A Retrospective Multicentric Study of Electrochemotherapy in the Treatment of Feline Nasal Planum Squamous Cell Carcinoma
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Selection
2.2. Staging
2.3. Electroporators, Electrical Parameters and Anesthesia Protocol
2.4. Treatment (ECT) Protocol
2.5. Follow-Up and Treatment Outcome
2.6. Statistics
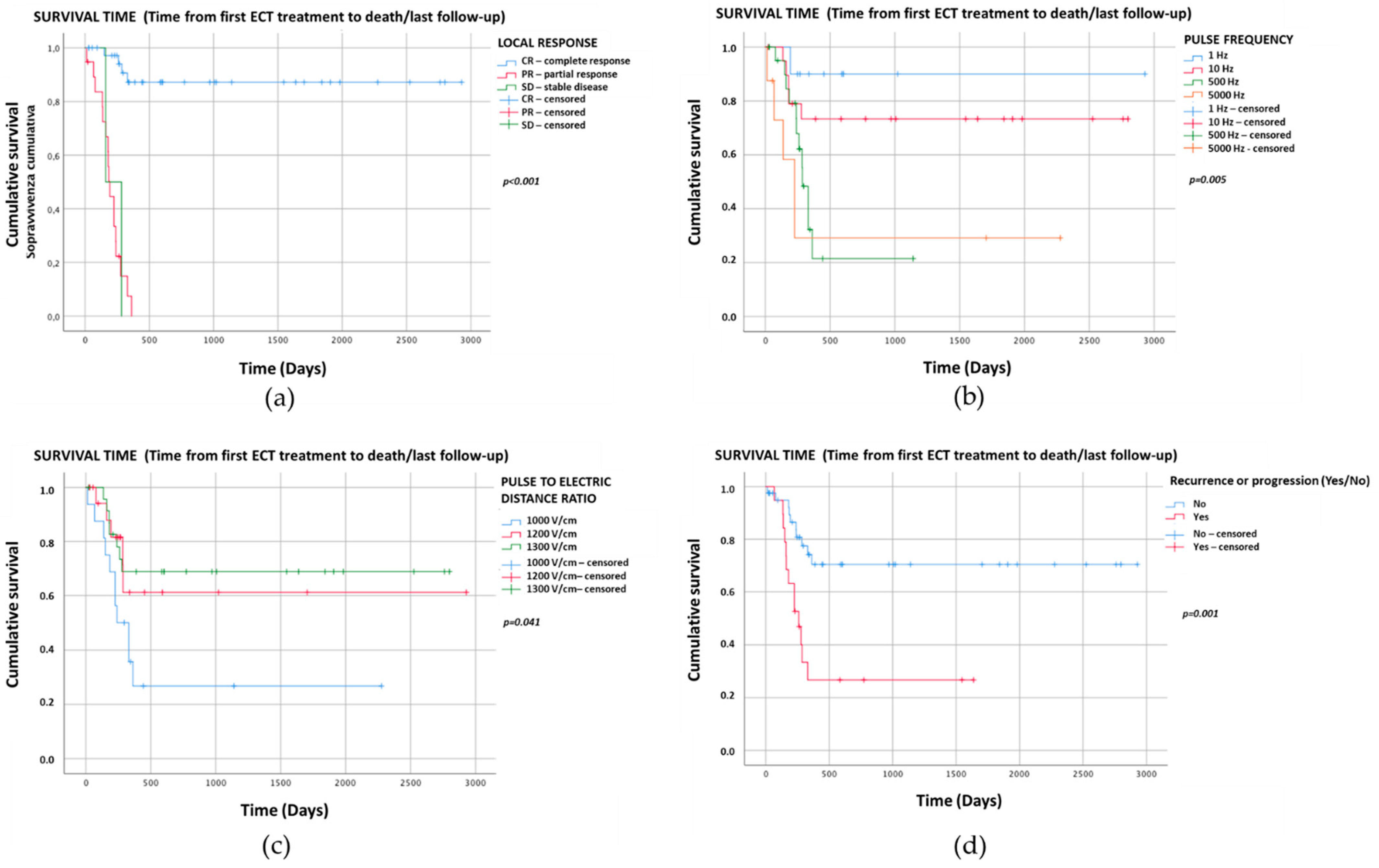
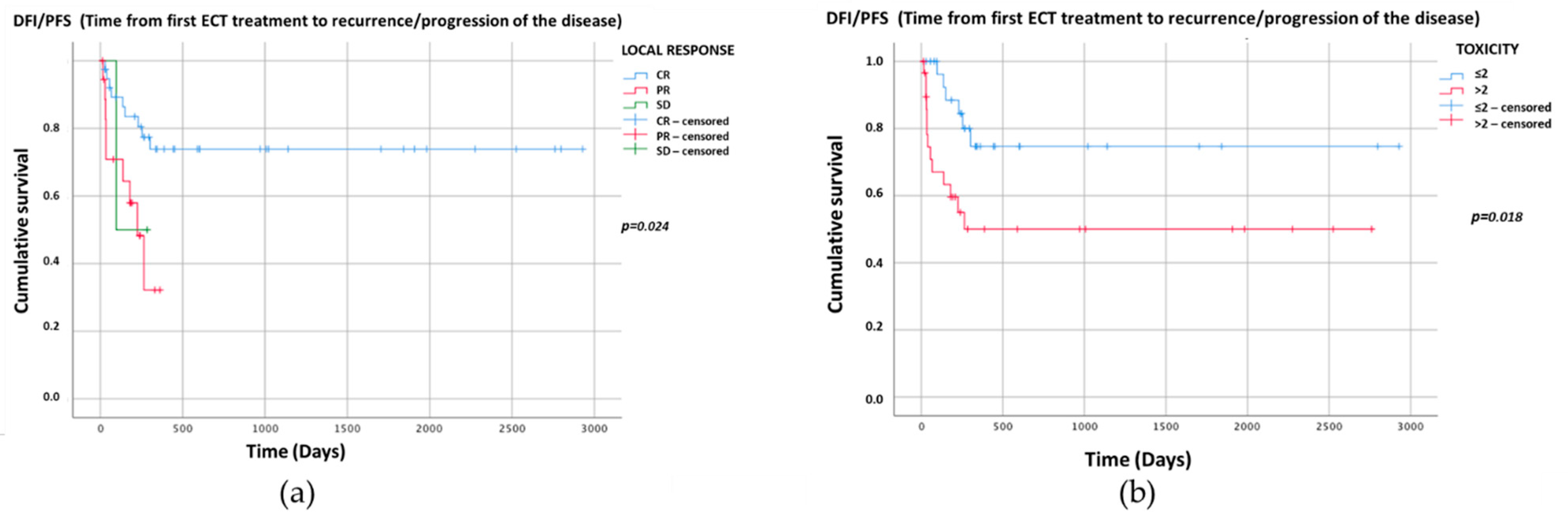
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bertino, G.; Sersa, G.; De Terlizzi, F.; Occhini, A.; Plaschke, C.C.; Groselj, A.; Langdon, C.; Grau, J.J.; McCaul, J.A.; Heuveling, D.; et al. European Research on Electrochemotherapy in Head and Neck Cancer (EURECA) project: Results of the treatment of skin cancer. Eur. J. Cancer 2016, 63, 41–52. [Google Scholar] [CrossRef]
- Murphy, S. Cutaneous Squamous Cell Carcinoma in the Cat: Current understanding and treatment approaches. J. Feline Med. Surg. 2013, 15, 401–407. [Google Scholar] [CrossRef]
- Hauck, M.L. Tumors of the Skin and Subcutaneous Tissues. In Withrow & MacEwen’s Small Animal Clinical Oncology, 5th ed.; Elsevier: St. Louis, MI, USA, 2013; pp. 305–320. [Google Scholar]
- Thomson, M. Squamous Cell Carcinoma of the Nasal Planum in Cats and Dogs. Clin. Tech. Small Anim. Pr. 2007, 22, 42–45. [Google Scholar] [CrossRef]
- Goldschmidt, M.H.; Goldschmidt, K.H. Epithelial and Melanocytic Tumors of the Skin. In Tumors in Domestic Animals; Wiley: Ames, IA, USA, 2016; Volume 2017, pp. 88–141. [Google Scholar]
- Hauck, M.L.; Oblak, M.L. Tumors of the Skin and Subcutaneous Tissues. In Withrow and MacEwen’s Small Animal Clinical Oncology; Elsevier: Amsterdam, The Netherlands, 2020; pp. 352–366. [Google Scholar]
- Munday, J.S.; Gwyther, S.; Thomson, N.A.; Malik, R. Bilateral pre-auricular papillary squamous cell carcinomas associated with papillomavirus infection in a domestic cat. Vet. Dermatol. 2016, 28, 232-e58. [Google Scholar] [CrossRef]
- Withrow, S.; Straw, R. Resection of the nasal planum in nine cats and five dogs. J. Am. Anim. Hosp. Assoc. 1990, 26, 219–222. [Google Scholar]
- Tozon, N.; Pavlin, D.; Sersa, G.; Dolinsek, T.; Cemazar, M. Electrochemotherapy with intravenous bleomycin injection: An observational study in superficial squamous cell carcinoma in cats. J. Feline Med. Surg. 2014, 16, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Clarke, R. Cryosurgical treatment of feline cutaneous squamous cell carcinoma. Aust. Vet. Pr. 1991, 21, 148–153. [Google Scholar]
- Lana, S.E.; Ogilvie, G.K.; Withrow, S.J.; Straw, R.C.; Rogers, K.S. Feline cutaneous squamous cell carcinoma of the nasal planum and the pinnae: 61 cases. J. Am. Anim. Hosp. Assoc. 1997, 33, 329–332. [Google Scholar] [CrossRef]
