Description of Plasma Penicillin G Concentrations after Intramuscular Injection in Double-Muscled Cows to Optimize the Timing of Antibiotherapy for Caesarean Section
Abstract
:1. Introduction
2. Materials and Methods
3. Results
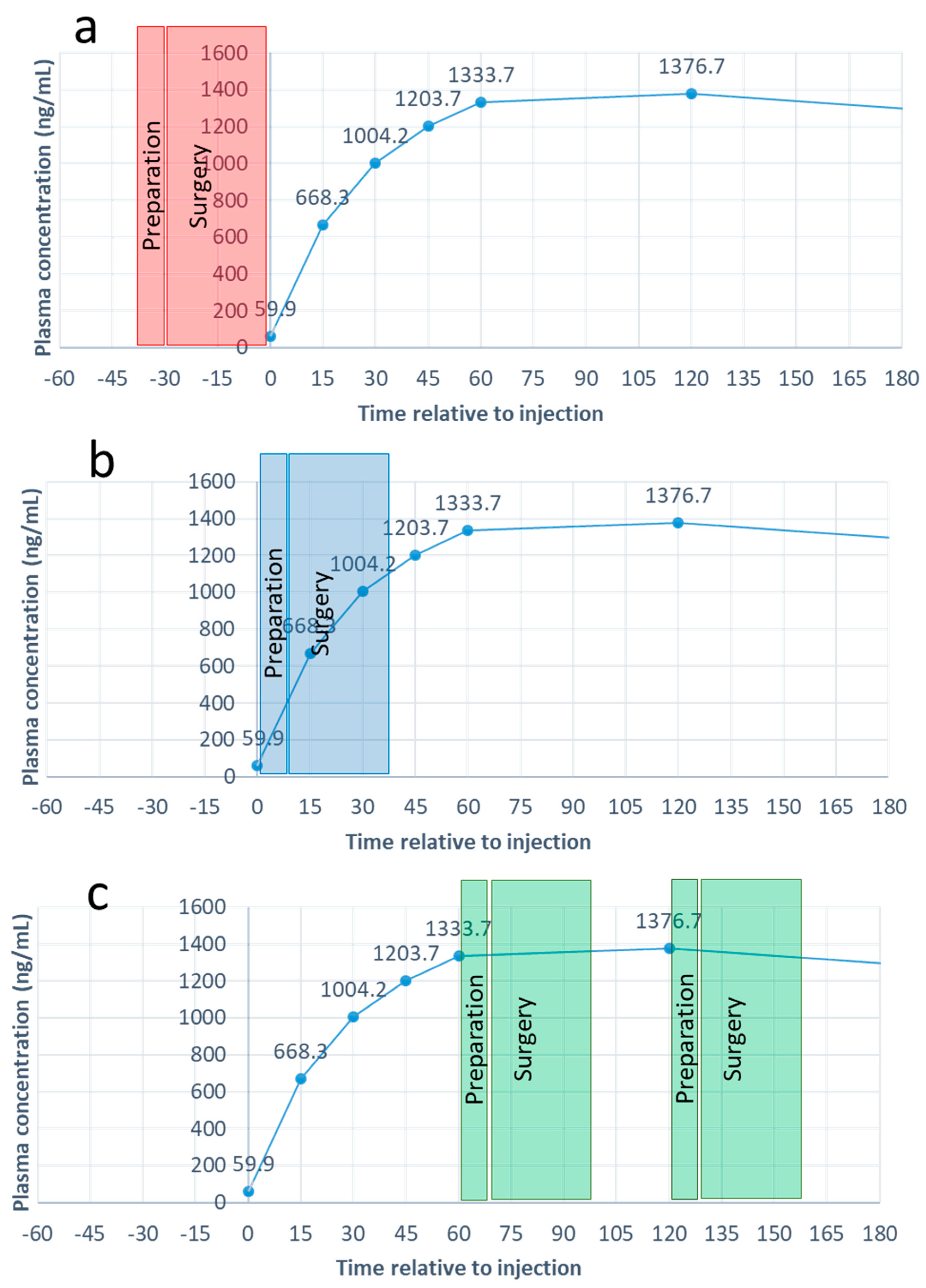
3.1. Plasma Concentrations of Penicillin G
3.2. Duration of Surgery Preparation and Realisation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Braun, U. Ultrasound as a decision-making tool in abdominal surgery in cows. Vet. Clin. Food Anim. Pract. 2005, 21, 33–53. [Google Scholar] [CrossRef] [PubMed]
- Hardefeldt, L.Y.; Browning, G.F.; Thursky, K.; Gilkerson, J.R.; Billman-Jacobe, H.; Stevenson, M.; Bailey, K. Cross-sectional study of antimicrobials used for surgical prophylaxis by bovine veterinary practitioners in Australia. Vet. Rec. 2017, 181, 426. [Google Scholar] [CrossRef]
- Hanzen, C.; Lourtie, O.; Ectors, F. La césarienne dans l’espèce bovine. Ann. Méd. Vét. 1999, 143, 65–90. [Google Scholar]
- Lyons, N.; Karvountzis, S.; Knight-Jones, T. Aspects of bovine caesarean section associated with calf mortality, dam survival and subsequent fertility. Vet. J. 2013, 197, 342–350. [Google Scholar] [CrossRef] [PubMed]
- Ajeel, A.A.; Mezeal, F.A.; khiad, A.J.; Abbidan, N.A. Caesarean section in ruminants referred to the Al-Muthanna Veterinary Hospital. MRVSA 2020, 8, 11–22. [Google Scholar]
- Djebala, S.; Moula, N.; Bayrou, C.; Sartelet, A.; Bossaert, P. Prophylactic antibiotic usage by Belgian veterinarians during elective caesarean section in Belgian blue cattle. Prev. Vet. Med. 2019, 172, 104785. [Google Scholar] [CrossRef] [PubMed]
- Herd Book Blanc Bleu Belge (HBBB). Caractéristiques. 2020. Available online: https://www.hbbbb.be/fr/pages/caracteristique (accessed on 1 January 2021).
- Mijten, P.; Bogaard, A.V.D.; Hazen, M.; De Kruif, A. Bacterial contamination of fetal fluids at the time of cesarean section in the cow. Theriogenology 1997, 48, 513–521. [Google Scholar] [CrossRef]
- Hanzen, C.; Théron, L.; Detilleux, J. Réalisation de la césarienne dans l’espèce bovine en Europe: L’intervention et ses conséquences. Bull. GTV 2011, 62, 61–72. [Google Scholar]
