. 2011, 21, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Ramos, R.A.; Latrofa, M.S.; Giannelli, A.; Lacasella, V.; Campbell, B.E.; Dantas-Torres, F.; Otranto, D. Detection of Anaplasma platys in dogs and Rhipicephalus sanguineus group ticks by a quantitative real-time PCR. Vet. Parasitol. 2014, 205, 285–288. [Google Scholar] [CrossRef]
- Dyachenko, V.; Pantchev, N.; Balzer, H.J.; Meyersen, A.; Straubinger, R.K. First case of Anaplasma platys infection in a dog from Croatia. Parasite Vector. 2012, 5, 49. [Google Scholar] [CrossRef] [Green Version]
- Barker, E.N.; Langton, D.A.; Helps, C.R.; Brown, G.; Malik, R.; Shaw, S.E.; Tasker, S. Haemoparasites of free-roaming dogs associated with several remote Aboriginal communities in Australia. BMC Vet. Res. 2012, 8, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hii, S.F.; Traub, R.J.; Thompson, M.F.; Henning, J.; O’Leary, C.A.; Burleigh, A.; Kopp, S.R. Canine tick-borne pathogens and associated risk factors in dogs presenting with and without clinical signs consistent with tick-borne diseases in northern Australia. Aust. Vet. J. 2015, 93, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Andersson, M.; Turcitu, M.A.; Stefanache, M.; Tamba, P.; Barbuceanu, F.; Chitimia, L. First evidence of Anaplasma platys and Hepatozoon canis co-infection in a dog from Romania-a case report. Ticks TickBorne Dis. 2013, 4, 317–319. [Google Scholar] [CrossRef]
- Kelly, P.J.; Lucas, H.; Eremeeva, M.E.; Dirks, K.G.; Rolain, J.M.; Yowell, C.; Thomas, R.; Douglas, T.; Dasch, G.A.; Raoult, D. Rickettsia felis, West Indies. Emerg. Infect. Dis. 2010, 16, 570–571. [Google Scholar] [CrossRef]
- Wei, L.; Kelly, P.; Ackerson, K.; Zhang, J.; El-Mahallawy, H.S.; Kaltenboeck, B.; Wang, C. First report of Babesia gibsoni in Central America and survey for vector-borne infections in dogs from Nicaragua. Parasit. Vectors 2014, 7, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Qurollo, B.A.; Chandrashekar, R.; Hegarty, B.C.; Beall, M.J.; Stillman, B.A.; Liu, J.; Thatcher, B.; Pultorak, E.; Cerrito, B.; Walsh, M.; et al. A serological survey of tick-borne pathogens in dogs in North America and the Caribbean as assessed by Anaplasma phagocytophilum, A. platys, Ehrlichia canis, E. chaffeensis, E. ewingii, and Borrelia burgdorferi species-specific peptides. Infect. Ecol. Epidemiol. 2014, 4, 24699. [Google Scholar] [CrossRef] [Green Version]
- Almazán, C.; González-Álvarez, V.H.; de Mera, I.G.F.; Cabezas-Cruz, A.; Rodríguez-Martínez, R.; de la Fuente, J. Molecular identification and characterization of Anaplasma platys and Ehrlichia canis in dogs in Mexico. Ticks TickBorne Dis. 2016, 7, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Aktas, M.; Altay, K.; Dumanli, N.; Kalkan, A. Molecular detection and identification of Ehrlichia and Anaplasma species in ixodid ticks. Parasitol. Res. 2009, 104, 1243–1248. [Google Scholar] [CrossRef] [PubMed]
- Bonilla, M.C.; Campos-Calderón, L.; Jiménez-Rocha, A.E.; Romero-Zúñiga, J.J.; Alberti, A.; Zobba, R.; Dolz, G. Characterization of Anaplasma spp. infection in dogs from Costa Rica. Vet. Parasitol. Reg. Stud. Rep. 2017, 8, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Soares, R.; Ramos, C.A.; Pedroso, T.; Babo-Terra, V.; Cleveland, H.; Araújo, F. Molecular survey of Anaplasma platys and Ehrlichia canis in dogs from Campo Grande, Mato Grosso do Sul, Brazil. Acad. Bras. Cienc. 2017, 89, 301–306. [Google Scholar] [CrossRef] [Green Version]
- da Silva, G.C.; Benitez, A.; Girotto, A.; Taroda, A.; Vidotto, M.C.; Garcia, J.L.; de Freitas, J.C.; Arlington, S.H.; Vidotto, O. Occurrence of Ehrlichia canis and Anaplasma platys in household dogs from northern Parana. Rev. Bras. Parasitol. Vet. 2012, 12, 379–385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lasta, C.S.; dos Santos, A.P.; Messick, J.B.; Oliveira, S.T.; Biondo, A.W.; Vieira, R.F.; Dalmolin, M.L.; González, F.H. Molecular detection of Ehrlichia canis and Anaplasma platys in dogs in Southern Brazil. Rev. Bras. Parasitol. Vet. 2013, 22, 360–366. [Google Scholar] [CrossRef]