- Théon, A.P.; Madewell, B.R.; Shearn, V.I.; Moulton, J.E. Prognostic factors associated with radiotherapy of squamous cell car-cinoma of the nasal plane in cats. J. Am. Vet. Med. Assoc. 1995, 206, 991–996. [Google Scholar] [PubMed]
- Goodfellow, M.; Hayes, A.; Murphy, S.; Brearley, M. A retrospective study of 90Strontium plesiotherapy for feline squamous cell carcinoma of the nasal planum. J. Feline Med. Surg. 2006, 8, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Hammond, G.M.; Gordon, I.K.; Theon, A.P.; Kent, M.S. Evaluation of strontium Sr 90 for the treatment of superficial squamous cell carcinoma of the nasal planum in cats: 49 cases (1990–2006). J. Am. Veter. Med. Assoc. 2007, 231, 736–741. [Google Scholar] [CrossRef] [PubMed]
- Van Vechten, M.K.; Théon, A.P. Strontium-90 plesiotherapy for treatment of early squamous cell carcinoma of the nasal planum in 25 cats. Proc. Vet. Can. Soc. 1993, 107, 108. [Google Scholar]
- Bexfield, N.H.; Stell, A.J.; Gear, R.N.; Dobson, J.M. Photodynamic Therapy of Superficial Nasal Planum Squamous Cell Carci-nomas in Cats: 55 Cases. J. Vet. Intern. Med. 2008, 22, 1385–1389. [Google Scholar] [CrossRef]
- Buchholz, J.; Wergin, M.; Walt, H.; Gräfe, S.; Bley, C.R.; Kaser-Hotz, B. Photodynamic therapy of feline cutaneous squamous cell carcinoma using a newly developed liposomal photosensitizes: Preliminary results concerning drug safety and efficacy. J. Vet. Intern. Med. 2007, 21, 770–775. [Google Scholar] [CrossRef] [PubMed]
- Théon, A.P.; VanVechten, M.K.; Madewell, B.R. Intratumoral administration of carboplatin for treatment of squamous cell carcinomas of the nasal plane in cats. Am. J. Vet. Res. 1996, 57, 205–210. [Google Scholar]
- Ogilvie, G.K.; Moore, A.S.; Obradovich, J.E.; Elmslie, R.E.; Vail, D.M.; Straw, R.C.; Salmon, M.D.; Klein, M.K.; Atwater, S.W.; Ciekot, P.E. Toxicoses and efficacy associated with administration of mitoxantrone to cats with malignant tumors. J. Am. Vet. Med. Assoc. 1993, 202, 1839–1844. [Google Scholar]
- De Vos, J.P.; Burm, A.G.O.; Focker, B.P. Results from the treatment of advanced stage squamous cell carcinoma of the nasal planum in cats, using a combination of intralesional carboplatin and superficial radiotherapy: A pilot study. Vet. Comp. Oncol. 2004, 2, 75–81. [Google Scholar] [CrossRef]
- Gasymova, E.; Meier, V.; Guscetti, F.; Cancedda, S.; Roos, M.; Bley, C.R. Retrospective clinical study on outcome in cats with nasal planum squamous cell carcinoma treated with an accelerated radiation protocol. BMC Vet. Res. 2017, 13, 86. [Google Scholar] [CrossRef]
- Cemazar, M.; Sersa, G. Recent Advances in Electrochemotherapy. Bioelectricity 2019, 1, 204–213. [Google Scholar] [CrossRef]
