Isolation and Characterization of Feline Wharton’s Jelly-Derived Mesenchymal Stem Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Cell Isolation
2.3. Reverse Transcriptase Polymerase Chain Reaction
2.4. Cumulative Population Doubling Level Analysis
2.5. Scratch Test, Colony Forming Unit Test
2.6. Karyotype Analysis
2.7. Flow Cytometry
2.8. Osteogenic Differentiation
2.9. Adipogenic Differentiation
2.10. Chondrogenic Differentiation
2.11. Quantitative Real-Time Polymerase Chain Reaction
2.12. Statistical Analysis
3. Results
3.1. Isolation and Culture of fWJ-MSCs
3.2. Scratch Test, Colony Forming Unit (CFU) Test
3.3. Expression Pattern of Stem Cell Markers
3.4. Analysis of Karyotype
3.5. Flow Cytometry
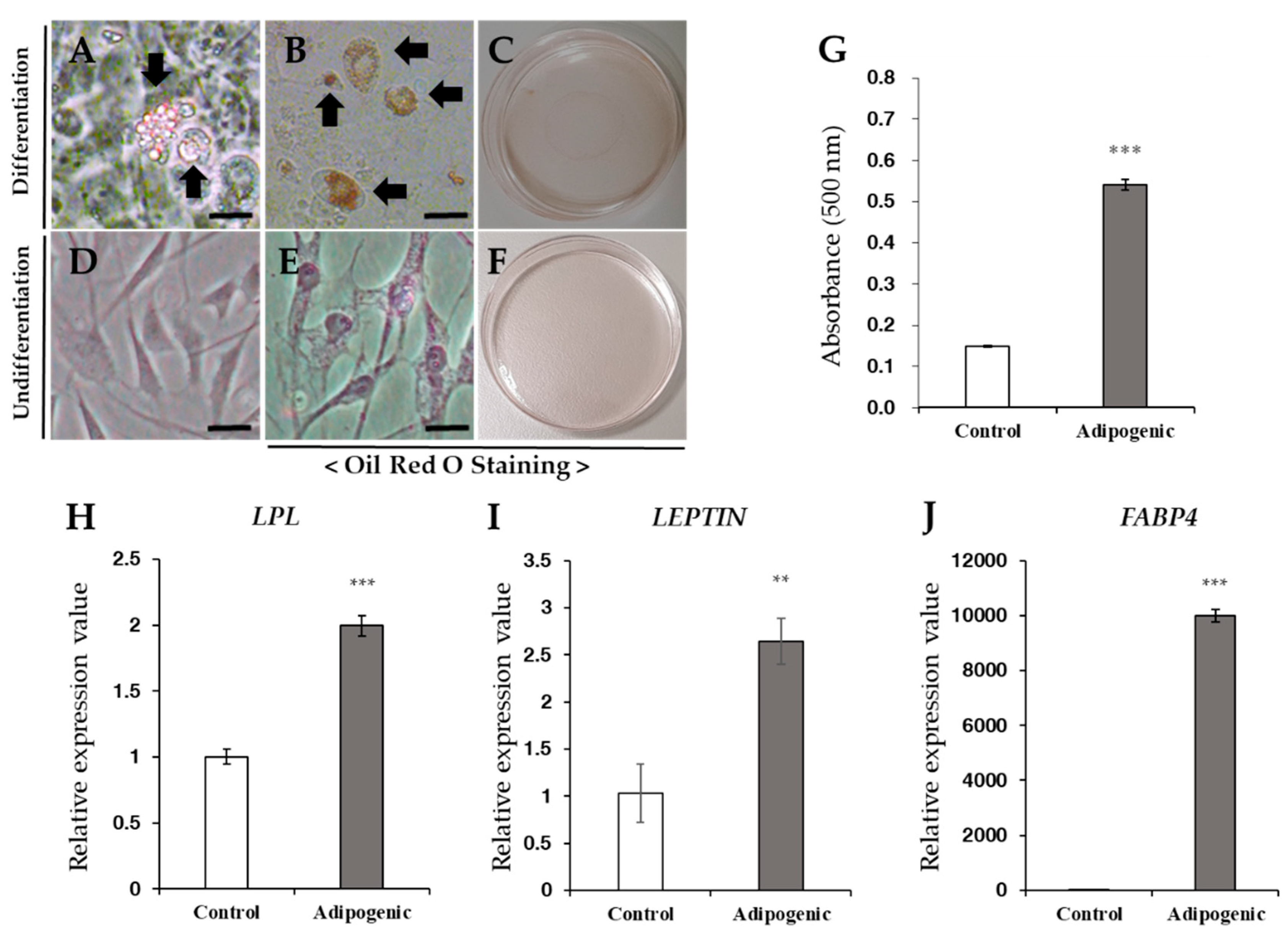
3.6. Induction of Differentiation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Diaz-Prado, S.; Muinos-Lopez, E.; Hermida-Gomez, T.; Rendal-Vazquez, M.E.; Fuentes-Boquete, I.; de Toro, F.J.; Blanco, F.J. Isolation and characterization of mesenchymal stem cells from human amniotic membrane. Tissue Eng. Part C Methods 2011, 17, 49–59. [Google Scholar] [CrossRef] [Green Version]
- La Rocca, G.; Anzalone, R.; Corrao, S.; Magno, F.; Loria, T.; Lo Iacono, M.; Di Stefano, A.; Giannuzzi, P.; Marasà, L.; Cappello, F.; et al. Isolation and characterization of Oct-4+/HLA-G+ mesenchymal stem cells from human umbilical cord matrix: Differentiation potential and detection of new markers. Histochem. Cell Biol. 2009, 131, 267–282. [Google Scholar] [CrossRef] [PubMed]
- Ullah, I.; Subbarao, R.B.; Rho, G.J. Human mesenchymal stem cells—Current trends and future prospective. Biosci. Rep. 2015, 35, e00191. [Google Scholar] [CrossRef]
- Huang, H.I.; Chen, S.K.; Ling, Q.D.; Chien, C.C.; Liu, H.T.; Chan, S.H. Multilineage differentiation potential of fibroblast-like stromal cells derived from human skin. Tissue Eng. Part A 2010, 16, 1491–1501. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.G.; Shinojima, N.; Hossain, A.; Gumin, J.; Yong, R.L.; Colman, H.; Marini, F.; Andreeff, M.; Lang, F.F. Isolation and perivascular localization of mesenchymal stem cells from mouse brain. Neurosurgery 2010, 67, 711–720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rada, T.; Reis, R.L.; Gomes, M.E. Distinct stem cells subpopulations isolated from human adipose tissue exhibit different chondrogenic and osteogenic differentiation potential. Stem Cell Rev. 2011, 7, 64–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tran, T.C.; Kimura, K.; Nagano, M.; Yamashita, T.; Ohneda, K.; Sugimori, H.; Sato, F.; Sakakibara, Y.; Hamada, H.; Yoshikawa, H.; et al. Identification of human placenta-derived mesenchymal stem cells involved in re-endothelialization. J. Cell Physiol. 2011, 226, 224–235. [Google Scholar] [CrossRef]