- McCown, M.E.; Alleman, A.; Sayler, K.A.; Chandrashekar, R.; Thatcher, B.; Tyrrell, P.; Stillman, B.; Beall, M.; Barbet, A.F. Point prevalence survey for tick-borne pathogens in military working dogs, shelter animals, and pet populations in northern Colombia. J. Spec. Oper. Med. 2014, 14, 81–85. [Google Scholar]
- Zaid, T.; Ereqat, S.; Nasereddin, A.; Al-Jawabreh, A.; Abdelkader, A.; Abdeen, Z. Molecular characterization of AnaplasmaandEhrlichiain ixodid ticks and reservoir hosts from Palestine: A pilot survey. Vet. Med. Sci. 2019, 5, 230–242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, B.; Ye, C.; Sun, E.; Wen, Y.; Qian, D.; Sun, H. First molecular evidence of Anaplasma spp. co-infection in stray dogs from Anhui, China. Acta. Trop. 2020, 206, 105453. [Google Scholar] [CrossRef]
- Cicuttin, G.; Boeri, E.; Beltrán, F.; Gury, D.; Federico, E. Molecular detection of Neorickettsiaristicii in Brazilian free-tailed bats (Tadaridabrasiliensis) from Buenos Aires, Argentina. Pesq. Vet. Bras. 2013, 33, 648–650. [Google Scholar] [CrossRef] [Green Version]
- Springer, A.; Montenegro, V.; Schicht, S.; Wölfel, S.; Schaper, S.; Chitimia-Dobler, L.; Siebert, S.; Strube, C. Detection of Rickettsia monacensis and Rickettsia amblyommatis in ticks collected from dogs in Costa Rica and Nicaragua. Ticks Tick Borne Dis. 2018, 9, 1565–1572. [Google Scholar] [CrossRef] [PubMed]
- Hmoon, M.M.; Htun, L.L.; Thu, M.J.; Chel, H.M.; Thaw, Y.N.; Win, S.Y.; Chan Soe, N.; Khaing, Y.; Thein, S.S.; Bawm, S. Molecular prevalence and identification of Ehrlichia canis and Anaplasma platys from Dogs in Nay Pyi Taw Area, Myanmar. Vet. Med. Int. 2021, 20, 8827206. [Google Scholar] [CrossRef] [PubMed]
- Chatanga, E.; Kainga, H.; Razemba, T.; Ssuna, R.; Swennen, L.; Hayashida, K.; Sugimoto, C.; Katakura, K.; Nonaka, N.; Nakao, R. Molecular detection and characterization of tick-borne hemoparasites and Anaplasmataceae in dogs in major cities of Malawi. Parasitol. Res. 2021, 120, 267–276. [Google Scholar] [CrossRef]
- Jimenez, I.A.; Vega Mariño, P.A.; Stapleton, G.S.; Prieto, J.B.; Bowman, D.D. Canine vector-borne disease in domestic dogs on Isla Santa Cruz, Galápagos. Vet. Parasitol. Reg. Stud. Rep. 2020, 19, 100373. [Google Scholar] [CrossRef]
- Alanazi, A.; Nguyen, V.; Alyousif, M.; Manoj, R.; Alouffi, A.; Donato, R.; Sazmand, A.; Mendoza-Roldan, J.; Torres, F.; Otranto, D. Ticks and associated pathogens in camels (Camelus dromedarius) from Riyadh Province, Saudi Arabia. Parasit. Vectors 2020, 13, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diakou, A.; Di Cesare, A.; Morelli, S.; Colombo, M.; Halos, L.; Simonato, G.; Tamvakis, A.; Beugnet, F.; Paoletti, B.; Traversa, D. Endoparasites and vector-borne pathogens in dogs from Greek islands: Pathogen distribution and zoonotic implications. PLoS Negl. Trop. Dis. 2019, 13, e0007003. [Google Scholar] [CrossRef] [Green Version]
- Licari, E.; Takács, N.; Solymosi, N.; Farkas, R. First detection of tick-borne pathogens of dogs from Malta. Ticks TickBorne Dis. 2017, 8, 396–399. [Google Scholar] [CrossRef]
- Starkey, L.A.; Newton, K.; Brunker, J.; Crowdis, K.; Edourad, E.; Meneus, P.; Little, S.E. Prevalence of vector-borne pathogens in dogs from Haiti. Vet. Parasitol. 2016, 224, 7–12. [Google Scholar] [CrossRef]
- Huggins, L.G.; Colella, V.; Koehler, A.V.; Schunack, B.; Traub, R.J. A multipronged next-generation sequencing metabarcoding approach unearths hyperdiverse and abundant dog pathogen communities in Cambodia. Transbound. Emerg. Dis. 2021, 10. [Google Scholar] [CrossRef]
- Proboste, T.; Kalema-Zikusoka, G.; Altet, L.; Solano-Gallego, L.; Fernández de Mera, I.G.; Chirife, A.D.; Muro, J.; Bach, E.; Piazza, A.; Cevidanes, A.; et al. Infection and exposure to vector-borne pathogens in rural dogs and their ticks, Uganda. Parasit. Vectors 2015, 8, 306. [Google Scholar] [CrossRef] [Green Version]