- Rangel, M.M.; Luz, J.C.; Oliveira, K.D.; Ojeda, J.; Freytag, J.O.; Suzuki, D.O. Electrochemotherapy in the treatment of neoplasms in dogs and cats. Austral. J. Vet. Sci. 2019, 51, 45–51. [Google Scholar] [CrossRef]
- Spugnini, E.P.; Azzarito, T.; Fais, S.; Fanciulli, M.; Baldi, A. Electrochemotherapy as First Line Cancer Treatment: Experiences from Veterinary Medicine in Developing Novel Protocols. Curr. Cancer Drug Targets 2015, 16, 43–52. [Google Scholar] [CrossRef]
- Miklavčič, D.; Mali, B.; Kos, B.; Heller, R.; Serša, G. Electrochemotherapy: From the drawing board into medical practice. Biomed. Eng. Online 2014, 13, 29. [Google Scholar] [CrossRef] [PubMed]
- Tozon, N.; Tratar, U.L.; Znidar, K.; Sersa, G.; Teissie, J.; Cemazar, M. Operating Procedures of the Electrochemotherapy for Treatment of Tumor in Dogs and Cats. J. Vis. Exp. 2016, 2016, e54760. [Google Scholar] [CrossRef]
- Serša, G.; Miklavčič, D.; Čemazarm, M.; Belehradek, J.; Jarm, T.; Mir, L.M. Electrochemotherapy with CDDP on LPB sarcoma: Comparison of the anti-tumor effectiveness in immunocompotent and immunodeficient mice. Bioelectrochem. Bioenerg. 1997, 43, 279–283. [Google Scholar] [CrossRef]
- Sersa, G.; Teissie, J.; Cemazar, M.; Signori, E.; Kamensek, U.; Marshall, G.; Miklavcic, D. Electrochemotherapy of tumors as in situ vaccination boosted by immunogene electrotransfer. Cancer Immunol. Immunother. 2015, 64, 1315–1327. [Google Scholar] [CrossRef] [PubMed]
- Lowe, R.; Gavazza, A.; Impellizeri, J.A.; Soden, D.M.; Lubas, G. The treatment of canine mast cell tumours with electrochemotherapy with or without surgical excision. Vet. Comp. Oncol. 2016, 15, 775–784. [Google Scholar] [CrossRef]
- Torrigiani, F.; Pierini, A.; Lowe, R.; Simčič, P.; Lubas, G. Soft tissue sarcoma in dogs: A treatment review and a novel approach using electrochemotherapy in a case series. Vet. Comp. Oncol. 2019, 17, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Simčič, P.; Lowe, R.; Granziera, V.; Pierini, A.; Torrigiani, F.; Lubas, G. Electrochemotherapy in treatment of canine oral non-tonsillar squamous cell carcinoma. A case series report. Vet. Comp. Oncol. 2019, 18, 428–432. [Google Scholar] [CrossRef]
- Spugnini, E.P.; Pizzuto, M.; Filipponi, M.; Romani, L.; Vincenzi, B.; Menicagli, F.; Lanza, A.; De Girolamo, R.; Lomonaco, R.; Fanciulli, M.; et al. Electroporation Enhances Bleomycin Efficacy in Cats with Periocular Carcinoma and Advanced Squamous Cell Carcinoma of the Head. J. Vet. Intern. Med. 2015, 29, 1368–1375. [Google Scholar] [CrossRef] [PubMed]
- Spugnini, E.P.; Renaud, S.M.; Buglioni, S.; Carocci, F.; Dragonetti, E.; Murace, R.; Cardelli, P.; Vincenzi, B.; Baldi, A.; Citro, G. Electrochemotherapy with cisplatin enhances local control after surgical ablation of fibrosarcoma in cats: An approach to improve the therapeutic index of highly toxic chemotherapy drugs. J. Transl. Med. 2011, 9, 152. [Google Scholar] [CrossRef]
