Left Pulmonary Artery Coarctation Associated with Pneumonia and Pulmonary Hypertension in a Cat
Abstract
:1. Introduction
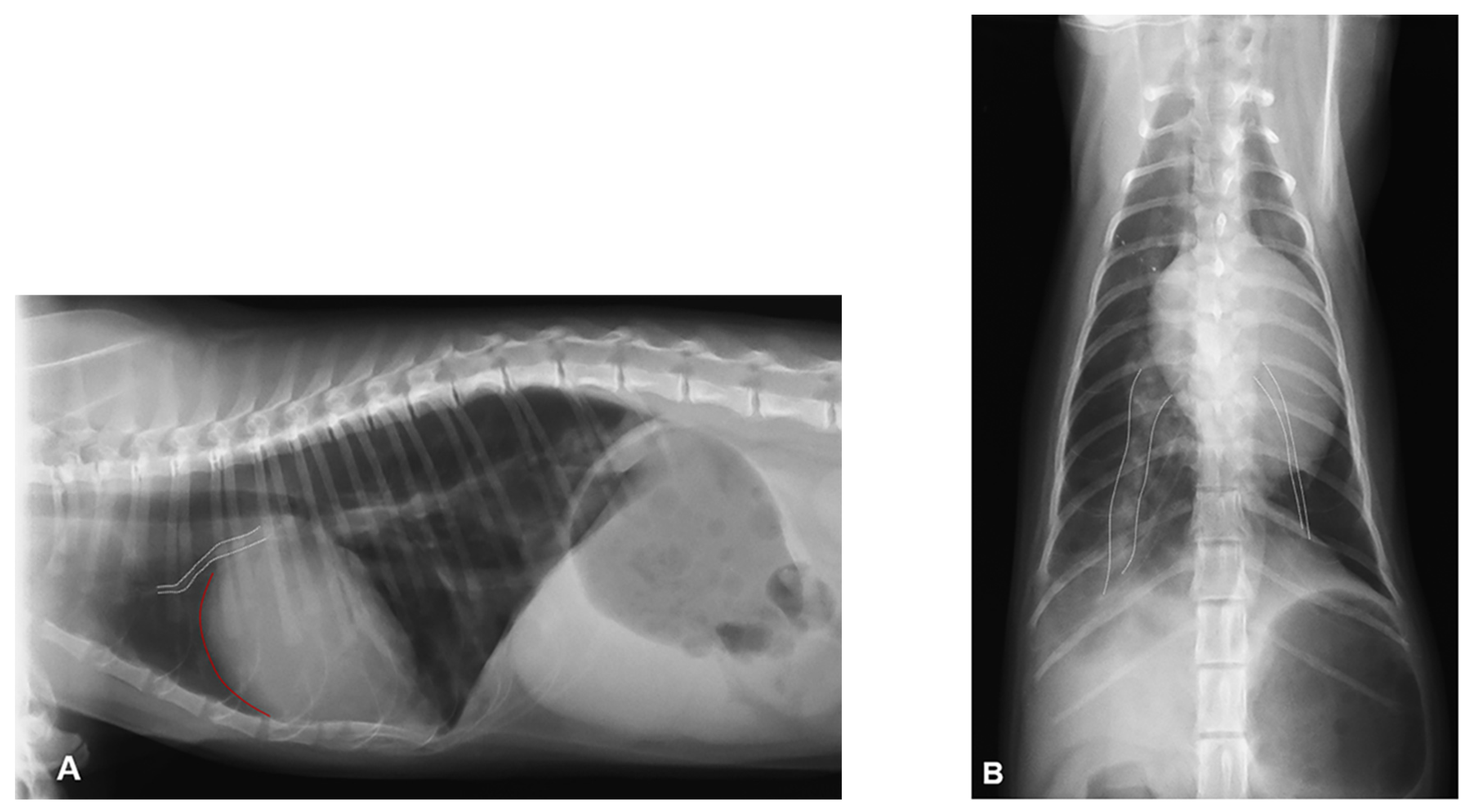
2. Case Description
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gay, B.B.J.; French, R.H.; Shuford, W.H.; Rogers, J.V.J. The Roentgenologic features of single and multiple coarctations of the pulmonary artery and branches. Am. J. Roentgenol. Radium. Ther. Nucl. Med. 1963, 90, 599–613. [Google Scholar]
- Scansen, B.A.; Schneider, M.; Bonagura, J.D. Sequential segmental classification of feline congenital heart disease. J. Vet. Cardiol. 2015, 17 (Suppl. 1), 10–52. [Google Scholar] [CrossRef] [PubMed]
- Schrope, D.P.; Kelch, W.J. Clinical and echocardiographic findings of pulmonary artery stenosis in seven cats. J. Vet. Cardiol. 2007, 9, 83–89. [Google Scholar] [CrossRef]
- Szatmári, V.; Freund, M.W.; Veldhuis Kroeze, E.J.B.; Strengers, J. Juxtaductal coarctation of both pulmonary arteries in a cat. J. Vet. Diagn Invest. 2010, 22, 812–816. [Google Scholar] [CrossRef] [Green Version]
- Galiè, N.; Humbert, M.; Vachiery, J.-L.; Gibbs, S.; Lang, I.; Torbicki, A.; Simonneau, G.; Peacock, A.; Vonk Noordegraaf, A.; Beghetti, M.; et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS). Eur. Heart J. 2016, 37, 67–119. [Google Scholar] [CrossRef]
- Reinero, C.; Visser, L.C.; Kellihan, H.B.; Masseau, I.; Rozanski, E.; Clercx, C.; Williams, K.; Abbott, J.; Borgarelli, M.; Scansen, B.A. ACVIM consensus statement guidelines for the diagnosis, classification, treatment, and monitoring of pulmonary hypertension in dogs. J. Vet. Intern. Med. 2020, 34, 549–573. [Google Scholar] [CrossRef]
- Vezzosi, T.; Schober, K.E. Doppler-derived echocardiographic evidence of pulmonary hypertension in cats with left-sided congestive heart failure. J. Vet. Cardiol. 2019, 23, 58–68. [Google Scholar] [CrossRef]
- Block, C.L.; Glassman, M.M. Pulmonary artery banding in a kitten with a partial atrioventricular septal defect. J Vet Cardiol. 2019, 24, 20–27. [Google Scholar] [CrossRef]
- Russell, D.S.; Scansen, B.A.; Himmel, L. Plexogenic pulmonary arteriopathy in a cat with non-restrictive ventricular septal defect and chronic pulmonary hypertension. J. Small Anim. Pract. 2015, 56, 524–529. [Google Scholar] [CrossRef] [PubMed]
- Novo-Matos, J.; Hurter, K.; Bektas, R.; Grest, P.; Glaus, T. Patent ductus arteriosus in an adult cat with pulmonary hypertension and right-sided congestive heart failure: Hemodynamic evaluation and clinical outcome following ductal closure. J. Vet. Cardiol. 2014, 16, 197–203. [Google Scholar] [CrossRef] [PubMed]
- MacPhail, C.M.; Innocenti, C.M.; Kudnig, S.T.; Veir, J.K.; Lappin, M.R. Atypical manifestations of feline inflammatory polyps in three cats. J. Feline Med. Surg. 2007, 9, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Evola, M.G.; Edmondson, E.F.; Reichle, J.K.; Biller, D.S.; Mitchell, C.W.; Valdés-Martínez, A. Radiographic and histopathologic characteristics of pulmonary fibrosis in nine cats. Vet. Radiol. Ultrasound. 2014, 55, 133–140. [Google Scholar] [CrossRef]