- Dumas, S.E.; French, H.M.; Lavergne, S.N.; Ramirez, C.R.; Brown, L.J.; Bromfield, C.R.; Garrett, E.F.; French, D.D.; Aldridge, B.M. Judicious use of prophylactic antimicrobials to reduce abdominal surgical site infections in periparturient cows: Part 1—A risk factor review. Vet. Rec. 2016, 178, 654–660. [Google Scholar] [CrossRef]
- Credille, B.; Woolums, A.; Giguère, S.; Robertson, T.; Overton, M.; Hurley, D. Prevalence of Bacteremia in Dairy Cattle with Acute Puerperal Metritis. J. Vet. Intern. Med. 2014, 28, 1606–1612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mijten, P. Puerperal Complications after Cesarean Section in Dairy Cows and in Double-Muscled Cows. Reprod. Domest. Anim. 1998, 33, 175–179. [Google Scholar] [CrossRef]
- Djebala, S.; Evrard, J.; Moula, N.; Gille, L.; Bayrou, C.; Eppe, J.; Casalta, H.; Sartelet, A.; Bossaert, P. Comparison between generalised peritonitis and parietal fibrinous peritonitis in cows after caesarean section. Vet. Rec. 2020, 187, 105867. [Google Scholar] [CrossRef] [PubMed]
- Ferraro, S.; DesRochers, A.; Nichols, S.; Francoz, D.; Babkine, M.; Lardé, H.; Roy, J.-P.; Fecteau, G. Clinical characteristics, treatment, and outcome for cattle that developed retroperitoneal abscesses following paralumbar fossa laparotomy: 32 cases (1995–2017). J. Am. Vet. Med. Assoc. 2020, 256, 814–821. [Google Scholar] [CrossRef] [PubMed]
- Djebala, S.; Evrard, J.; Gregoire, F.; Thiry, D.; Bayrou, C.; Moula, N.; Sartelet, A.; Bossaert, P. Infectious Agents Identified by Real-Time PCR, Serology and Bacteriology in Blood and Peritoneal Exudate Samples of Cows Affected by Parietal Fibrinous Peritonitis after Caesarean Section. Vet. Sci. 2020, 7, 134. [Google Scholar] [CrossRef] [PubMed]
- Classen, D.C.; Evans, R.S.; Pestotnik, S.L.; Horn, S.D.; Menlove, R.L.; Burke, J.P. The Timing of Prophylactic Administration of Antibiotics and the Risk of Surgical-Wound Infection. N. Engl. J. Med. 1992, 326, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Baaqeel, H.; Baaqeel, R. Timing of administration of prophylactic antibiotics for caesarean section: A systematic review and meta-analysis. BJOG Int. J. Obstet. Gynaecol. 2012, 120, 661–669. [Google Scholar] [CrossRef] [Green Version]
- Kalaichelvan, V.; Marickar, Y. Cesarean Section and Prophylactic Antibiotics. IOSR J. Pharm. Biol. Sci. 2014, 9, 51–54. [Google Scholar] [CrossRef]