- Tozon, N.; Kodre, V.; Serša, G.; Čemažar, M. Effective treatment of perianal tumors in dogs with electrochemothera-py. Anticancer Res. 2005, 25, 839–845. [Google Scholar] [PubMed]
- Maglietti, F.; Tellado, M.; Olaiz, N.; Michinski, S.; Marshall, G. Minimally invasive electrochemotherapy procedure for treating nasal duct tumors in dogs using a single needle electrode. Radiol. Oncol. 2017, 51, 422–430. [Google Scholar] [CrossRef] [PubMed]
- Kranjc, S.; Kamensek, U.; Cemazar, M.; Sersa, G. Combined Treatment of Electrochemotherapy with Irradiation. In Handbook of Electroporation; Springer Nature: Cham, Switzerland, 2016; pp. 1–17. [Google Scholar]
- Milevoj, N.; Tratar, U.L.; Nemec, A.; Brožič, A.; Žnidar, K.; Serša, G.; Čemažar, M.; Tozon, N. A combination of electrochemotherapy, gene electrotransfer of plasmid encoding canine IL-12 and cytoreductive surgery in the treatment of canine oral malignant melanoma. Res. Vet. Sci. 2019, 122, 40–49. [Google Scholar] [CrossRef]
- Spugnini, E.P.; Vincenzi, B.; Citro, G.; Tonini, G.; Dotsinsky, I.; Mudrov, N.; Baldi, A. Electrochemotherapy for the treatment of squamous cell carcinoma in cats: A preliminary report. Vet. J. 2009, 179, 117–120. [Google Scholar] [CrossRef]
- Spugnini, E.P.; Baldi, A.; Vincenzi, B.; Bongiorni, F.; Bellelli, C.; Citro, G.; Porrello, A. Intraoperative versus postoperative electrochemotherapy in high grade soft tissue sarcomas: A preliminary study in a spontaneous feline model. Cancer Chemother. Pharmacol. 2006, 59, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Spugnini, E.P.; Dotsinsky, I.; Mudrov, N.; Citro, G.; Caruso, G.; Cardelli, P.; Baldi, A. Electrochemotherapy-induced radiation recall in a cat. In Vivo 2009, 22, 751–754. [Google Scholar]
- Palareti, G.; Legnani, C.; Cosmi, B.; Antonucci, E.; Erba, N.; Poli, D.; Testa, S.; Tosetto, A.; De Micheli, V.; Ghirarduzzi, A.; et al. Comparison between different D - D imer cutoff values to assess the individual risk of recurrent venous thromboembolism: Analysis of results obtained in the DULCIS study. Int. J. Lab. Hematol. 2015, 38, 42–49. [Google Scholar] [CrossRef]
- Groselj, A.; Bosnjak, M.; Strojan, P.; Krzan, M.; Cemazar, M.; Sersa, G. Efficiency of electrochemotherapy with reduced bleomycin dose in the treatment of nonmelanoma head and neck skin cancer: Preliminary results. Head Neck 2018, 40, 120–125. [Google Scholar] [CrossRef]
- Nguyen, S.M.; Thamm, D.H.; Vail, D.M.; London, C.A. Response evaluation criteria for solid tumours in dogs (v1.0): A Veterinary Cooperative Oncology Group (VCOG) consensus document. Vet. Comp. Oncol. 2013, 13, 176–183. [Google Scholar] [CrossRef]
