First Molecular Confirmation of Equine Ocular Setaria digitata in China
Abstract
:1. Introduction
2. Materials and Methods
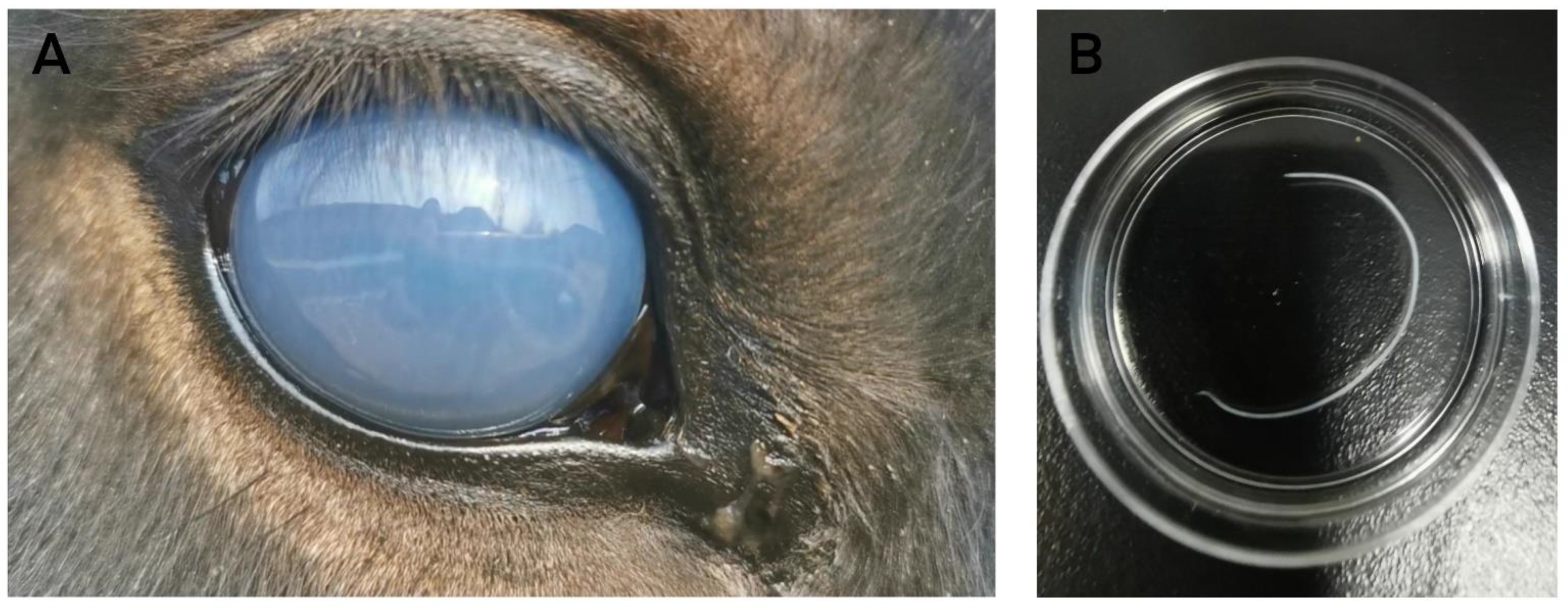
2.1. Case Report
2.2. Worm Identification
3. Results and Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Azawi, A.K.A.L.; Fadhl, R.A.; Fadhl, R.S. Epidemiological study of Setaria equina infection in donkeys. Iraq Vet. J. 2012, 36, 93–97. [Google Scholar]
- Bazargani, T.; Eslami, A.; Gholami, G.R.; Molai, A.; Ghafari-Charati, J.; Dawoodi, J.; Ashrafi, J. Cerebrospinal nematodiasis of cattle, sheep and goats in Iran. Iran. J. Parasitol. 2008, 3, 16–20. [Google Scholar]
- Jayakumar, K.; Dharmaceelan, S.; Rajendran, N.; Senthilkumar, S.; Kathirvel, S.; Nagarajan, L.; Kumaresan, A. Ocular setariasis in a pony. Indian Vet. J. 2012, 89, 64–66. [Google Scholar]
- Radwan, A.M.; Ahmed, N.E.; Elakabawy, L.M.; Ramadan, M.Y.; Elmadawy, R.S. Prevalence and pathogenesis of some filarial nematodes infecting donkeys in Egypt. Vet. World 2016, 9, 888–892. [Google Scholar] [CrossRef] [PubMed]
- Voronin, D.; Abeykoon, A.M.; Gunawardene, Y.I.; Dassanayake, R.S. Absence of Wolbachia endobacteria in Sri Lankan isolates of the nematode parasite of animals Setaria digitata. Vet. Parasitol. 2015, 207, 350–354. [Google Scholar] [CrossRef] [PubMed]
- Tung, K.C.; Cheng, F.P.; Lai, C.H.; Wang, K.S.; Wang, J.S.; Lee, W.M. Demonstration of vector competence of Culex quinquefasciatus (Diptera: Culicidae) for Setaria digitata. Vet. Parasitol. 2004, 123, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Perumal, A.N.I.; Gunawardene, Y.I.N.S.; Dassanyake, R.S. Setaria digitata in advancing our knowledge of human lymphatic filariasis. J. Helminthol. 2016, 90, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Peng, T.L.; Armiladiana, M.; Ruhil, H.H.; Maizan, M.; Choong, S.S. First report of equine Setaria digitata (von Linstow 1906) infestation in Malaysia. Vet. Parasitol. Reg. Stud. Reports. 2019, 17, 100310. [Google Scholar] [CrossRef] [PubMed]
- Sathu, S. Intraocular parasites in horses. A report of five cases. Indian Vet. J. 1974, 5, 225. [Google Scholar]
- Ladoucer, C.A.; Kazacos, K.R. Thelazia lacrimalis in horses in India. J. Am. Vet. Med. Assoc. 1981, 178, 301–302. [Google Scholar]
- Parrah, J.D.; Buchoo, B.A.; Moulvi, B.A. Ocular filariasis in equines: A study of 9 cases. Centaur 2004, 4, 70–71. [Google Scholar]
- Tamilmahan, P.; Zama, M.M.S.; Pathak, R.; Muneeswaran, N.S.; Karthik, K. A retrospective study of ocular occurrences in domestic animals: 799 cases. Vet. World 2013, 6, 274–276. [Google Scholar] [CrossRef]
- Shin, S.S.; Cho, K.O.; Wee, S.H. Ocular infection of cattle with Setaria digitata. J. Vet. Med. Sci. 2002, 64, 7–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Basak, S.K.; Hazra, T.K.; Bhattacharya, D. Persistent corneal oedema secondary to presumed dead adult worm in the anterior chamber. Indian J. Ophthalmol. 2007, 55, 679. [Google Scholar] [CrossRef]
- Paglia, D.T.; Miller, P.E.; Dubielzig, R.R. James Wardrop and equine recurrent uveitis. Arch. Ophthalmol. 2004, 122, 1218–1223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rhee, J.K.; Choi, E.Y.; Park, B.K.; Jang, B.G. Application of scanning electron microscopy in assessing the prevalence of some Setaria species in Korean cattle. Korean J. Parasitol. 1994, 32, 1–6. [Google Scholar] [CrossRef]