- Hamel, D.; Shukullari, E.; Rapti, D.; Silaghi, C.; Pfister, K.; Rehbein, S. Parasites and vector-borne pathogens in client-owned dogs in Albania. Blood pathogens and seroprevalences of parasitic and other infectious agents. Parasitol. Res. 2016, 115, 489–499. [Google Scholar] [CrossRef]
- Kamani, J.; Morick, D.; Mumcuoglu, K.; Harrus, S. Prevalence and diversity of Bartonella species in commensal rodents and ectoparasites from Nigeria, West Africa. PLoS Neglect. Trop. Dis. 2013, 7, e2246. [Google Scholar] [CrossRef]
- Alho, A.M.; Lima, C.; Latrofa, M.S.; Colella, V.; Ravagnan, S.; Capelli, G.; Madeira de Carvalho, L.; Cardoso, L.; Otranto, D. Molecular detection of vector-borne pathogens in dogs and cats from Qatar. Parasit. Vectors 2017, 10, 298. [Google Scholar] [CrossRef] [Green Version]
- Modarelli, J.J.; Tomeček, J.M.; Piccione, J.; Ferro, P.J.; Esteve-Gasent, M.D. Molecular prevalence and ecoregion distribution of select tick-borne pathogens in Texas dogs. Transbound. Emerg. Dis. 2019, 66, 1291–1300. [Google Scholar] [CrossRef] [PubMed]
- Manoj, R.; Iatta, R.; Latrofa, M.S.; Capozzi, L.; Raman, M.; Colella, V.; Otranto, D. Canine vector-borne pathogens from dogs and ticks from Tamil Nadu, India. Acta. Trop. 2020, 203, 105308. [Google Scholar] [CrossRef]
- Motoi, Y.; Satoh, H.; Inokuma, H.; Kiyuuna, T.; Muramatsu, Y.; Ueno, H.; Morita, C. First detection of Ehrlichia platys in dogs and ticks in Okinawa, Japan. Microbiol. Immunol. 2001, 45, 89–91. [Google Scholar] [CrossRef] [PubMed]
- Baldridge, G.D.; Scoles, G.; Burkhardt, N.Y.; Schloeder, B.; Kurtti, T.J.; Munderloh, U.G. Transovarial transmission of Francisella-like endosymbionts and Anaplasma phagocytophilum variants in Dermacentor albipictus (Acari: Ixodidae). J. Med. Entomol. 2009, 46, 625–632. [Google Scholar] [CrossRef] [Green Version]
- Dugat, T.; Lagrée, A.C.; Maillard, R.; Boulouis, H.J.; Haddad, N. Opening the black box of Anaplasma phagocytophilum diversity: Current situation and future perspectives. Front. Cell. Infect. Microbiol. 2015, 5, 61. [Google Scholar] [CrossRef]
- Fine, A.B.; Sweeney, J.D.; Nixon, C.P.; Knoll, B.M. Transfusion-transmitted anaplasmosis from a leukoreduced platelet pool. Transfusion 2016, 56, 699–704. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.S.; Khalaf, J.M. First identification of Anaplasma platys and Anaplasma phagocytophlium in the blood of dogs in Baghdad Governorate. Plant Arch. 2020, 20, 393–397. [Google Scholar]
- Yousefi, A.M.R.C.; Golmohammadi, A.; Azami, S. Molecular detection of Anaplasma Phagocytophilum as a zoonotic agent in owned and stray dogs in Tehran, Iran. Arch. Razi. Inst. 2019, 74, 33–38. [Google Scholar]
- Rojero-Vázquez, E.; Gordillo-Pérez, G.; Weber, M. Infection of Anaplasma phagocytophilum and Ehrlichia spp. in opossums and dogs in Campeche, Mexico: The role of tick infestation. Front. Ecol. Evol. 2017, 5, 161–163. [Google Scholar] [CrossRef] [Green Version]
- Henn, J.B.; Gabriel, M.W.; Kasten, R.W.; Brown, R.N.; Theis, J.H.; Foley, J.E.; Chomel, B.B. Gray foxes (Urocyon cinereoargenteus) as a potential reservoir of a Bartonella clarridgeiae-like bacterium and domestic dogs as part of a sentinel system for surveillance of zoonotic arthropod-borne pathogens in northern California. J. Clin. Microbiol. 2007, 45, 2411–2418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santos, H.A.; Pires, M.S.; Vilela, J.A.; Santos, T.M.; Faccini, J.L.; Baldani, C.D.; Thomé, S.M.; Sanavria, A.; Massard, C.L. Detection of Anaplasma phagocytophilum in Brazilian dogs by real-time polymerase chain reaction. J. Vet. Diagn. Investing. 2011, 23, 770–774. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elfving, K.; Malmsten, J.; Dalin, A.M.; Nilsson, K. Serologic and Molecular Prevalence of Rickettsia helvetica and Anaplasma phagocytophilum in Wild Cervids and Domestic Mammals in the Central Parts of Sweden. Vector Borne Zoonotic Dis. 2015, 15, 529–533. [Google Scholar] [CrossRef] [PubMed]