- Dos Anjos, D.S.; Sierra, O.R.; Spugnini, E.P.; De Nardi, A.B.; Fonseca-Alves, C.E. Comparison of two different doses of bleomycin in electrochemotherapy protocols for feline cutaneous squamous cell carcinoma nonsegregated from ultraviolet light exposure. Sci. Rep. 2020, 10, 1–9. [Google Scholar] [CrossRef]
- Cunha, S.C.; Carvalho, L.A.V.; Canary, P.C.; Reisner, M.; Corgozinho, K.B.; Souza, H.J.; Ferreira, A.M.R. Radiation therapy for feline cutaneous squamous cell carcinoma using a hypofractionated protocol. J. Feline Med. Surg. 2010, 12, 306–313. [Google Scholar] [CrossRef]
- Magne, M.L.; Rodriguez, C.O.; Autry, S.A.; Edwards, B.F.; Theon, A.P.; Madewell, B.R. Photodynamic therapy of facial squamous cell carcinoma in cats using a new photosensitizer. Lasers Surg Med. 1997, 20, 202–209. [Google Scholar] [CrossRef]
- Mir, L.M.; Gehl, J.; Sersa, G.; Collins, C.G.; Garbay, J.-R.; Billard, V.; Geertsen, P.F.; Rudolf, Z.; O’Sullivan, G.C.; Marty, M. Standard operating procedures of the electrochemotherapy: Instructions for the use of bleomycin or cisplatin administered either systemically or locally and electric pulses delivered by the CliniporatorTM by means of invasive or non-invasive electrodes. Eur. J. Cancer Suppl. 2006, 4, 14–25. [Google Scholar] [CrossRef]
- Quaglino, P.; Matthiessen, L.W.; Curatolo, P.; Muir, T.; Bertino, G.; Kunte, C.; Odili, J.; Rotunno, R.; Humphreys, A.C.; Letulé, V.; et al. Predicting patients at risk for pain associated with electrochemotherapy. Acta Oncol. 2015, 54, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Miklavčič, D.; Pucihar, G.; Pavlovec, M.; Ribarič, S.; Mali, M.; Maček-Lebar, A.; Petkovšek, M.; Nastran, J.; Kranjc, S.; Čemažar, M.; et al. The effect of high frequency electric pulses on muscle contractions and antitumor efficiency in vivo for a potential use in clinical electrochemotherapy. Bioelectrochemistry 2005, 65, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Mali, B.; Miklavcic, D.; Campana, L.G.; Cemazar, M.; Sersa, G.; Snoj, M.; Jarm, T. Tumor size and effectiveness of electrochemotherapy. Radiol. Oncol. 2013, 47, 32–41. [Google Scholar] [CrossRef] [PubMed]
| Facility (n° Cats) | Electroporator | ECT Frequency (Hz) (N° Cats) | Amplitude to Electrode Distance Ratio (V/cm) (N° Cats) | Electrode (N° Cats) | Anesthesia Protocol |
|---|---|---|---|---|---|
| SVG 1 (12) | Electrovet EZ 7 | 500 (12) | 1000 (10) | N 11 (12) | Premedication: Dexmedetomidine 2 mcg/kg, IM 15 and Methadone 0.2 mg/kg, IM Induction: Propofol 2 mg/kg, IV Maintenance: Isoflurane |
| 1300 (2) | |||||
| AVC 2 (10) | Oncovet 8 | 1 (8) | 1200 (10) | N 12(10) | Premedication/induction: Medetomidine 0.10–0.15 mg/kg, IM and Butorphanol 0.1–0.5 mg/kg, IV; Atipamezole 0.5 mL/kg, IV to reverse |
| 5000 (2) | |||||
