Monitoring of Organochlorine Pesticide and Polychlorinated Biphenyl Residues in Common Swifts (Apus apus) in the Region of Hannover, Lower Saxony, Germany
Abstract
:1. Introduction
2. Materials and Methods
2.1. Birds and Ethical Statement
2.2. Samples
2.3. Chemical Analysis
2.4. Statistical Analysis
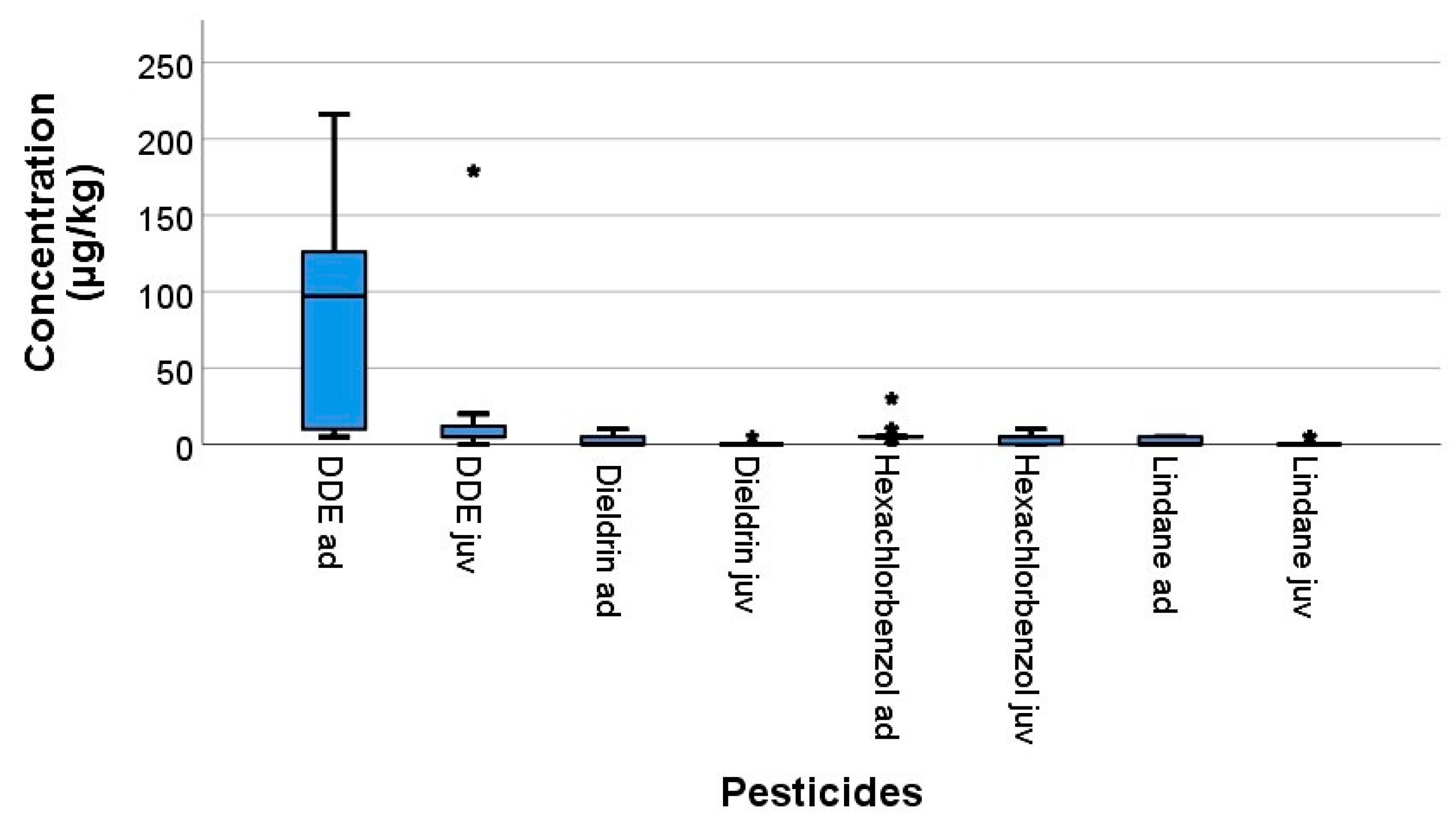
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Both, C.; Bouwhuis, S.; Lessells, C.; Visser, M.E. Climate change and population declines in a long-distance migratory bird. Nature 2006, 441, 81–83. [Google Scholar] [CrossRef] [Green Version]
- Henny, C.J.; Ward, F.P.; Riddle, K.; Prouty, R.M. Migratory peregrine falcons, Falco peregrinus, accumulate pesticides in Latin America during winter. Can. Field-Nat. 1982, 96, 333–338. [Google Scholar]
- Henny, C.J.; Kaiser, J.L.; Grove, R.A.; Johnson, B.L.; Letcher, R.J. Polybrominated diphenyl ether flame retardants in eggs may reduce reproductive success of ospreys in Oregon and Washington, USA. Ecotoxicology 2009, 18, 802–813. [Google Scholar] [CrossRef] [PubMed]
- DeGraaf, R.M.; Rappole, J.H. Neotropical Migratory Birds: Natural History, Distribution, and Population Change; Cornell University Press: London, UK, 1995. [Google Scholar]
- Chen, D.; Hale, R.C. A global review of polybrominated diphenyl ether flame retardant contamination in birds. Environ. Int. 2010, 36, 800–811. [Google Scholar] [CrossRef] [PubMed]
- Fry, D.M. Reproductive effects in birds exposed to pesticides and industrial chemicals. Environ. Health Perspect. 1995, 103, 165–171. [Google Scholar] [PubMed]
- Jones, K.C.; De Voogt, P. Persistent organic pollutants (POPs): State of the science. Environ. Pollut. 1999, 100, 209–221. [Google Scholar] [CrossRef]
- Kunisue, T.; Watanabe, M.; Subramanian, A.; Sethuraman, A.; Titenko, A.M.; Qui, V.; Prudente, M.; Tanabe, S. Accumulation features of persistent organochlorines in resident and migratory birds from Asia. Environ. Pollut. 2003, 125, 157–172. [Google Scholar] [CrossRef]