- Bollig, C.; Nothacker, M.; Lehane, C.; Motschall, E.; Lang, B.; Meerpohl, J.J.; Schmucker, C.M. Prophylactic antibiotics before cord clamping in cesarean delivery: A systematic review. Acta Obstet. Gynecol. Scand. 2017, 97, 521–535. [Google Scholar] [CrossRef] [Green Version]
- Antimicrobial Consumption and Resistance in Animals (AMCRA), 2020. Traitement Antibactérien Péri-Opératoire. Available online: https://formularium.amcra.be/i/79 (accessed on 5 January 2021).
- Ziv, G.; Wanner, M.; Nicolet, J. Distribution of penicillin G, dihydrostreptomycin, oxytetracycline, and chloramphenicol in serum and subcutaneous chamber fluid. J. Vet. Pharmacol. Ther. 1982, 5, 59–69. [Google Scholar] [CrossRef]
- Papich, M.G.; Korsrud, G.O.; Boison, J.O.; Yates, W.D.G.; MacNeil, J.D.; Janzen, E.D.; Cohen, R.D.H.; Landry, D.A. A study of the disposition of procaine penicillin G in feedlot steers following intramuscular and subcutaneous injection. J. Vet. Pharmacol. Ther. 1993, 16, 317–327. [Google Scholar] [CrossRef]
- Conlon, P.D.; Butler, D.G.; Burger, J.P.; Gervais, M.D. Evaluation of route and frequency of administration of three antimicrobial drugs in cattle. Can. Vet. J. 1993, 34, 606–610. [Google Scholar] [PubMed]
- Dubreuil, P.; Daigneault, J.; Couture, Y.; Guay, P.; Landry, D. Penicillin concentrations in serum, milk, and urine following intramuscular and subcutaneous administration of increasing doses of procaine penicillin G in lactating dairy cows. Can. J. Vet. Res. 2001, 65, 173–180. [Google Scholar] [PubMed]
- Greenblatt, D.J. Elimination Half-Life of Drugs: Value and Limitations. Annu. Rev. Med. 1985, 36, 421–427. [Google Scholar] [CrossRef]
- Toutain, P.; del Castillo, J.; Bousquet-Mélou, A. The pharmacokinetic–pharmacodynamic approach to a rational dosage regimen for antibiotics. Res. Vet. Sci. 2002, 73, 105–114. [Google Scholar] [CrossRef]
- Klein, W.R.; Firth, E.C.; Kievits, J.M.C.A.; De Jager, J.C. Intra-abdominal versus intramuscular application of two ampicillin preparations in cows. J. Vet. Pharmacol. Ther. 1989, 12, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Europeans Commission. Commission Decision of 14 August 2002 Implementing Council Directive 96/23/EC Concerning the Performance of Analytical Methods and the Interpretation of Results, January 2004 (2002/657/EC). Available online: https://ec.europa.eu/food/sites/food/files/safety/docs/cs_vetmed (accessed on 11 January 2021).