- Wei, Y.; Li, Y.; Chen, C.; Stoelzel, K.; Kaufmann, A.M.; Albers, A.E. Human skeletal muscle-derived stem cells retain stem cell properties after expansion in myosphere culture. Exp. Cell Res. 2011, 317, 1016–1027. [Google Scholar] [CrossRef]
- Simoes, I.N.; Boura, J.S.; dos Santos, F.; Andrade, P.Z.; Cardoso, C.M.; Gimble, J.M.; Da Da Silva, C.L.; Cabral, J.M.S. Human mesenchymal stem cells from the umbilical cord matrix: Successful isolation and ex vivo expansion using serum-/xeno-free culture media. Biotechnol. J. 2013, 8, 448–458. [Google Scholar] [CrossRef]
- Filioli Uranio, M.; Valentini, L.; Lange-Consiglio, A.; Caira, M.; Guaricci, A.C.; L’Abbate, A.; Catacchio, C.R.; Ventura, M.; Cremonesi, F.; Dell’Aquila, M.E. Isolation, proliferation, cytogenetic, and molecular characterization and in vitro differentiation potency of canine stem cells from foetal adnexa: A comparative study of amniotic fluid, amnion, and umbilical cord matrix. Mol. Reprod. Dev. 2011, 78, 361–373. [Google Scholar] [CrossRef]
- Martin, D.R.; Cox, N.R.; Hathcock, T.L.; Niemeyer, G.P.; Baker, H.J. Isolation and characterization of multipotential mesenchymal stem cells from feline bone marrow. Exp. Hematol. 2002, 30, 879–886. [Google Scholar] [CrossRef]
- Corradetti, B.; Lange-Consiglio, A.; Barucca, M.; Cremonesi, F.; Bizzaro, D. Size-sieved subpopulations of mesenchymal stem cells from intervascular and perivascular equine umbilical cord matrix. Cell Prolif. 2011, 44, 330–342. [Google Scholar] [CrossRef]
- Lange-Consiglio, A.; Corradetti, B.; Bertani, S.; Notarstefano, V.; Perrini, C.; Marini, M.G.; Arrighi, S.; Bosi, G.; Belloli, A.; Pravettoni, D.; et al. Peculiarity of Porcine Amniotic Membrane and Its Derived Cells: A Contribution to the Study of Cell Therapy from a Large Animal Model. Cell Reprogram. 2015, 17, 472–483. [Google Scholar] [CrossRef] [PubMed]
- Corradetti, B.; Meucci, A.; Bizzaro, D.; Cremonesi, F.; Lange Consiglio, A. Mesenchymal stem cells from amnion and amniotic fluid in the bovine. Reproduction 2013, 145, 391–400. [Google Scholar] [CrossRef]
- Meirelles Lda, S.; Nardi, N.B. Murine marrow-derived mesenchymal stem cell: Isolation, in vitro expansion, and characterization. Br. J. Haematol. 2003, 123, 702–711. [Google Scholar] [CrossRef] [PubMed]
- Arzi, B.; Clark, K.C.; Sundaram, A.; Spriet, M.; Verstraete, F.J.M.; Walker, N.J.; Loscar, M.R.; Fazel, N.; Murphy, W.J.; Vapniarsky, N.; et al. Therapeutic Efficacy of Fresh, Allogeneic Mesenchymal Stem Cells for Severe Refractory Feline Chronic Gingivostomatitis. Stem Cells Transl. Med. 2017, 6, 1710–1722. [Google Scholar] [CrossRef] [PubMed]