- Peakall, D.B. DDE-induced eggshell thinning: An environmental detective story. Environ. Rev. 1993, 1, 13–20. [Google Scholar] [CrossRef]
- Buck, A.; Carrillo-Hidalgo, J.; Camarero, P.R.; Mateo, R. Organochlorine pesticides and polychlorinated biphenyls in common kestrel eggs from the Canary Islands: Spatiotemporal variations and effects on eggshell and reproduction. Chemosphere 2020, 261, 127722. [Google Scholar] [CrossRef]
- Beyer, W.N.; Meador, J.P. Environmental Contaminants in Biota: Interpreting Tissue Concentrations; CRC Press: Boca Raton, FL, USA, 2011; pp. 425–516. [Google Scholar]
- Bustnes, J.O.; Bardsen, B.J.; Moe, B.; Herzke, D.; Hanssen, S.A.; Sagerup, K.; Bech, C.; Nordstad, T.; Chastel, O.; Tartu, S.; et al. Temporal variation in circulating concentrations of organochlorine pollutants in a pelagic seabird breeding in the high Arctic. Environ. Toxicol. Chem. 2017, 36, 442–448. [Google Scholar] [CrossRef] [Green Version]
- Colabuono, F.I.; Taniguchi, S.; Montone, R.C. Organochlorine contaminants in albatrosses and petrels during migration in South Atlantic Ocean. Chemosphere 2012, 86, 701–708. [Google Scholar] [CrossRef] [PubMed]
- WHO. World Malaria Report 2018; World Health Organization: Geneva, Switzerland, 2018; pp. 16–21. [Google Scholar]
- WHO. World Malaria Report 2019; World Health Organization: Geneva, Switzerland, 2019; pp. 46–51. [Google Scholar]
- Mochungong, P.; Zhu, J. DDTs, PCBs and PBDEs contamination in Africa, Latin America and south-southeast Asia—A review. AIMS Environ. Sci. 2015, 2, 374–399. [Google Scholar] [CrossRef]
- Barbash, J.; Resek, E. Pesticides in Ground Water: Current Understanding of Distribution and Major Influences; U.S. Geological Survey: Reston, VA, USA, 1996; pp. 1–4.