- Vargas-Hernandez, G.; André, M.R.; Cendales, D.M.; de Sousa, K.C.M.; Gonçalves, L.R.; Rondelli, M.C.H.; Machado, R.Z.; Tinucci-Costa, M. Molecular detection of Anaplasma species in dogs in Colombia. Rev. Bras. Parasitol. Vet. 2016, 25, 459–464. [Google Scholar] [CrossRef] [Green Version]
- Çetinkaya, H.; Matur, E.; Akyazi, İ.; Ekiz, E.E.; Aydin, L.; Toparlak, M. Serological and molecular investigation of Ehrlichia spp. and Anaplasma spp. in ticks and blood of dogs, in the Thrace Region of Turkey. Ticks Tick Borne Dis. 2016, 7, 706–714. [Google Scholar] [CrossRef] [PubMed]
- Rikihisa, Y. The tribe Ehrlichieae and ehrlichial diseases. Clin. Microbiol. Rev. 1991, 4, 286–308. [Google Scholar] [CrossRef]
- Latrofa, M.S.; Dantas-Torres, F.; deCaprariis, D.; Cantacessi, C.; Capelli, G.; Lia, R.P.; Breitschwerdt, E.B.; Otranto, D. Vertical transmission of Anaplasma platys and Leishmania infantum in dogs during the first half of gestation. Parasit. Vector. 2016, 9, 269–275. [Google Scholar] [CrossRef] [Green Version]
- Matei, I.A.; D’Amico, G.; Yao, P.K.; Ionică, A.M.; Kanyari, P.W.N.; Daskalaki, A.A.; Dumitrache, M.O.; Sándor, A.D.; Gherman, C.M.; Qablan, M.; et al. Molecular detection of Anaplasma platys infection in free-roaming dogs and ticks from Kenya and Ivory Coast. Parasit. Vectors 2016, 9, 157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stuen, S.; Okstad, W.; Sagen, A.M. Intrauterine transmission of Anaplasma phagocytophilum in persistently infected lambs. Vet. Sci. 2018, 5, 25–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Villar, M.; López, V.; Ayllón, N.; Cabezas-Cruz, A.; López, J.A.; Vázquez, J.; Alberdi, P.; de la Fuente, J. The intracellular bacterium Anaplasma phagocytophilum selectively manipulates the levels of vertebrate host proteins in the tick vector Ixodes scapularis. Parasit. Vectors 2016, 9, 1–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Snellgrove, A.N.; Krapiunaya, I.; Ford, S.L.; Stanley, H.M.; Wickson, A.G.; Hartzer, K.L.; Levin, M.L. Vector competence of Rhipicephalus sanguineus sensu stricto for Anaplasma platys. Ticks Tick Borne Dis. 2020, 11, 101517. [Google Scholar] [CrossRef]
- De Tommasi, A.S.; Baneth, G.; Breitschwerdt, E.B.; Stanneck, D.; Dantas-Torres, F.; Otranto, D.; de Caprariis, D. Anaplasmaplatys in bone marrow megakaryocytes of young dogs. J. Clin. Microbial. 2014, 52, 2231–2234. [Google Scholar] [CrossRef] [Green Version]
- Rikihisa, Y. Mechanisms of obligatory intracellular infection with Anaplasma phagocytophilum. Clin. Microbiol. Rev. 2011, 24, 469–489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bradfield, J.F.; Vore, S.J.; Pryor, W.H.J. Ehrlichia platys infection in dogs. Lab. Anim. Sci. 1996, 46, 565–568. [Google Scholar]
- Bouzouraa, T.; René-Martellet, M.; Chêne, J.; Attipa, C.; Lebert, I.; Chalvet-Monfray, K.; Cadoré, J.L.; Halos, L.; Chabanne, L. Clinical and laboratory features of canine Anaplasma platys infection in 32 naturally infected dogs in the Mediterranean basin. Ticks Tick Borne Dis. 2016, 7, 1256–1264. [Google Scholar] [CrossRef]
- Kohn, B.; Galke, D.; Beelitz, P.; Pfister, K. Clinical features of canine granulocytic anaplasmosis in 18 naturally infected dogs. J. Vet. Intern. Med. 2008, 22, 1289–1295. [Google Scholar] [CrossRef]
- Nair, A.D.; Cheng, C.; Ganta, C.K.; Sanderson, M.W.; Alleman, A.R.; Munderloh, U.G.; Ganta, R.R. Comparative experimental infection study in dogs with Ehrlichia canis, E. chaffeensis, Anaplasma platys and A. phagocytophilum. PLoS ONE 2016, 11, e0148239. [Google Scholar]
- Bjöersdorff, A.; Svendenius, L.; Owens, J.H.; Massung, R.F. Feline granulocytic ehrlichiosis—a report of a new clinical entity and characterisation of the infectious agent. J. Small Anim. Pract. 1999, 40, 20–24. [Google Scholar] [CrossRef] [PubMed]