- Quimby, J.M.; Webb, T.L.; Randall, E.; Marolf, A.; Valdes-Martinez, A.; Dow, S.W. Assessment of intravenous adipose-derived allogeneic mesenchymal stem cells for the treatment of feline chronic kidney disease: A randomized, placebo-controlled clinical trial in eight cats. J. Feline Med. Surg. 2016, 18, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Webb, T.L.; Webb, C.B. Stem cell therapy in cats with chronic enteropathy: A proof-of-concept study. J. Feline Med. Surg. 2015, 17, 901–908. [Google Scholar] [CrossRef]
- Trzil, J.E.; Masseau, I.; Webb, T.L.; Chang, C.H.; Dodam, J.R.; Liu, H.; Quimby, J.M.; Dow, S.W.; Reinero, C.R. Intravenous adipose-derived mesenchymal stem cell therapy for the treatment of feline asthma: A pilot study. J. Feline Med. Surg. 2016, 18, 981–990. [Google Scholar] [CrossRef]
- Webb, T.L.; Quimby, J.M.; Dow, S.W. In vitro comparison of feline bone marrow-derived and adipose tissue-derived mesenchymal stem cells. J. Feline Med. Surg. 2012, 14, 165–168. [Google Scholar] [CrossRef]
- Iacono, E.; Cunto, M.; Zambelli, D.; Ricci, F.; Tazzari, P.L.; Merlo, B. Could fetal fluid and membranes be an alternative source for mesenchymal stem cells (MSCs) in the feline species? A preliminary study. Vet. Res. Commun. 2012, 36, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.W.; Staples, M.; Shinozuka, K.; Pantcheva, P.; Kang, S.D.; Borlongan, C.V. Wharton’s jelly-derived mesenchymal stem cells: Phenotypic characterization and optimizing their therapeutic potential for clinical applications. Int. J. Mol. Sci. 2013, 14, 11692–11712. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seshareddy, K.; Troyer, D.; Weiss, M.L. Method to isolate mesenchymal-like cells from Wharton’s Jelly of umbilical cord. Methods Cell Biol. 2008, 86, 101–119. [Google Scholar]
- Troyer, D.L.; Weiss, M.L. Wharton’s jelly-derived cells are a primitive stromal cell population. Stem Cells 2008, 26, 591–599. [Google Scholar] [CrossRef] [Green Version]
- Seo, M.S.; Park, S.B.; Kang, K.S. Isolation and characterization of canine Wharton’s jelly-derived mesenchymal stem cells. Cell Transpl. 2012, 21, 1493–1502. [Google Scholar] [CrossRef] [Green Version]
- Kono, S.; Kazama, T.; Kano, K.; Harada, K.; Uechi, M.; Matsumoto, T. Phenotypic and functional properties of feline dedifferentiated fat cells and adipose-derived stem cells. Vet. J. 2014, 199, 88–96. [Google Scholar] [CrossRef] [Green Version]
- Zhang, N.; Dietrich, M.A.; Lopez, M.J. Therapeutic doses of multipotent stromal cells from minimal adipose tissue. Stem Cell Rev. 2014, 10, 600–611. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.R.; Lee, J.; Byeon, J.S.; Gu, N.Y.; Lee, J.; Cho, I.S.; Cha, S.-H. Extensive characterization of feline intra-abdominal adipose-derived mesenchymal stem cells. J. Vet. Sci. 2017, 18, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Vidane, A.S.; Souza, A.F.; Sampaio, R.V.; Bressan, F.F.; Pieri, N.C.; Martins, D.S.; Meirelles, F.V.; Miglino, M.A. Cat amniotic membrane multipotent cells are nontumorigenic and are safe for use in cell transplantation. Stem Cells Cloning 2014, 7, 71–78. [Google Scholar] [PubMed] [Green Version]