- Åkesson, S.; Klaassen, R.; Holmgren, J.; Fox, J.W.; Hedenström, A. Migration routes and strategies in a highly aerial migrant, the common swift Apus apus, revealed by light-level geolocators. PLoS ONE 2012, 7, e41195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wellbrock, A.H.J.; Bauch, C.; Rozman, J.; Witte, K. ‘Same procedure as last year?‘ Repeatedly tracked swifts show individual consistency in migration pattern in successive years. J. Avian. Biol. 2017, 48, 897–903. [Google Scholar] [CrossRef]
- Hedenström, A.; Norevik, G.; Warfvinge, K.; Andersson, A.; Bäckman, J.; Åkesson, S. Annual 10-Month Aerial Life Phase in the Common Swift Apus apus. Curr. Biol. 2016, 26, 3066–3070. [Google Scholar] [CrossRef] [Green Version]
- Weitnauer, E.; Scherner, E.R. Mauersegler. In Handbuch der Vögel Mitteleuropas; Glutz von Blotzheim, U.N., Bauer, K.M., Eds.; Akademische Verlagsgesellschaft: Wiesbaden, Germany, 1980; Volume 9, pp. 669–712. [Google Scholar]
- NABU-Bundesverband. 15 Jahre Vogelzählung und Citizen Science im NABU, Ergebnisse der “Stunde der Gartenvögel” und der “Stunde der Wintervögel”; Eversfrank: Berlin, Germany, 2019; pp. 23–41. [Google Scholar]
- Miniero, R.; Carere, C.; De, E.F.; Iacovella, N.; Rodriguez, F.; Alleva, E.; Di, A.D. The use of common swift (Apus apus), an aerial feeder bird, as a bioindicator of persistent organic microcontaminants. Ann. Ist. Super. Sanita 2008, 44, 187–194. [Google Scholar] [PubMed]
- Rodriguez, F.; Carere, C.; Dell’Omo, G.; Iacovella, N.; Turrio-Baldassarri, L.; Volpi, F.; di Domenico, A. The Common Swift (Apus apus): A synanthropic bird species for monitoring airborne contaminants? Organohalogen Compd. 1996, 28, 308–313. [Google Scholar]
- Haupt, C. Mauersegler in Menschenhand: Erste Hilfe—Aufzucht und Pflege—Tierärztliche Versorgung; Deutsche Gesellschaft für Mauersegler e.V.: Frankfurt am Main, Germany, 2001. [Google Scholar]
- Ahrens, R.; Weber, C. DDT und die Stockholmer Konvention—Staaten am Rande der Legalität; Pestizid Aktions-Netzwerk e. V. (PAN Germany): Hamburg, Germany, 2009; pp. 5–39. [Google Scholar]
- Waldron, A.; Naber, E. Importance of feed as an unavoidable source of pesticide contamination in poultry meat and eggs: 2. Residues in eggs and tissues. Poult. Sci. 1974, 53, 1428–1435. [Google Scholar] [CrossRef]
- Stickel, W.H.; Stickel, L.F.; Dyrland, R.A.; Hughes, D.L. DDE in birds: Lethal residues and loss rates. Arch. Environ. Contam. Toxicol. 1984, 13, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Bailey, S.; Bunyan, P.; Rennison, B.; Taylor, A. The metabolism of 1,1-di (p-chlorophenyl)-2,2-dichloroethylene and 1,1-di (p-chlorophenyl)-2-chloroethylene in the pigeon. Toxicol. Appl. Pharmacol. 1969, 14, 23–32. [Google Scholar] [CrossRef]
- Luzardo, O.P.; Ruiz-Suárez, N.; Henríquez-Hernández, L.A.; Valerón, P.F.; Camacho, M.; Zumbado, M.; Boada, L.D. Assessment of the exposure to organochlorine pesticides, PCBs and PAHs in six species of predatory birds of the Canary Islands, Spain. Sci. Total Environ. 2014, 472, 146–153. [Google Scholar] [CrossRef]
- Cifuentes, J.M.; Becker, P.H.; Sommer, U.; Pacheco, P.; Schlatter, R. Seabird eggs as bioindicators of chemical contamination in Chile. Environ. Pollut. 2003, 126, 123–137. [Google Scholar] [CrossRef]
- Mendenhall, V.M.; Klaas, E.E.; McLane, M.A.R. Breeding success of barn owls (Tyto alba) fed low levels of DDE and dieldrin. Arch. Environ. Contam. Toxicol. 1983, 12, 235–240. [Google Scholar] [CrossRef]
- Bartuszevige, A.; Capparella, A.; Harper, R.; Frick, J.; Criley, B.; Doty, K.; Erhart, E. Organochlorine pesticide contamination in grassland-nesting passerines that breed in North America. Environ. Pollut. 2002, 117, 225–232. [Google Scholar] [CrossRef]