- Savidge, C.; Ewing, P.; Andrews, J.; Aucoin, D.; Lappin, M.R.; Moroff, S. Anaplasma phagocytophilum infection of domestic cats: 16 cases from the northeastern USA. J. Feline Med. Surg. 2016, 18, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Sim, R.R.; Joyner, P.H.; Padilla, L.R.; Anikis, P.; Aitken-Palmer, C. Clinical disease associated with Anaplasma phagocytophilum infection in captive Przewalski’s horses (Equus ferus przewalskii). J. Zoo Wildl. Med. 2017, 48, 497–505. [Google Scholar] [CrossRef]
- Ismail, N.; Bloch, K.C.; McBride, J.W. Human ehrlichiosis and anaplasmosis. Clin. Lab. Med. 2010, 30, 261–292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Khoury, L.; Furie, R. Inflammatory arthritis: A unique presentation of human anaplasmosis. Clin. Rheumatol. 2019, 38, 257–259. [Google Scholar] [CrossRef]
- Palmer, G.H.; Abbott, J.R.; French, D.M.; McElwain, T.F. Persistence of Anaplasma ovis infection and conservation of the msp-2 and msp-3 multigene families within the genus Anaplasma. Infect. Immun. 1998, 66, 6035–6039. [Google Scholar] [CrossRef] [Green Version]
- Kocan, K.M.; de la Fuente, J.; Blouin, E.F.; Coetzee, J.F.; Ewing, S.A. The natural history of Anaplasma marginale. Vet. Parasitol. 2010, 167, 95–107. [Google Scholar] [CrossRef]
- Eddlestone, S.M.; Gaunt, S.D.; Neer, T.M.; Boudreaux, C.M.; Gill, A.; Haschke, E.; Corstvet, R.E. PCR detection of Anaplasma platys in blood and tissue of dogs during acute phase of experimental infection. Exp. Parasitol. 2007, 115, 205–210. [Google Scholar] [CrossRef]
- OIE. Bovine Anaplasmosis. In Manual of Diagnostic Tests and Vaccines for Terestrial Animals; OIE: Paris, France, 2015; Chapter 2.4.1. [Google Scholar]
- Choi, S.; Cho, Y.U.; Kim, S.H. Morulae in neutrophils: A diagnostic clue for human granulocytic anaplasmosis. IDcases 2019, 15, e00506. [Google Scholar] [CrossRef]
- Tokarz, R.; Tagliafierro, T.; Cucura, D.M.; Rochlin, I.; Sameroff, S.; Lipkin, W.I. Detection of Anaplasma phagocytophilum, Babesia microti, Borrelia burgdorferi, Borrelia miyamotoi and Powassan virus in ticks by a multiplex real-time reverse transcription-PCR assay. mSphere 2017, 2, e00151-17. [Google Scholar] [CrossRef] [Green Version]
- Silaghi, C.; Santos, A.S.; Gomes, J.; Christova, I.; Matei, I.A.; Walder, G.; Domingos, A.; Bell-Sakyi, L.; Sprong, H.; von Loewenich, F.D.; et al. Guidelines for the direct detection of Anaplasma spp. in diagnosis and epidemiological studies. Vector Borne Zoonotic Dis. 2017, 17, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Hebels, D.G.; van Herwijnen, M.H.; Brauers, K.J.; de Kok, T.M.; Chalkiadaki, G.; Kyrtopoulos, S.A.; Kleinjans, J.C. Elimination of heparin interference during microarray processing of fresh and biobank-archived blood samples. Environ. Mol. Mutagen. 2014, 55, 482–491. [Google Scholar] [CrossRef]
- Sanchez-Fito, M.T.; Oltra, E. Optimized treatment of heparinized blood fractions to make them suitable for analysis. Biopreserv. Biobank. 2015, 13, 287–295. [Google Scholar] [CrossRef]
- Massung, R.F.; Slater, K.; Owens, J.H.; Nicholson, W.L.; Mather, T.N.; Solberg, V.B.; Olson, J.G. Nested PCR assay for detection of granulocytic ehrlichiae. J. Clin. Microbiol. 1998, 36, 1090–1095. [Google Scholar] [CrossRef] [Green Version]
- Gaunt, S.D.; Beall, M.J.; Stillman, B.A.; Lorentzen, L.; Diniz, P.P.V.P.; Chandrashekar, R.; Breitschwerdt, E.B. Experimental infection and co-infection of dogs with Anaplasma platys and Ehrlichia canis: Hematologic, serologic and molecular findings. Parasit. Vector. 2010, 3, 33–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szekeres, S.; Coipan, C.E.; Rigó, K.; Majoros, G.; Jahfari, S.; Sprong, H.; Földvári, G. Candidatus Neoehrlichia mikurensis and Anaplasma phagocytophilum in natural rodent and tick communities in Southern Hungary. Ticks TickBorne Dis. 2015, 6, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Njiru, Z.K. Loop-mediated isothermal amplification technology: Towards point of care diagnostics. PLoS Negl. Trop. Dis. 2012, 10, e0004348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, C.; Lin, Y.; Tsang, C.; Chung, Y. A loop-mediated isothermal amplification (LAMP) assay for rapid detection of Anaplasma phagocytophilum infection in dogs. Turk. J. Vet. Anim. Sci. 2012, 36, 205–210. [Google Scholar]