- Lin, S.Z.; Chang, Y.J.; Liu, J.W.; Chang, L.F.; Sun, L.Y.; Li, Y.S.; Luo, G.H.; Liao, C.H.; Chen, P.H.; Chen, T.M.; et al. Transplantation of human Wharton’s Jelly-derived stem cells alleviates chemically induced liver fibrosis in rats. Cell Transpl. 2010, 19, 1451–1463. [Google Scholar] [CrossRef] [Green Version]
- Carlin, R.; Davis, D.; Weiss, M.; Schultz, B.; Troyer, D. Expression of early transcription factors Oct-4, Sox-2 and Nanog by porcine umbilical cord (PUC) matrix cells. Reprod. Biol. Endocrinol. 2006, 4, 8. [Google Scholar] [CrossRef] [Green Version]
- Cardoso, T.C.; Ferrari, H.F.; Garcia, A.F.; Novais, J.B.; Silva-Frade, C.; Ferrarezi, M.C.; Andrade, A.L.; Gameiro, R. Isolation and characterization of Wharton’s jelly-derived multipotent mesenchymal stromal cells obtained from bovine umbilical cord and maintained in a defined serum-free three-dimensional system. BMC Biotechnol. 2012, 12, 18. [Google Scholar] [CrossRef] [Green Version]
- Iacono, E.; Brunori, L.; Pirrone, A.; Pagliaro, P.P.; Ricci, F.; Tazzari, P.L.; Merlo, B. Isolation, characterization and differentiation of mesenchymal stem cells from amniotic fluid, umbilical cord blood and Wharton’s jelly in the horse. Reproduction 2012, 143, 455–468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bai, C.; Li, X.; Hou, L.; Zhang, M.; Guan, W.; Ma, Y. Biological characterization of chicken mesenchymal stem/progenitor cells from umbilical cord Wharton’s jelly. Mol. Cell Biochem. 2013, 376, 95–102. [Google Scholar] [CrossRef]
- Li, W.W.; Wei, Y.H.; Li, H.; Lai, D.M.; Lin, T.N. Isolation and characterization of a novel strain of mesenchymal stem cells from mouse umbilical cord: Potential application in cell-based therapy. PLoS ONE 2013, 8, e74478. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Bai, J.; Ji, X.; Li, R.; Xuan, Y.; Wang, Y. Comprehensive characterization of four different populations of human mesenchymal stem cells as regards their immune properties, proliferation and differentiation. Int. J. Mol. Med. 2014, 34, 695–704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shaer, A.; Azarpira, N.; Aghdaie, M.H.; Esfandiari, E. Isolation and characterization of Human Mesenchymal Stromal Cells Derived from Placental Decidua Basalis; Umbilical cord Wharton’s Jelly and Amniotic Membrane. Pak. J. Med. Sci. 2014, 30, 1022–1026. [Google Scholar] [PubMed]
- Chen, M.Y.; Lie, P.C.; Li, Z.L.; Wei, X. Endothelial differentiation of Wharton’s jelly-derived mesenchymal stem cells in comparison with bone marrow-derived mesenchymal stem cells. Exp. Hematol. 2009, 37, 629–640. [Google Scholar] [CrossRef]