- Vos, J.G.; Dybing, E.; Greim, H.A.; Ladefoged, O.; Lambré, C.; Tarazona, J.V.; Brandt, I.; Vethaak, A.D. Health effects of endocrine-disrupting chemicals on wildlife, with special reference to the European situation. Crit. Rev. Toxicol. 2000, 30, 71–133. [Google Scholar] [CrossRef]
- Mnif, W.; Hassine, A.I.H.; Bouaziz, A.; Bartegi, A.; Thomas, O.; Roig, B. Effect of endocrine disruptor pesticides: A review. Int. J. Environ. Res. Public Health 2011, 8, 2265–2303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Newton, I.; Galbraith, E. Organochlorines and mercury in the eggs of golden eagles Aquila chrysaetos from Scotland. Ibis 1991, 133, 115–120. [Google Scholar] [CrossRef]
- Ratcliffe, D.A. Decrease in eggshell weight in certain birds of prey. Nature 1967, 215, 208–210. [Google Scholar] [CrossRef] [PubMed]
- Bustnes, J.; Bakken, V.; Erikstad, K.; Mehlum, F.; Skaare, J. Patterns of incubation and nest-site attentiveness in relation to organochlorine (PCB) contamination in glaucous gulls. J. Appl. Ecol. 2001, 38, 791–801. [Google Scholar] [CrossRef]
- Hoogesteijn, A.L.; DeVoogd, T.J.; Quimby, F.W.; De Caprio, T.; Kollias, G.V. Reproductive impairment in zebra finches (Taeniopygia guttata). Environ. Toxicol. Chem. 2005, 24, 219–223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grzimek, B. Grzimek’s Animal Life Encyclopedia Birds II, 2nd ed.; Michael Hutchins, J.A.J., Bock, W.J., Olendorf, D., Eds.; Gale Group: Farmington Hills, MI, USA, 2002; Volume 9, pp. 429–430. [Google Scholar]
- Sibley, D.; Elphick, C.; Dunning, J.B. The Sibley Guide to Bird Life & Behavior; Alfred A. Knopf: New York, NY, USA, 2001; pp. 353–356. [Google Scholar]
- Poma, G.; Yin, S.; Tang, B.; Fujii, Y.; Cuykx, M.; Covaci, A. Occurrence of selected organic contaminants in edible insects and assessment of their chemical safety. Environ. Health Perspect. 2019, 127, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Maul, J.D.; Belden, J.B.; Schwab, B.A.; Whiles, M.R.; Spears, B.; Farris, J.L.; Lydy, M.J. Bioaccumulation and trophic transfer of polychlorinated biphenyls by aquatic and terrestrial insects to tree swallows (Tachycineta bicolor). Environ. Toxicol. Chem. 2006, 25, 1017–1025. [Google Scholar] [CrossRef] [Green Version]
- Fortmann, H.; Gunreben, M.; Kleefisch, B.; Meesenburg, H.; Meiwes, K.-J.; Merkel, D.; Schneider, J.; Severin, K. Bodenqualitätszielkonzept Niedersachsen. Teil 2: Schwermetalle, Organische Belastungen und Säurebildner; Landesamt für Bergbau, Energie und Geologie (LBEG): Hannover, Germany, 2007; pp. 19–32. [Google Scholar]
- Fortmann, H.; Meesenburg, H. Organische Schadstoffe in Waldböden Niedersachsens: Bodendauerbeobachtung in Niedersachsen; Landesamt für Bergbau, Energie und Geologie (LBEG): Hannover, Germany, 2007; pp. 20–83. [Google Scholar]
- Ballin, U.; Bartelt, E.; Bisenius, S.; Bruns-Weller, E.; Effkemann, S.; Heemken, O.P.; Knoll, A.; Melles, D.; Meyer, L.; Neuhaus, H.; et al. Schadstoffmonitoring in Flussfischen aus niedersächsischen Flussabschnitten (Teil I: Schadstoffe). Niedersächsisches Landesamt Verbrauch. Lebensm. 2020, 4, 1–16. [Google Scholar]
- Legler, M.; Wolken, S.; Epe, C.; Thomas, P.; Kummerfeld, N. Cestodes and intestinal trematodes of Common Swifts (Apus apus) in the area of Hannover (Germany) and the use of Praziquantel for treatment. APUSlife 2011, 4843, 1–10. [Google Scholar]
- Legler, M.; Leonhard, W.; Koch, N.J.; Kummerfeld, N. Mercury concentrations in feathers of Common Swifts (Apus apus). Berl. Muench. Tieraerztl. Wochenschr. 2015, 128, 340–344. [Google Scholar]
- Wentz, D.A.; Brigham, M.E.; Chasar, L.C.; Lutz, M.A.; Krabbenhoft, D.P. Mercury in the Nation’s Streams-Levels, Trends, and Implications; US Geological Survey: Reston, VA, USA, 2014; pp. 2330–5703.