- Li, H.T.; Sun, L.S.; Chen, Z.M.; Hu, J.S.; Ye, C.D.; Jia, K.; Wang, H.; Yuan, L.G.; Zhang, G.H.; Li, S. Detection of Anaplasma platys in dogs using real-time loop-mediated isothermal amplification. Vet. J. 2014, 199, 468–470. [Google Scholar] [CrossRef]
- Nelson, C.M.; Herron, M.J.; Felsheim, R.F.; Schloeder, B.R.; Grindle, S.M.; Chavez, A.O.; Kurtti, T.J.; Munderloh, U.G. Whole genome transcription profiling of Anaplasma phagocytophilum in human and tick host cells by tiling array analysis. BMC Genomics 2008, 9, 364. [Google Scholar] [CrossRef] [Green Version]
- Munderloh, U.G.; Lynch, M.J.; Herron, M.J.; Palmer, A.T.; Kurtti, T.J.; Nelson, R.D.; Goodman, J.L. Infection of endothelial cells with Anaplasma marginale and A. phagocytophilum. Vet. Microbiol. 2004, 101, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Shimada, M.; Takamoto, N.; Su, H.; Sasahara, H.; Shimamura, Y.; Ando, S.; Ohashi, N. Predomination shift of different P44-expressing Anaplasma phagocytophilum in infected HL-60, THP-1, NB4, and RF/6A cell lines. Jpn. J. Infect. Dis. 2018, 72, 73–80. [Google Scholar] [CrossRef]
- Massung, R.F.; Levin, M.L.; Munderloh, U.G.; Silverman, D.J.; Lynch, M.J.; Gaywee, J.K.; Kurtti, T.J. Isolation and propagation of the Ap-Variant 1 strain of Anaplasma phagocytophilum in a tick cell line. J. Clin. Microbiol. 2007, 45, 2138–2143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bell-Sakyi, L.; Darby, A.; Baylis, M.; Makepeace, B.L. The Tick Cell Biobank: A global resource for in vitro research on ticks, other arthropods and the pathogens they transmit. Ticks TickBorne Dis. 2018, 9, 1364–1371. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, C.; Vale, F.L.; Vieira, M.S.; Perinotto, W.M.D.S.; Auad, A.M.; Dolisnki, C.; Furlong, J.; Bittencourt, V.R.E.P.; Cristina de Azevedo Prata, M. Efficacy of Heterorhabditis baujardi (Rhabditida: Heterorhabditidae) against Rhipicephalus microplus (Acari: Ixodidae) in presence of susceptible and alternate insect hosts. Biocontrol Sci. Technol. 2020, 30, 1316–1329. [Google Scholar] [CrossRef]
- Samish, M.; Ginsberg, H.; Glazer, I. Biological control of ticks. J. Parasitol. 2004, 129, S389–S403. [Google Scholar] [CrossRef]
- Ben Said, M.; Galai, Y.; Canales, M.; Nijhof, A.M.; Mhadhbi, M.; Jedidi, M.; de la Fuente, J.; Darghouth, M.A. Hd86, the Bm86 tick protein ortholog in Hyalomma scupense (syn. H. detritum): Expression in Pichia pastoris and analysis of nucleotides and amino acids sequences variations prior to vaccination trials. Vet. Parasitol. 2012, 183, 215–223. [Google Scholar] [PubMed] [Green Version]
- Galai, Y.; Canales, M.; Saïd, M.B.; Gharbi, M.; Mhadhbi, M.; Jedidi, M.; de la Fuente, J.; Darghouth, M.A. Efficacy of Hyalomma scupense (Hd86) antigen against Hyalomma excavatum and H. scupense tick infestations in cattle. Vaccine 2012, 30, 7084–7089. [Google Scholar] [CrossRef] [Green Version]
- Bhowmick, B.; Han, Q. Understanding tick biology and its implications in anti-tick and transmission blocking vaccines against tick-borne pathogens. Front. Vet. Sci. 2020, 7, 319–322. [Google Scholar] [CrossRef] [PubMed]
- Contreras, M.; Villar, M.; De La Fuente, J. A vaccinomics approach for the identification of tick protective antigens for the control of Ixodes ricinus and Dermacentor reticulatus infestations in companion animals. Front. Physiol. 2019, 10, 977. [Google Scholar] [CrossRef] [Green Version]
- De La Fuente, J.; Blouin, E.F.; Manzano-Roman, R.; Naranjo, V.; Almazán, C.; De La Lastra, J.M.P.; Zivkovic, Z.; Massung, R.F.; Jongejan, F.; Kocan, K.M. Differential expression of the tick protective antigen subolesin in Anaplasma marginale and A. phagocytophilum infected host cells. Ann. N. Y. Acad. Sci. 2008, 1149, 27–35. [Google Scholar] [CrossRef]