- European Medicines Agency. Science Medicine Health. VICH Topic GL49: Studies to Evaluate the Metabolism and Residues Kinetics of Veterinary Drugs in Human Food-Producing Animals: Validation of Analytical Methods Used in Residue Depletion Studies-EMEA/CVMP/VICH/463202/2009, January 2016. Available online: https://www.ema.europa.eu/en/vich-gl49-studies-evaluate-metabolism-residue-kinetics-veterinary-drugs-food-producing-animals (accessed on 1 January 2021).
- Prescott, J.F. Beta-lactam Antibiotics: Penam Penicillins. In Antimicrobial Therapy in Veterinary Medicine, 5th ed.; Giguère, G., Prescott, J.F., Dowling, P.M., Eds.; Wiley: Chichester, UK, 2013; pp. 135–152. [Google Scholar]
- Chantziaras, I.; Boyen, F.; Callens, B.; Dewulf, J. Correlation between veterinary antimicrobial use and antimicrobial resistance in food-producing animals: A report on seven countries. J. Antimicrob. Chemother. 2014, 69, 827–834. [Google Scholar] [CrossRef] [Green Version]
- Callens, B.; Cargnel, M.; Sarrazin, S.; Dewulf, J.; Hoet, B.; Vermeersch, K.; Wattiau, P.; Welby, S. Associations between a decreased veterinary antimicrobial use and resistance in commensal Escherichia coli from Belgian livestock species (2011–2015). Prev. Vet. Med. 2018, 157, 50–58. [Google Scholar] [CrossRef] [Green Version]
- Prescott, J.F.; Gannon, V.P.; Kittler, G.; Hlywka, G. Antimicrobial Drug Susceptibility of Bacteria Isolated from Disease Processes in Cattle, Horses, Dogs and Cats. Can. Vet. J. 1984, 25, 289–292. [Google Scholar]
- Gustin, P. Antibactériens. In Répertoire Commenté des Médicaments à Usage Vétérinaire; Gustin, P., Ed.; Centre Belge d’Information Pharmacothérapeutique CBIP-Vétérinaire: Bruxelles, Belgium, 2017; pp. 1–46. [Google Scholar]
- Auckenthaler, R. Pharmacokinetics and pharmacodynamics of oral -lactam antibiotics as a two-dimensional approach to their efficacy. J. Antimicrob. Chemother. 2002, 50, 13–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McKellar, Q.; Bruni, S.F.S.; Jones, D.G. Pharmacokinetic/pharmacodynamic relationships of antimicrobial drugs used in veterinary medicine. J. Vet. Pharmacol. Ther. 2004, 27, 503–514. [Google Scholar] [CrossRef]
- Bratzler, D.W.; Houck, P.M. Antimicrobial prophylaxis for surgery: An advisory statement from the National Surgical Infection Prevention Project. Am. J. Surg. 2005, 189, 395–404. [Google Scholar] [CrossRef] [PubMed]
- Association Générale de l’Industrie du Médicament. Propriétés Pharmacologiques. Available online: https://www.e-compendium.be/fr/notices/scientifique/4566 (accessed on 1 January 2021).
- Mansfield, M.A.; Jones, A.D.; Kuldau, G.A. Contamination of Fresh and Ensiled Maize by Multiple Penicillium Mycotoxins. Phytopathology 2008, 98, 330–336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- European Committee on Antimicrobial Susceptibility Testing (EUCAST). Antimicrobial Wild Type Distributions of Microorganisms. Available online: https://mic.eucast.org/Eucast2/SearchController/search.jsp?action=performSearch&BeginIndex=0&Micdif=mic&NumberIndex=50&Antib=43&Specium=-1 (accessed on 1 January 2021).