- Speir, S.L.; Chumchal, M.M.; Drenner, R.W.; Cocke, W.G.; Lewis, M.E.; Whitt, H.J. Methyl mercury and stable isotopes of nitrogen reveal that a terrestrial spider has a diet of emergent aquatic insects. Environ. Toxicol. Chem. 2014, 33, 2506–2509. [Google Scholar] [CrossRef] [PubMed]
- Åkesson, S.; Bianco, G.; Hedenström, A. Negotiating an ecological barrier: Crossing the Sahara in relation to winds by common swifts. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2016, 371. [Google Scholar] [CrossRef] [Green Version]
- Tigges, U. The breeding cycle in calendar form of the common swift Apus apus across its eurasian breeding range–a testable hypothesis. Podoces 2006, 1, 27–33. [Google Scholar]
- Driver, D.; Brewer, R.N.; Cottier, G.J. Pesticide residues in eggs and chicks from laying hens fed low levels of several chlorinated hydrocarbon pesticides. Poult. Sci. 1976, 55, 1544–1549. [Google Scholar] [CrossRef]

| List of Analysed Chemicals | Quantification Limits (mg/kg) |
|---|---|
| 2,4′-DDE | 0.005 |
| 2,4′-DDT | 0.005 |
| 2,4′-DDD | 0.005 |
| 4,4′-DDE | 0.005 |
| 4,4′-DDT | 0.005 |
| 4,4′-DDD | 0.005 |
| Alachlor | 0.010 |
| Aldrin | 0.005 |
| Alpha-Endosulfan | 0.005 |
| Beta-Endosulfan | 0.005 |
| Beta-Hexachlorocyclohexane | 0.005 |
| Bromophos-ethyl | 0.010 |
| Bromophos-methyl | 0.010 |
| Bromopropylate | 0.010 |
| Buprofezin | 0.010 |
| Chlorobenzilate | 0.010 |
| Chlorfenson | 0.010 |
| Chloroneb | 0.010 |
| Chlorpyrifos-ethyl | 0.010 |
| Chlorpyrifos-methyl | 0.010 |
| Chlorthal-dimethyl | 0.010 |
| Chlorothalonil | 0.010 |
| Chlorthion | 0.010 |
| Delta-Hexachlorocyclohexane | 0.005 |
| Dicofol | 0.010 |
| Dieldrin | 0.005 |
| Endosulfan sulfate | 0.005 |
| Endrin | 0.005 |
| Epsilon-Hexachlorocyclohexane | 0.010 |
| Fenson | 0.010 |
| Gamma-Chlordane | 0.005 |
| Heptachlor | 0.005 |
| Heptachlorepoxide-cris | 0.005 |
| Heptachlorepoxide-trans | 0.005 |
| Hexachlorobenzene (HCB) | 0.005 |
| Isodrin | 0.005 |
| Jodfenphos | 0.010 |
| Lindane (Gamma-Hexachlorocyclohexane) | 0.005 |
| Methoxychlor | 0.005 |
| Mirex | 0.005 |
| Nitrofen | 0.010 |
| Parathion-ethyl | 0.010 |
| Parathion-methyl | 0.010 |
| Pentachloroaniline | 0.010 |
| PCB IUPAC-Nr.28 | 0.005 |
| PCB IUPAC-Nr.52 | 0.005 |
| PCB IUPAC-Nr.101 | 0.005 |
| PCB IUPAC-Nr.138 | 0.005 |
| PCB IUPAC-Nr.153 | 0.005 |
| PCB IUPAC-Nr.180 | 0.005 |
| Compounds | 2016 | 2017 | 2018 | |||
|---|---|---|---|---|---|---|
| Adult Swifts (n d = 5) | Juvenile Swifts (n d = 5) | Adult Swifts (n d = 5) | Juvenile Swifts (n d = 8) | Adult Swifts (n d = 11) | Juvenile Swifts (n d = 7) | |
| 4,4′-DDE | 10 ± 6 | 6 ± 4 | 59 ± 85 | 7 ± 6 | 126 ± 41 | 34 ± 64 |