| VCB 3 (26) | VET CP 125 9 | 5000 (6) | 1000 (6) | N 13 (26) | Premedication: Methadone 0.3 mg/kg, IM Induction: Propofol 5 mg/kg, IV Maintenance: Isoflurane. |
| BTX ECM 830 9 | 10 (20) | 1300 (20) | |||
| OVG 4 (10) | Electrovet EZ 7 | 500 (10) | 1200 (10) | N 11 (9) | Premedication: Methadone 0.2mg/kg, IM or Butorphanol 0.2 mg/kg, IM both associated with Ketamine 5 mg/kg, IM and Dexmedetomidine 40 mcg/kg, IM (Atipamezole to reverse) Induction: Propofol 4.5mg/kg, IV Maintenance: Isoflurane |
| P 14 (1) | |||||
| CVM 5 (2) | Electrovet S13 7 | 1 (1) | 1300 (2) | P 14 (2) | Premedication: Medetomidine 0.001 mg/kg, IM, Midazolam 0.2 mg/kg, IM and Methadone 0.2 mg/kg, IM Induction: Propofol 4.5 mg/kg, IV Maintenance: Isoflurane |
| GVS p 6 (1) | Cliniporator 10 | 5000 (1) | 400 (1) | N 12(1) | Premedication: Dexmedetomidine 0.04 mg/kg, IM (Atipamezole to reverse) Induction: Propofol 4 mg/kg, IV Maintenance: Isoflurane |
| Facility/Electroporator (N° Cats) | Size Range (cm) | N° of ECT (N° Cats) | Outcome (N° Cats) | Toxicity (N° Cats) | Post-ECT Therapy |
|---|---|---|---|---|---|
| SVG/Electrovet EZ (12) | 0.2–4 | 1 ECT (4) 2 ECT (6) 3 ECT (1) 4 ECT (1) | CR 1 (7) PR 2 (5) | 0 (11) 3 (1) | If necessary: Meloxicam single dose 0.2 mg/kg, then for a few days, as needed, 0.1 mg/kg, Amoxicillin and clavulanic acid 20 mg/kg. |
| AVC/Oncovet (10) | 0.3–3 | 1 ECT (10) | CR 1 (9) PR 2 (1) | 0 (1) 2 (7) 3 (1) 4 (1) | Amoxicillin and clavulanic acid 7–10 mg/kg, IV one dose, then Clindamycin 5.5 mg/kg, PO for 7 days, Meloxicam 0.3 mg/kg, SC, Dexamethasone 0.3 mg/kg, SC single dose 24 h after ECT. |
| VCB/VET CP 125 (6) | 2.1–5 | 1 ECT (4) 2 ECT (1) 3 ECT (1) | CR 1 (1) PR 2 (5) | 4 (3) 5 (4) | After ECT: Dipyrone 25 mg/kg, PO and Ketoprofen 1 mg/kg, PO (single dose); Home: Tramadol 1 mg/kg, PO SID 3 days, Dipyrone 25 mg/kg, PO SID 3–5 days, Ketoprofen 1 mg/kg, PO SID 3–5 days and Amoxicillin and clavulanic acid 15 mg/kg, PO BID 7 days. Persisting necrosis (toxicity grade 4 or 5): Amoxicillin and clavulanic acid 15 mg/kg, PO BID 7 days. Pain: Tramadol 1 mg/kg, PO SID 3 days (severe cases), Dipyrone 25 mg/kg, PO SID 3-5 days, Ketoprofen 1 mg/kg, PO SID 3–5 days. Severe cases: hospitalization 1-3 days with Methadone 0.1 mg/kg, SC BID, Dipyrone 25 mg/kg, IV SID, Ketoprofen 1 mg/kg, SC SID 3–5 days, Ampicillin and sulbactam 15 mg/kg BID 7 days IV. If hospitalization less than 7 days, antibiotic is replaced by Amoxicillin and clavulanic acid 15 mg/kg PO BID until the protocol is completed. |
| VCB/BTX ECM 830 (20) | 0.8–3.6 | 1 ECT (12) 2 ECT (5) 3 ECT (3) | CR 1 (14) PR 2 (6) | 2 (2) 3 (1) 4 (10) 5 (7) | |