- Wu, L.F.; Wang, N.N.; Liu, Y.S.; Wei, X. Differentiation of Wharton’s jelly primitive stromal cells into insulin-producing cells in comparison with bone marrow mesenchymal stem cells. Tissue Eng. Part A 2009, 15, 2865–2873. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.L.; Liu, Y.J.; Yang, S.G.; Zhao, Q.J.; Wang, X.; Gong, W.; Han, Z.B.; Xu, Z.S.; Lu, Y.X.; Liu, D.; et al. Isolation and characterization of human umbilical cord mesenchymal stem cells with hematopoiesis-supportive function and other potentials. Haematologica 2006, 91, 1017–1026. [Google Scholar] [PubMed]
- Fong, C.Y.; Richards, M.; Manasi, N.; Biswas, A.; Bongso, A. Comparative growth behaviour and characterization of stem cells from human Wharton’s jelly. Reprod. Biomed. Online 2007, 15, 708–718. [Google Scholar] [CrossRef]
- Nekanti, U.; Mohanty, L.; Venugopal, P.; Balasubramanian, S.; Totey, S.; Ta, M. Optimization and scale-up of Wharton’s jelly-derived mesenchymal stem cells for clinical applications. Stem Cell Res. 2010, 5, 244–254. [Google Scholar] [CrossRef] [Green Version]
- Friedman, R.; Betancur, M.; Boissel, L.; Tuncer, H.; Cetrulo, C.; Klingemann, H. Umbilical cord mesenchymal stem cells: Adjuvants for human cell transplantation. Biol. Blood Marrow. Transpl. 2007, 13, 1477–1486. [Google Scholar] [CrossRef] [Green Version]
- Le Blanc, K. Immunomodulatory effects of fetal and adult mesenchymal stem cells. Cytotherapy 2003, 5, 485–489. [Google Scholar] [CrossRef]
- Van Pham, P.; Truong, N.C.; Le, P.T.; Tran, T.D.; Vu, N.B.; Bui, K.H.; Phan, N.K. Isolation and proliferation of umbilical cord tissue derived mesenchymal stem cells for clinical applications. Cell Tissue Bank. 2016, 17, 289–302. [Google Scholar] [CrossRef]
- Sato, K.; Yamawaki-Ogata, A.; Kanemoto, I.; Usui, A.; Narita, Y. Isolation and characterisation of peripheral blood-derived feline mesenchymal stem cells. Vet. J. 2016, 216, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Arzi, B.; Kol, A.; Murphy, B.; Walker, N.J.; Wood, J.A.; Clark, K.; Verstraete, F.J.M.; Borjesson, D.L. Feline foamy virus adversely affects feline mesenchymal stem cell culture and expansion: Implications for animal model development. Stem Cells Dev. 2015, 24, 814–823. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Spaas, J.H.; De Schauwer, C.; Cornillie, P.; Meyer, E.; Van Soom, A.; Van de Walle, G.R. Culture and characterisation of equine peripheral blood mesenchymal stromal cells. Vet. J. 2013, 195, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Taechangam, N.; Iyer, S.S.; Walker, N.J.; Arzi, B.; Borjesson, D.L. Mechanisms utilized by feline adipose-derived mesenchymal stem cells to inhibit T lymphocyte proliferation. Stem Cell Res. Ther. 2019, 10, 188. [Google Scholar] [CrossRef]