- Baron Toaldo, M.; Guglielmini, C.; Diana, A.; Giunti, M.; Dondi, F.; Cipone, M. Reversible pulmonary hypertension in a cat. J. Small Anim. Pract. 2011, 52, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Vezzosi, T.; Perrucci, S.; Parisi, F.; Morelli, S.; Maestrini, M.; Mennuni, G.; Traversa, D.; Poli, A. Fatal Pulmonary Hypertension and Right-Sided Congestive Heart Failure in a Kitten Infected with Aelurostrongylus abstrusus. Animals 2020, 10, 2263. [Google Scholar] [CrossRef] [PubMed]
- Oldach, M.S.; Gunther-Harrington, C.T.; Balsa, I.M.; McLarty, E.M.; Wakeman, K.A.; Phillips, K.L.; Honkavaara, J.; Visser, L.C.; Stern, J.A. Aberrant migration and surgical removal of a heartworm (Dirofilaria immitis) from the femoral artery of a cat. J. Vet. Intern. Med. 2018, 32, 792–796. [Google Scholar] [CrossRef] [PubMed]
- Jaffey, J.A.; Williams, K.J.; Masseau, I.; Krueger, M.; Reinero, C. Vasoproliferative process resembling pulmonary capillary hemangiomatosis in a cat. BMC Vet. Res. 2017, 13, 72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Søndergaard, T. Coarctation of the pulmonary artery. Dan Med. Bull. 1954, 1, 46–48. [Google Scholar] [PubMed]
- Elzenga, N.J.; von Suylen, R.J.; Frohn-Mulder, I.; Essed, C.E.; Bos, E.; Quaegebeur, J.M. Juxtaductal pulmonary artery coarctation. An underestimated cause of branch pulmonary artery stenosis in patients with pulmonary atresia or stenosis and a ventricular septal defect. J. Thorac. Cardiovasc. Surg. 1990, 100, 416–424. [Google Scholar] [CrossRef]
- Gittenberger-de Groot, A.C.; Peterson, J.C.; Wisse, L.J.; Roest, A.A.W.; Poelmann, R.E.; Bökenkamp, R.; Elzenga, N.J.; Hazekamp, M.; Bartelings, M.M.; Jongbloed, M.R.M.; et al. Pulmonary ductal coarctation and left pulmonary artery interruption; pathology and role of neural crest and second heart field during development. PLoS ONE 2020, 15, e0228478. [Google Scholar] [CrossRef] [PubMed]
- Brink, J.; MacIver, R.; Lee, M.G.; Konstantinov, I.E.; Cheung, M.; Brizard, C.P.; d’Udekem, Y. Neonatal pulmonary artery reconstruction during shunting to treat and prevent juxtaductal coarctation. Ann. Thorac. Surg. 2015, 99, 641–647. [Google Scholar] [CrossRef] [PubMed]
- McElhinney, D.B.; Reddy, V.M.; Moore, P.; Hanley, F.L. Bilateral branch pulmonary artery obstruction due to kinking at insertion sites of bilateral ductus arteriosus. Ann. Thorac. Surg. 1997, 64, 537–539. [Google Scholar] [CrossRef]
- Franch, R.H.; Gay, B.B.J. Congenital stenosis of the pulmonary artery branches. A classification, with postmortem findings in two cases. Am. J. Med. 1963, 35, 512–529. [Google Scholar] [CrossRef]
- Vezzosi, T.; Domenech, O.; Costa, G.; Marchesotti, F.; Venco, L.; Zini, E.; Del Palacio, M.J.F. Echocardiographic evaluation of the right ventricular dimension and systolic function in dogs with pulmonary hypertension. J. Vet. Intern. Med. 2018, 32, 1541–1548. [Google Scholar] [CrossRef]