- de la Fuente, J.; Ayoubi, P.; Blouin, E.F.; Almazán, C.; Naranjo, V.; Kocan, K.M. Anaplasmosis: Focusing on host-vector-pathogen interactions for vaccine development. Ann. N. Y. Acad. Sci. 2006, 1078, 416–423. [Google Scholar] [CrossRef]
- Ojogun, N.; Kahlon, A.; Ragland, S.A.; Troese, M.J.; Mastronunzio, J.E.; Walker, N.J.; VieBrock, L.; Thomas, R.J.; Borjesson, D.L.; Fikrig, E.; et al. Anaplasma phagocytophilum outer membrane protein A interacts with sialylated glycoproteins to promote infection of mammalian host cells. Infect. Immun. 2012, 80, 3748–3760. [Google Scholar] [CrossRef] [Green Version]
- Kahlon, A.; Ojogun, N.; Ragland, S.A.; Seidman, D.; Troese, M.J.; Ottens, A.K.; Fikring, E.; Carlyon, J.A. Anaplasma phagocytophilum Asp14 is an invasin that interacts with mammalian host cells via its C terminus to facilitate infection. Infect. Immun. 2013, 81, 65–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seidman, D.; Ojogun, N.; Walker, N.J.; Mastronunzio, J.; Kahlon, A.; Hebert, K.S.; Karandashova, S.; Miller, D.P.; Tegels, B.K.; Marconi, R.T.; et al. Anaplasma phagocytophilum surface protein AipA mediates invasion of mammalian host cells. Cell. Microbiol. 2014, 16, 1133–1145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, M.; Xu, W.; Zhang, L.; Liu, Z.; Zhu, J.; Li, Y.; Wu, S.; Niu, H. Identification of novel immunoreactive proteins and delineation of a specific epitope of Anaplasma phagocytophilum. Microb. Pathog. 2018, 125, 183–188. [Google Scholar] [CrossRef]
- Fukui, Y.; Ohkawa, S.; Inokuma, H. First molecular detection and phylogenetic analysis of Anaplasma phagocytophilum from a clinical case of canine granulocytic anaplasmosis in Japan. Jpn. J. Infect. Dis. 2018, 71, 302–305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dantas-Torres, F.; Otranto, D. Anaplasmosis. In Arthropod Borne Disease Switzerland; Marcondes, C.B., Ed.; Springer: Berlin/Heidelberg, Germany, 2017; pp. 215–222. [Google Scholar]
- Yancey, C.B.; Diniz, P.P.V.P.; Breitschwerdt, E.B.; Hegarty, B.C.; Wiesen, C.; Qurollo, B.A. Doxycycline treatment efficacy in dogs with naturally occurring Anaplasma phagocytophilum infection. J. Small Anim. Pract. 2018, 59, 286–293. [Google Scholar] [CrossRef]
- Hansmann, Y.; Jaulhac, B.; Kieffer, P.; Martinot, M.; Wurtz, E.; Dukic, R.; Argemi, X.; De Martino, S. Value of PCR, serology, and blood smears for human granulocytic anaplasmosis diagnosis, France. Emerg. Infect. Dis. 2019, 25, 996. [Google Scholar] [CrossRef] [PubMed]


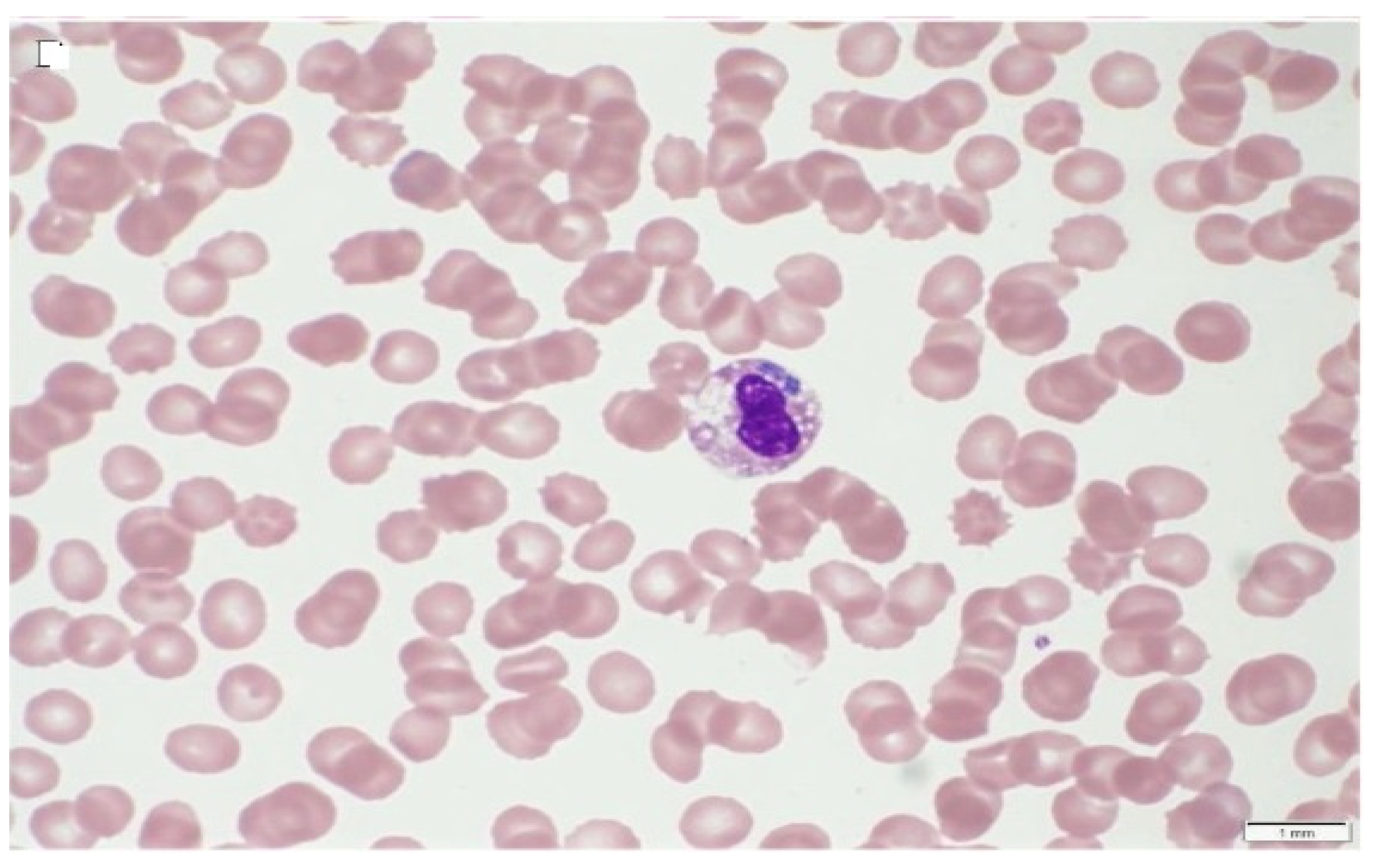
| Anaplasma Species | Infecting Cells | Vertebrate Hosts | Potential Vectors | References |
|---|---|---|---|---|
| A. platys | Platelets | Dogs and camels | Rhipicephalus | [22,23] |
| A. phagocytophilum | Granulocytes | Domestic and wild ruminants, horses, dogs, cats, rabbits, rodents, insectivores, wild swine, and humans | Ixodes, Dermacentor, Hyalomma, Rhipicephalus | [13] |
| A. marginale | Erythrocytes | Domestic ruminants | Rhipicephalus, Ixodes, Dermacentor | [24] |
| A. centrale | Erythrocytes | Domestic and wild ruminants | Rhipicephalus, Ixodes, Haemaphysalis | [1] |
| A. ovis | Erythrocytes | Domestic and wild ruminants and humans | Rhipicephalus, Dermacentor, Hyalomma | [1] |
| A. bovis | Monocytes | Domestic and wild ruminants and small mammals | Haemaphysalis, Rhipicephalus, Amblyomma | [25] |
| A. capra | Erythrocytes | Domestic and wild ruminants and humans | Haemaphysalis | [26,27] |
| A. odocoilei | Platelets | Wild ruminants | Not known | [28] |
| Candidatus A. camelii | Not known | Camels | Not known | [29] |
| Candidatus A. boleense | Not known | Not known | Hyalomma | [30] |
| Candidatus A. corsicanum | Not known | Domestic ruminants | Not known | [31] |
| Candidatus A. mediterraneum | Not known | Domestic ruminants | Not known | [31] |
| Candidatus A. sphenisci | Not known | African penguins | Not known | [32] |
| Candidatus A. rodmosense | Not known | Rodents | Not known | [33] |