- An, J.H.; Song, W.J.; Li, Q.; Kim, S.M.; Yang, J.I.; Ryu, M.O.; Ryung, N.A.; Dong Ha, B.; Jung, Y.C.; Youn, H.Y. Prostaglandin E2 secreted from feline adipose tissue-derived mesenchymal stem cells alleviate DSS-induced colitis by increasing regulatory T cells in mice. BMC Vet. Res. 2018, 14, 354. [Google Scholar] [CrossRef] [PubMed]




| Genes | Forward Primer (5′-3′) | Reverse Primer (5′-3′) | Product Size |
|---|---|---|---|
| OCT4 | GCCCGAAAGAGAAAGCGAAC | CGACGATTGCAGAACCACAC | 161 bp |
| SOX2 | GCCCTGCAGTACAACTCCAT | TGGAGTGGGAGGAAGAGGTA | 175 bp |
| KLF4 | ACCAAGAGCTCATGCCACCT | AAGGCTTCTCACCTGTGTGG | 183 bp |
| MYC | AGGAGAAACGAGCTGAAACG | GTTCTCGTCGCTTCCTCAAC | 181 bp |
| GAPDH | GTGGAGGGACTCATGACCAC | GTGAGCTTCCCATTCAGCTC | 176 bp |
| Marker | Antibody | Company/Catalog number |
|---|---|---|
| CD105 | MOUSE ANTI HUMAN CD105 | BIO-RAD/MCA1557 |
| CD90 | PE Mouse Anti-Human CD90 | BD Pharmingen/555596 |
| CD45 | FITC Mouse Anti-Human CD45 | BD Pharmingen/555482 |
| CD44 | PE anti-mouse/human CD44 Antibody | BioLegend/103024 |
| CD34 | FITC Mouse Anti-Human CD34 | BD Pharmingen/555821 |
| CD14 | MOUSE ANTI HUMAN CD14 | BIO-RAD/MCA1568 |
| Genes | Forward Primer (5′-3′) | Reverse Primer (5′-3′) | Product Size |
|---|---|---|---|
| MSX2 | GCCTCCAAGACACATGAGC | CCTGGGTCTCTGTGAGGTTC | 185 bp |
| COL1A1 | GAGCGGAGAATACTGGATCG | ATGCTCTCGCCATACCAGAC | 180 bp |
| LPL | TGGCGGAGGAATTTCACTAT | AGGAGAAAGGCGACTTGGAG | 176 bp |
| LEPTIN | AGCAGCTTGGCTGACAATTT | CCAGCAATCACTCCTGGTCT | 178 bp |
| FABP4 | CATCAGTGTGAATGGGGATG | CCACTTCTGCACCTGTACCA | 169 bp |
| COL2A1 | CCCTAGAGGTCCTCCTGGTC | CAAAGGCAGACATGTCGATG | 188 bp |
| GAPDH | GTGGAGGGACTCATGACCAC | GTGAGCTTCCCATTCAGCTC | 176 bp |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seo, M.-S.; Kang, K.-K.; Oh, S.-K.; Sung, S.-E.; Kim, K.-S.; Kwon, Y.-S.; Yun, S. Isolation and Characterization of Feline Wharton’s Jelly-Derived Mesenchymal Stem Cells. Vet. Sci. 2021, 8, 24. https://doi.org/10.3390/vetsci8020024
Seo M-S, Kang K-K, Oh S-K, Sung S-E, Kim K-S, Kwon Y-S, Yun S. Isolation and Characterization of Feline Wharton’s Jelly-Derived Mesenchymal Stem Cells. Veterinary Sciences. 2021; 8(2):24. https://doi.org/10.3390/vetsci8020024
Chicago/Turabian StyleSeo, Min-Soo, Kyung-Ku Kang, Se-Kyung Oh, Soo-Eun Sung, Kil-Soo Kim, Young-Sam Kwon, and Sungho Yun. 2021. "Isolation and Characterization of Feline Wharton’s Jelly-Derived Mesenchymal Stem Cells" Veterinary Sciences 8, no. 2: 24. https://doi.org/10.3390/vetsci8020024
APA StyleSeo, M.-S., Kang, K.-K., Oh, S.-K., Sung, S.-E., Kim, K.-S., Kwon, Y.-S., & Yun, S. (2021). Isolation and Characterization of Feline Wharton’s Jelly-Derived Mesenchymal Stem Cells. Veterinary Sciences, 8(2), 24. https://doi.org/10.3390/vetsci8020024