| Sample | Time (minute) | Cow 1 (ng/mL) | Cow 2 (ng/mL) | Cow 3 (ng/mL) | Cow 4 (ng/mL) | Cow 5 (ng/mL) | Cow 6 (ng/mL) | Cow 7 (ng/mL) | Cow 8 (ng/mL) | Cow 9 (ng/mL) | Cow 10 (ng/mL) | Cow 11 (ng/mL) | Cow 12 (ng/mL) | Mean ± SE (ng/mL) | Mean ± SD (ng/mL) [23] |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 0 | 4.1 | 62.1 | 17.7 | 33.7 | 3.7 | 13.3 | 70.7 | 39.2 | 291.9 | 75.3 | 22.3 | 85.3 | 59.9 ± 22.6 a | ND |
| 2 | 15 | 466.1 | 759.1 | 644.6 | 373.9 | 462.8 | 501.4 | 572.8 | 405.6 | 1226.9 | 898.0 | 846.4 | 862.4 | 668.3 ± 73.7 b | 820 ± 510 |
| 3 | 30 | 826.0 | 1622.0 | 868.7 | 668.6 | 662.7 | 823.8 | 633.4 | 772.1 | 1790.5 | 1187.9 | 1079.7 | 1115.3 | 1004.2 ± 108.5 c | 880 ± 440 |
| 4 | 45 | 947.5 | 1788.7 | 1132.4 | 871.8 | 823.3 | 1062.2 | 672.0 | 716.2 | 2438.5 | 1275.5 | 1478.6 | 1237.1 | 1203.6 ± 146.0 cd | 800 ± 400 |
| 5 | 60 | 982.1 | 2252.5 | 1181.6 | 889.3 | 970.4 | 1189.4 | 744.4 | 931.6 | 2587.2 | 1427.9 | 1453.3 | 1394.7 | 1333.7 ± 161.6 d | 740 ± 100 |
| 6 | 120 | 884.5 | 2882.5 | 1173.5 | 840.4 | 1159.3 | 1375.3 | 857.2 | 1014.3 | 2241.0 | 1370.1 | 1459.5 | 1262.8 | 1376.7 ± 175.2 d | 770 ± 350 |
| 7 | 240 | 966.0 | 1596.4 | 1035.0 | 918.9 | 1213.7 | 1180.5 | 1087.9 | 843.4 | 1300.3 | 1660.1 | 1502.5 | 1300.7 | 1217.1 ± 76.7 d | 740 ± 420 |
| 8 | 480 | 769.2 | 1360.8 | 667.8 | 840.2 | 1061.4 | 940.4 | 880.2 | 705.1 | 668.7 | 1442.1 | 1642.6 | 1046.4 | 1002.1 ± 93.2 ce | 850 ± 180 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Djebala, S.; Croubels, S.; Cherlet, M.; Martinelle, L.; Thiry, D.; Moula, N.; Sartelet, A.; Bossaert, P. Description of Plasma Penicillin G Concentrations after Intramuscular Injection in Double-Muscled Cows to Optimize the Timing of Antibiotherapy for Caesarean Section. Vet. Sci. 2021, 8, 67. https://doi.org/10.3390/vetsci8050067
Djebala S, Croubels S, Cherlet M, Martinelle L, Thiry D, Moula N, Sartelet A, Bossaert P. Description of Plasma Penicillin G Concentrations after Intramuscular Injection in Double-Muscled Cows to Optimize the Timing of Antibiotherapy for Caesarean Section. Veterinary Sciences. 2021; 8(5):67. https://doi.org/10.3390/vetsci8050067
Chicago/Turabian StyleDjebala, Salem, Siska Croubels, Marc Cherlet, Ludovic Martinelle, Damien Thiry, Nassim Moula, Arnaud Sartelet, and Philippe Bossaert. 2021. "Description of Plasma Penicillin G Concentrations after Intramuscular Injection in Double-Muscled Cows to Optimize the Timing of Antibiotherapy for Caesarean Section" Veterinary Sciences 8, no. 5: 67. https://doi.org/10.3390/vetsci8050067
APA StyleDjebala, S., Croubels, S., Cherlet, M., Martinelle, L., Thiry, D., Moula, N., Sartelet, A., & Bossaert, P. (2021). Description of Plasma Penicillin G Concentrations after Intramuscular Injection in Double-Muscled Cows to Optimize the Timing of Antibiotherapy for Caesarean Section. Veterinary Sciences, 8(5), 67. https://doi.org/10.3390/vetsci8050067