| Domestic Canid | Countries (Regions) | Prevalences (%) | Methods(Target Genes) | References |
|---|---|---|---|---|
| Dog | Iraq | 55.6 | Blood smear | [113] |
| Iran | 2.0 | PCR a (msp4) | [114] | |
| Mexico | 27 | PCRa (16S rRNA) | [115] | |
| USA (California) | 7.6 | RT-PCRb (msp2) | [116] | |
| Brazil | 7.1 | RT-PCRb (msp2) | [117] | |
| USA (South) | 2.1 | ELISAc | [83] | |
| USA (Mid-Atlantic) | 5.4 | ELISAc | [83] | |
| USA (Northeast) | 13 | ELISAc | [83] | |
| USA (Midwest) | 1.9 | ELISAc | [83] | |
| USA (West) | 2.0 | ELISAc | [83] | |
| Canada | 1.1 | ELISAc | [83] | |
| Caribbean | 3.4 | ELISAc | [83] | |
| Sweden | 17.0 | IFATd | [118] | |
| Colombia | 1.1 | PCRa (16S rRNA) | [119] | |
| Costa Rica | 0.3 | PCRa (16SrRNA, groEL) | [86] | |
| India | 0.4 | PCRa (16S/18S rRNA) | [108] | |
| Turkey | 4.0 | nPCRe (16S rRNA) | [120] |
| Disease | Clinical Findings | Diagnosis | Treatment | Control |
|---|---|---|---|---|
| Canine cyclic thrombocytopenia | Dogs usually remain asymptomatic; however, fever, lethargy, anorexia, weight loss, anemia, icterus, petechiae, nasal discharge, lymphadenopathy, and lymphadenomegaly may beobserved [5] | Stained blood smear, thrombocytopenia, serology, and PCR/DNA sequencing [5] | Doxycycline @5–10 mg kg−1 q12–24 h for 8–10 days orenrofloxacin @ 5mg kg−1, q12 h for 14–21 days [5] | Tick elimination, collar, pour-on or spot-on acaricidal products for R. sanguineus sensu lato ticks, knowledge of tick seasonality, andecology [5] |
| Canine granulocytic anaplasmosis | Non-specific signs, fever, anemia, anorexia, dullness, and thrombocytopenia [5] | Morulae in stained blood smear, thrombocytopenia, leucopenia, elevated liver enzymes, serology, andPCR/DNA sequencing [1,5] | Doxycycline 5mg/kg bid for 28 days [172] | Vector control, habitat modification, rearing tick-resistant breeds, and chemotherapy [5] |
| Human granulocyticanaplasmosis | Fever, headache, myalgias, and chills [123] | Morulae in stained blood smear, thrombocytopenia, leucopenia, elevated liver enzymes, serology/IFA, and PCR/DNA sequencing [95,173] | Doxycycline @ 100mg, orally, twice dailyfor 10–14 days or rifampicin @ 20 mgkg−1 day−1 orallyfor children, otherwise 300 mg orally, twice dailyfor 5–7 days [47] | Humans: Minimizing high-risk tick exposure activities (hiking, gardening, etc.), blood transfusion, immune suppression, identificationof reservoirs and vectors, and their control [1] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Atif, F.A.; Mehnaz, S.; Qamar, M.F.; Roheen, T.; Sajid, M.S.; Ehtisham-ul-Haque, S.; Kashif, M.; Ben Said, M. Epidemiology, Diagnosis, and Control of Canine Infectious Cyclic Thrombocytopenia and Granulocytic Anaplasmosis: Emerging Diseases of Veterinary and Public Health Significance. Vet. Sci. 2021, 8, 312. https://doi.org/10.3390/vetsci8120312
Atif FA, Mehnaz S, Qamar MF, Roheen T, Sajid MS, Ehtisham-ul-Haque S, Kashif M, Ben Said M. Epidemiology, Diagnosis, and Control of Canine Infectious Cyclic Thrombocytopenia and Granulocytic Anaplasmosis: Emerging Diseases of Veterinary and Public Health Significance. Veterinary Sciences. 2021; 8(12):312. https://doi.org/10.3390/vetsci8120312
Chicago/Turabian StyleAtif, Farhan Ahmad, Saba Mehnaz, Muhammad Fiaz Qamar, Taleeha Roheen, Muhammad Sohail Sajid, Syed Ehtisham-ul-Haque, Muhammad Kashif, and Mourad Ben Said. 2021. "Epidemiology, Diagnosis, and Control of Canine Infectious Cyclic Thrombocytopenia and Granulocytic Anaplasmosis: Emerging Diseases of Veterinary and Public Health Significance" Veterinary Sciences 8, no. 12: 312. https://doi.org/10.3390/vetsci8120312
APA StyleAtif, F. A., Mehnaz, S., Qamar, M. F., Roheen, T., Sajid, M. S., Ehtisham-ul-Haque, S., Kashif, M., & Ben Said, M. (2021). Epidemiology, Diagnosis, and Control of Canine Infectious Cyclic Thrombocytopenia and Granulocytic Anaplasmosis: Emerging Diseases of Veterinary and Public Health Significance. Veterinary Sciences, 8(12), 312. https://doi.org/10.3390/vetsci8120312