| OVG/Electrovet EZ (10) | 0.5–6 | 1 ECT (8) 2 ECT (1) 3 ECT (1) | CR 1 (6) PR 2 (2) SD 3 (2) | 1 (2) 2 (5) 3 (1) 4 (1) 5 (1) | Meloxicam 0.05 mg/kg, PO and Amoxicillin and clavulanic acid 20 mg/kg, PO for 5–7 days. For wound cleaning and crust removal: saline and local ointments: VEA cream PF® (antioxidant cream with Vit. E and Polyphenol), One Vet spray® (neem oil, St John’s wort oil and olive oil), Iruxol® (collagenase and chloramphenicol) or Hypermix® (neem oil and St John’s wort oil) |
| CVM/Electrovet S13 (2) | 0.6–3 | 1 ECT (1) 2 ECT (1) | CR 1 (2) | 0 (1) 2 (1) | Meloxicam 0.3 mg/kg SC for 2–3 days. |
| GVS/Cliniporator (1) | 0.5 | 2 ECT (1) | CR 1 (1) | 0 (1) | None |
| UNIVARIATE—Logistic Binary Regression | |||||
|---|---|---|---|---|---|
| Tumor-Specific Survival (N° Cats) | |||||
| Yes | No | ||||
| Variables | 1 p | 2 OR | 3 95% CI | ||
| Gender | |||||
| 4 MN | 9 | 21 | 0.187 | 2.04 | 0.71–5.9 |
| 5 FN | 14 | 16 | Reference category | ||
| Local treatment response | |||||
| 6 CR | 4 | 35 | Reference category | ||
| 7 PR | 17 | 2 | <0.001 | 74.4 | 12.37–447.05 |
| 8 SD | 2 | 0 | 0.999 | 9 H | |
| Pulse frequency (Hz) | |||||
| 1 | 1 | 9 | 0.035 | 0.07 | 0.01–0.82 |
| 10 | 5 | 15 | 0.072 | 0.2 | 0.04–1.15 |
| 500 | 12 | 10 | 0.70 | 0.72 | 0.14–3.78 |
| 5000 | 5 | 3 | Reference category | ||
| Amplitude to electrode distance ratio (V/cm) | |||||
| 1000 | 11 | 5 | Reference category | ||
| 1200 | 5 | 15 | 0.011 | 0.15 | 0.04–0.66 |
| 1300 | 7 | 17 | 0.016 | 0.19 | 0.05–0.74 |
| Toxicity score | |||||
| 0–2 | 10 | 20 | Reference category | ||
| 3–5 | 13 | 17 | 0.43 | 1.53 | 0.45–4.36 |
| Recurrence/progression | |||||
| Yes | 13 | 6 | 0.002 | 6.72 | 2.02–22.34 |
| No | 10 | 31 | Reference category | ||
| Age (years) | 0.806 | 1.02 | 0.85–1.23 | ||
| Tumor size (cm) | 0.074 | 1.52 | 0.96–2.40 | ||
| MULTIVARIATE—Logistic Binary Regression | |||||
| Variables | 1 p | 2 OR | 3 95%CI | ||
| Local treatment response | |||||
| 6 CR | 4 | 35 | 0.001 | Reference category | |
| 7 PR | 17 | 2 | <0.001 | 179.28 | 10.97–2938.89 |
| 8 SD | 2 | 0 | 0.999 | 10 VH | |
| Recurrence/progression | |||||
| Yes | 13 | 6 | 0.017 | 27.4 | 1.80–386.95 |
| No | 10 | 31 | Reference category | ||
| UNIVARIATE—Logistic Binary Regression | |||||
|---|---|---|---|---|---|
| Local Treatment Response (N° Cats) | |||||
| 6 CR | 7 PR/8 SD | ||||
| Variables | 1 p | 2 OR | 3 95% CI | ||
| Gender | |||||
| 4 MN | 21 | 9 | 0.418 | 1.56 | 0.53–4.53 |
| 5 FN | 18 | 12 | Reference category | ||
| Pulse frequency (Hz) | |||||
| 1 | 9 | 1 | 0.035 | 0.07 | 0.01–0.82 |
| 10 | 14 | 6 | 0.122 | 0.26 | 0.46–1.44 |
| 500 | 13 | 9 | 0.301 | 0.42 | 0.08–2.20 |
| 5000 | 3 | 5 | Reference category | ||
| Amplitude to electrode distance ratio (V/cm) | |||||
| 1000 | 7 | 9 | Reference category | ||
| 1200 | 15 | 5 | 0.061 | 0.26 | 0.06–1.07 |
| 1300 | 17 | 7 | 0.092 | 0.32 | 0.09–1.20 |
| Toxicity score | |||||