- Kreutzer, J.; Landzberg, M.J.; Preminger, T.J.; Mandell, V.S.; Treves, S.T.; Reid, L.M.; Lock, J.E. Isolated peripheral pulmonary artery stenoses in the adult. Circulation 1996, 93, 1417–1423. [Google Scholar] [CrossRef] [PubMed]
- Singampalli, K.L.; Jui, E.; Shani, K.; Ning, Y.; Connell, J.P.; Birla, R.K.; Bollyky, P.L.; Caldarone, C.A.; Keswani, S.G.; Grande-Allen, K.J. Congenital Heart Disease: An Immunological Perspective. Front. Cardiovasc. Med. 2021, 8, 701375. [Google Scholar] [CrossRef] [PubMed]
- Jordan, T.J.; Scansen, B.A.; Kent, A.M.; Hitchcock, L.S.; Russell, D.S. Isolated unilateral absence of the right pulmonary artery in two cats visualized by computed tomography angiography. JFMS Open Rep. 2016, 2, 2055116916671480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Healy, F.; Hanna, B.D.; Zinman, R. Pulmonary complications of congenital heart disease. Paediatr. Respir. Rev. 2012, 13, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Adatia, I.; Kothari, S.S.; Feinstein, J.A. Pulmonary hypertension associated with congenital heart disease: Pulmonary vascular disease: The global perspective. Chest 2010, 137 (Suppl. 6), 52–61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reinero, C. Interstitial lung diseases in dogs and cats part I: The idiopathic interstitial pneumonias. Vet. J. 2019, 243, 48–54. [Google Scholar] [CrossRef]
- Cadoré, J.L.; Steiner-Laurent, S.; Greenland, T.; Mornex, J.F.; Loire, R. Interstitial lung disease in feline immunodeficiency virus (FIV) infected cats. Res. Vet. Sci. 1997, 62, 287–288. [Google Scholar] [CrossRef]
- Bacha, E.A.; Kreutzer, J. Comprehensive management of branch pulmonary artery stenosis. J. Interv. Cardiol. 2001, 14, 367–375. [Google Scholar] [CrossRef] [PubMed]
- McDevitt, H.; Stauthammer, C.; Leeder, D.; Hanson, M.; Olson, J.; Gruenstein, D. Palliative balloon angioplasty in a cat with right pulmonary arterial branch stenoses and concurrent absence of the left pulmonary artery. J. Vet. Cardiol. 2013, 15, 211–216. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valente, C.; Tursi, M.; Poser, H.; Guglielmini, C. Left Pulmonary Artery Coarctation Associated with Pneumonia and Pulmonary Hypertension in a Cat. Vet. Sci. 2021, 8, 325. https://doi.org/10.3390/vetsci8120325
Valente C, Tursi M, Poser H, Guglielmini C. Left Pulmonary Artery Coarctation Associated with Pneumonia and Pulmonary Hypertension in a Cat. Veterinary Sciences. 2021; 8(12):325. https://doi.org/10.3390/vetsci8120325
Chicago/Turabian StyleValente, Carlotta, Massimiliano Tursi, Helen Poser, and Carlo Guglielmini. 2021. "Left Pulmonary Artery Coarctation Associated with Pneumonia and Pulmonary Hypertension in a Cat" Veterinary Sciences 8, no. 12: 325. https://doi.org/10.3390/vetsci8120325
APA StyleValente, C., Tursi, M., Poser, H., & Guglielmini, C. (2021). Left Pulmonary Artery Coarctation Associated with Pneumonia and Pulmonary Hypertension in a Cat. Veterinary Sciences, 8(12), 325. https://doi.org/10.3390/vetsci8120325