| (10; 5–20) | (5; 0–10) | (5; 5–200) | (5; 0–20) | (124; 60–216) | (14; 0–179) | |
| 5/5 | 4/5 | 5/5 | 7/8 | 11/11 | 6/7 | |
| Dieldrin | 2 ± 4.5 | 0.6 ± 2 | 3 ± 3 | |||
| (0; 0–10) | (0; 0–5) | (5; 0–5) | ||||
| 0/5 | 0/5 | 1/5 | 1/8 | 6/11 | 0/7 | |
| HCB | 2 ± 4 | 2 ± 4 | 10 ± 12 | 3 ± 3 | 5 ± 1.5 | 5 ± 0 |
| (0; 0–10) | (0; 0–10) | (5; 0–30) | (5; 0–5) | (5; 5–10) | (5; 5–5) | |
| 1/5 | 1/5 | 4/5 | 5/8 | 11/11 | 7/7 | |
| Lindane | 1 ± 2 | 1 ± 2 | 1 ± 2 | 3 ± 2.5 | ||
| (0; 0–5) | (0; 0–5) | (0; 0–5) | (5; 0–5) | |||
| 0/5 | 1/5 | 1/5 | 2/8 | 7/11 | 0/7 | |
| PCB138 | 5 ± 0 | 10 ± 17 | 37 ± 48 | 28 ± 28 | 18 ± 18 | 17 ± 7 |
| (5; 5–5) | (5; 0–40) | (25; 5–120) | (20; 0–72) | (11; 5–63) | (16; 9–28) | |
| 5/5 | 3/5 | 5/5 | 7/8 | 11/11 | 7/7 | |
| PCB153 | 6 ± 2 | 4 ± 4 | 118 ± 141 | 60 ± 59 | 47 ± 36 | 32 ± 19 |
| (5; 5–10) | (5; 0–10) | (62; 24–360) | (42; 0–167) | (39; 14–134) | (23; 14–67) | |
| 5/5 | 3/5 | 5/5 | 7/8 | 11/11 | 7/7 | |
| PCB180 | 5 ± 0 | 10 ± 17 | 74 ± 90 | 25 ± 25 | 31 ± 17 | 17 ± 17 |
| (5; 5–5) | (5; 0–40) | (47; 10–230) | (15; 0–72) | (28; 8–55) | (11; 5–53) | |
| 5/5 | 3/5 | 5/5 | 7/8 | 11/11 | 7/7 | |
| PCB101 | 10 | |||||
| 0/5 | 0/5 | 0/5 | 1/8 | 0/11 | 0/7 | |
| Compounds | Adult Swifts (n d = 21) | Juvenile Swifts (n d = 20) | pe |
|---|---|---|---|
| 4,4′-DDE | 83 ± 70 (97; 5–216) | 17 ± 39 (5; 0–179) | 0.002 |
| Dieldrin | 2 ± 3 (0; 0–10) | 0.3 ± 1 (0; 0–5) | 0.023 |
| HCB | 6 ± 6 (5; 0–30) | 3 ± 2 (5; 0–10) | 0.086 |
| Lindane | 2 ± 3 (0; 0–5) | 0.8 ± 2 (0; 0–5) | 0.099 |
| PCB138 | 20 ± 30 (9; 5–120) | 20 ± 20 (13; 0–70) | 0.771 |
| PCB153 | 50 ± 80 (30; 5–360) | 40 ± 50 (20; 0–170) | 0.417 |
| PCB180 | 35 ± 50 (19; 5–230) | 18 ± 20 (10; 0–72) | 0.128 |
| PCB101 | - | 10 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tiyawattanaroj, W.; Witte, S.; Fehr, M.; Legler, M. Monitoring of Organochlorine Pesticide and Polychlorinated Biphenyl Residues in Common Swifts (Apus apus) in the Region of Hannover, Lower Saxony, Germany. Vet. Sci. 2021, 8, 87. https://doi.org/10.3390/vetsci8050087
Tiyawattanaroj W, Witte S, Fehr M, Legler M. Monitoring of Organochlorine Pesticide and Polychlorinated Biphenyl Residues in Common Swifts (Apus apus) in the Region of Hannover, Lower Saxony, Germany. Veterinary Sciences. 2021; 8(5):87. https://doi.org/10.3390/vetsci8050087
Chicago/Turabian StyleTiyawattanaroj, Warakorn, Stefan Witte, Michael Fehr, and Marko Legler. 2021. "Monitoring of Organochlorine Pesticide and Polychlorinated Biphenyl Residues in Common Swifts (Apus apus) in the Region of Hannover, Lower Saxony, Germany" Veterinary Sciences 8, no. 5: 87. https://doi.org/10.3390/vetsci8050087