| 0–2 | 24 | 6 | Reference category | ||
| 3–5 | 15 | 15 | 0.018 | 4.00 | 1.27–12.5 |
| Age (years) | 0.34 | 1.10 | 0.91–1.33 | ||
| Tumor size (cm) | 0.001 | 3.08 | 1.59–5.96 | ||
| MULTIVARIATE—Logistic binary regression | |||||
| Variables | 1p | 2OR | 395%CI | ||
| Tumor size (cm) | 0.008 | 4.50 | 1.48–13.67 | ||
| UNIVARIATE—Logistic Binary Regression | |||||
|---|---|---|---|---|---|
| Toxicity (N° Cats) | |||||
| 0–2 | 3–5 | ||||
| Variables | 1 p | 2 OR | 3 95% CI | ||
| Gender | |||||
| 4 MN | 17 | 13 | 0.187 | 2.04 | 0.71–5.9 |
| 5 FN | 13 | 17 | Reference category | ||
| Pulse frequency (Hz) | |||||
| 1 | 8 | 2 | 0.29 | 0.08 | 0.01–0.77 |
| 10 | 2 | 18 | 0.32 | 3,00 | 0.34–26.19 |
| 500 | 18 | 4 | 0.008 | 0.07 | 0.01–0.51 |
| 5000 | 2 | 6 | Reference category | ||
| Amplitude to electrode distance ratio (V/cm) | |||||
| 1000 | 10 | 6 | Reference category | ||
| 1200 | 15 | 5 | 0.421 | 0.56 | 0.13–2.33 |
| 1300 | 5 | 19 | 0.01 | 6.30 | 1.54–26.0 |
| Age (years) | 0.077 | 0.84 | 0.70–1.02 | ||
| Tumor size (cm) | <0.001 | 5.84 | 2.43–14.04 | ||
| MULTIVARIATE—Logistic Binary Regression | |||||
| Variables | 1 p | 2 OR | 3 95%CI | ||
| Tumor size (cm) | 0.005 | 3.75 | 1.49–9.44 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simčič, P.; Pierini, A.; Lubas, G.; Lowe, R.; Granziera, V.; Tornago, R.; Valentini, F.; Alterio, G.; Cochi, M.; Rangel, M.M.M.; et al. A Retrospective Multicentric Study of Electrochemotherapy in the Treatment of Feline Nasal Planum Squamous Cell Carcinoma. Vet. Sci. 2021, 8, 53. https://doi.org/10.3390/vetsci8030053
Simčič P, Pierini A, Lubas G, Lowe R, Granziera V, Tornago R, Valentini F, Alterio G, Cochi M, Rangel MMM, et al. A Retrospective Multicentric Study of Electrochemotherapy in the Treatment of Feline Nasal Planum Squamous Cell Carcinoma. Veterinary Sciences. 2021; 8(3):53. https://doi.org/10.3390/vetsci8030053
Chicago/Turabian StyleSimčič, Petra, Alessio Pierini, George Lubas, Ron Lowe, Valentina Granziera, Raimondo Tornago, Fabio Valentini, Giulia Alterio, Matteo Cochi, Marcelo Monte Mor Rangel, and et al. 2021. "A Retrospective Multicentric Study of Electrochemotherapy in the Treatment of Feline Nasal Planum Squamous Cell Carcinoma" Veterinary Sciences 8, no. 3: 53. https://doi.org/10.3390/vetsci8030053
APA StyleSimčič, P., Pierini, A., Lubas, G., Lowe, R., Granziera, V., Tornago, R., Valentini, F., Alterio, G., Cochi, M., Rangel, M. M. M., de Oliveira, K. D., Ostrand Freytag, J., Quadros, P. G., Sponza, E., Gattino, F., Impellizeri, J. A., & Torrigiani, F. (2021). A Retrospective Multicentric Study of Electrochemotherapy in the Treatment of Feline Nasal Planum Squamous Cell Carcinoma. Veterinary Sciences, 8(3), 53. https://doi.org/10.3390/vetsci8030053