- Sundar, S.T.B.; D’Souza, P.E. Morphological characterization of Setaria worms collected from cattle. J. Parasit. Dis. 2015, 39, 572–576. [Google Scholar] [CrossRef] [Green Version]
- Shin, J.; Ahn, K.-S.; Jeong, H.-S.; Kim, B.-S.; Choi, E.; Shin, S.-S.; Suh, G.-H.; Kim, H.-J. First blindness cases of horses infected with Setaria digitata (Nematoda: Filarioidea) in the Republic of Korea. Korean J. Parasitol. 2017, 55, 667–761. [Google Scholar] [CrossRef]
- Maharana, B.R.; Potliya, S.; Ganguly, A.; Bisla, R.S.; Mishra, C.; Ganguly, I. First report of the isolation and phylogenetic characterization of equine Setaria digitata from India based on mitochondrial COI, 12S rDNA, and nuclear ITS2 sequence data. Parasitol. Res. 2020, 119, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Yatawara, L.; Wickramasinghe, S.; Rajapakse, R.P.; Agatsuma, T. The complete mitochondrial genome of Setaria digitata (Nematoda: Filarioidea): Mitochondrial gene content, arrangement and composition compared with other nematodes. Mol. Biochem. Parasitol. 2010, 173, 32–38. [Google Scholar] [CrossRef]
- Liu, G.; Li, J.; Zhu, X. Characterization of the complete mitochondrial genome of Setaria digitata (Nematoda: Setariidae) from China. J. Helminthol. 2017, 91, 772–776. [Google Scholar] [CrossRef]
- Lavach, J.D. Parasitic diseases. In Large Animal Ophthalmology; Mosby: Philadelphia, PA, USA, 1990; pp. 260–263. [Google Scholar]
- Moore, C.P.; Sarazan, R.D.; Whitley, R.D.; Jackson, W.F. Equine ocular parasites: A review. Equine Vet. J. Suppl. 1983, 2, 76–85. [Google Scholar] [CrossRef]
- Raziq, S.A. A preliminary clinical trial on the use of diethylcarbamazine citrate for the treatment of equine filariasis. Pak. Vet. J. 1989, 9, 93–96. [Google Scholar]
- Muhammad, G.; Saqib, M. Successful treatment of ocular equine microfilariasis (Setaria species) with ivermectin. Vet. Rec. 2007, 160, 25–26. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Ahn, D.C.; Jin, H.P.; Yu, D.; Chae, J.; Yoo, J.; Sim, C.H.; Choi, K.-S.; Park, Y.-J.; Park, B.-K. Ocular setariasis by Setaria digitata in a horse in Korea. Korean J. Vet. Serv. 2018, 41, 15–19. [Google Scholar]
- Nabie, R.; Spotin, A.; Rouhani, S. Subconjunctival setariasis due to Setaria equina infection; a case report and a literature review. Parasitol. Int. 2017, 66, 930–932. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, F.; Liu, B.; Chen, S.; Yi, Z.; Liu, X.; Zhu, Y.; Li, J. First Molecular Confirmation of Equine Ocular Setaria digitata in China. Vet. Sci. 2021, 8, 55. https://doi.org/10.3390/vetsci8040055
Yu F, Liu B, Chen S, Yi Z, Liu X, Zhu Y, Li J. First Molecular Confirmation of Equine Ocular Setaria digitata in China. Veterinary Sciences. 2021; 8(4):55. https://doi.org/10.3390/vetsci8040055
Chicago/Turabian StyleYu, Feng, Bo Liu, Shulei Chen, Ziwen Yi, Xianyong Liu, Yiping Zhu, and Jing Li. 2021. "First Molecular Confirmation of Equine Ocular Setaria digitata in China" Veterinary Sciences 8, no. 4: 55. https://doi.org/10.3390/vetsci8040055
APA StyleYu, F., Liu, B., Chen, S., Yi, Z., Liu, X., Zhu, Y., & Li, J. (2021). First Molecular Confirmation of Equine Ocular Setaria digitata in China. Veterinary Sciences, 8(4), 55. https://doi.org/10.3390/vetsci8040055







