Effects of Lactobacillus rhamnosus and Enterococcus faecalis Supplementation as Direct-Fed Microbials on Rumen Microbiota of Boer and Speckled Goat Breeds
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals, Treatments and Sampling
2.2. DNA Extraction, PCR Amplification and MiSeq Sequencing
2.3. Data Analysis
3. Results
3.1. OTU Clustering and Taxonomic Annotation of the Goat Rumen Microbiome
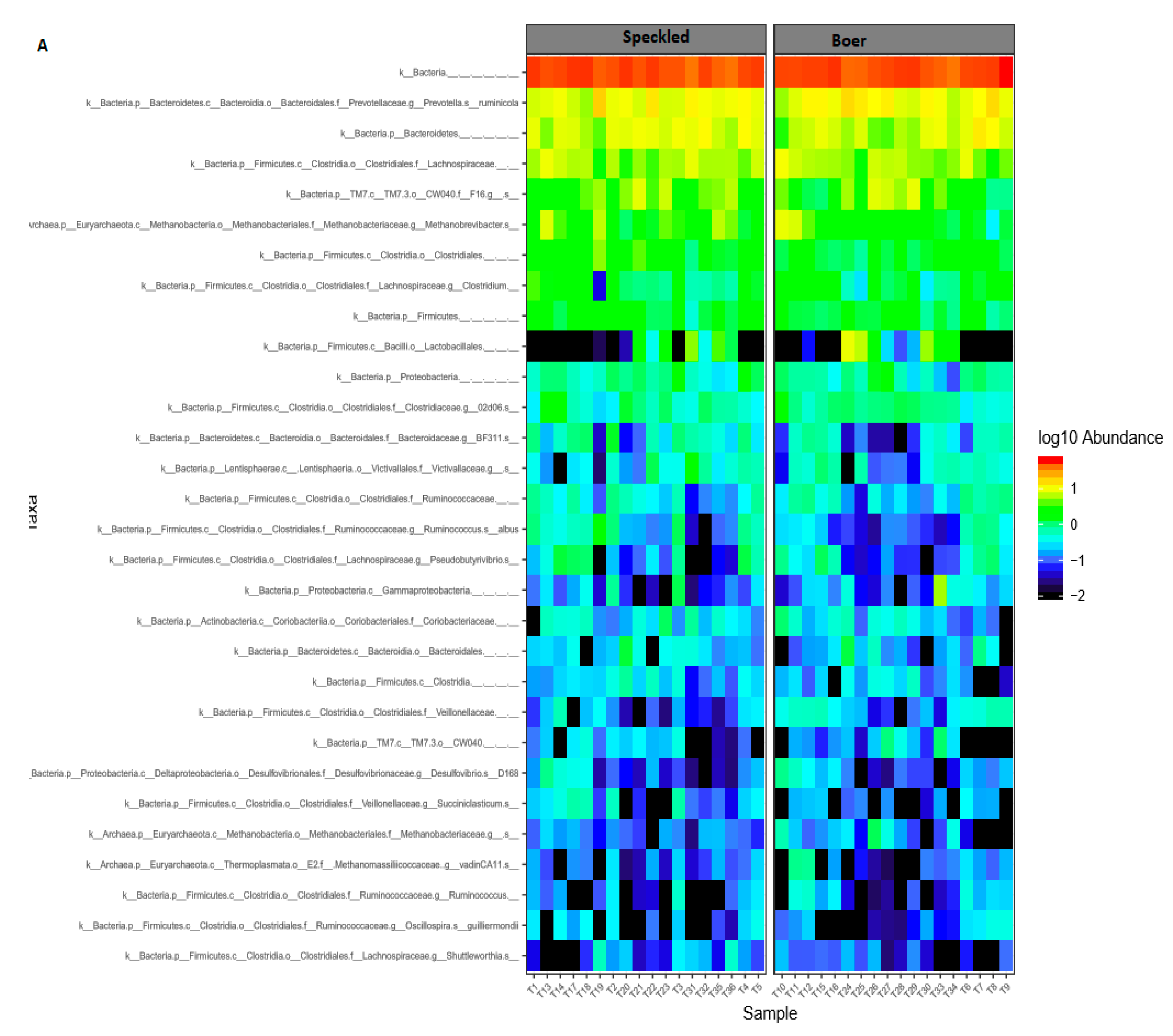
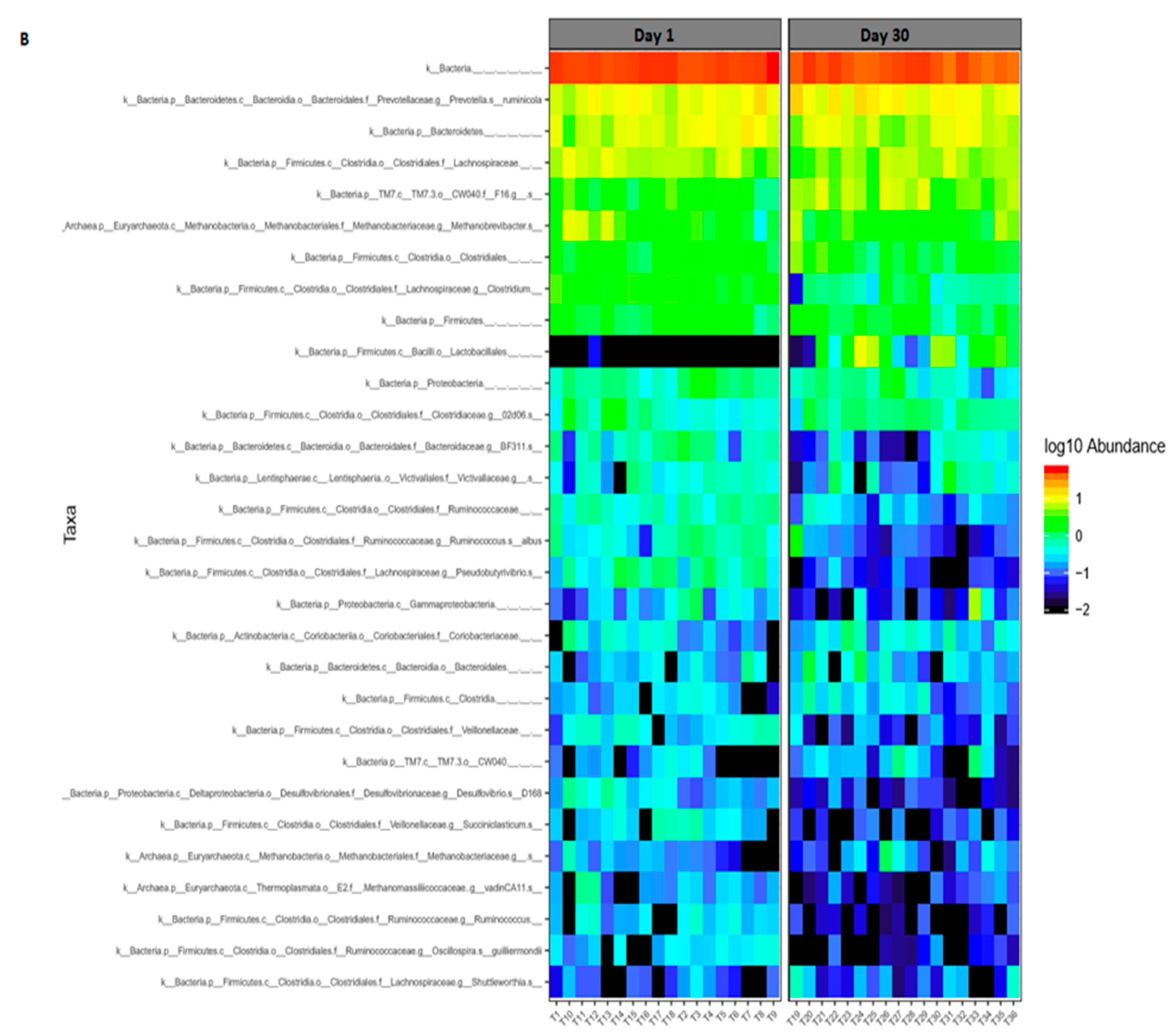
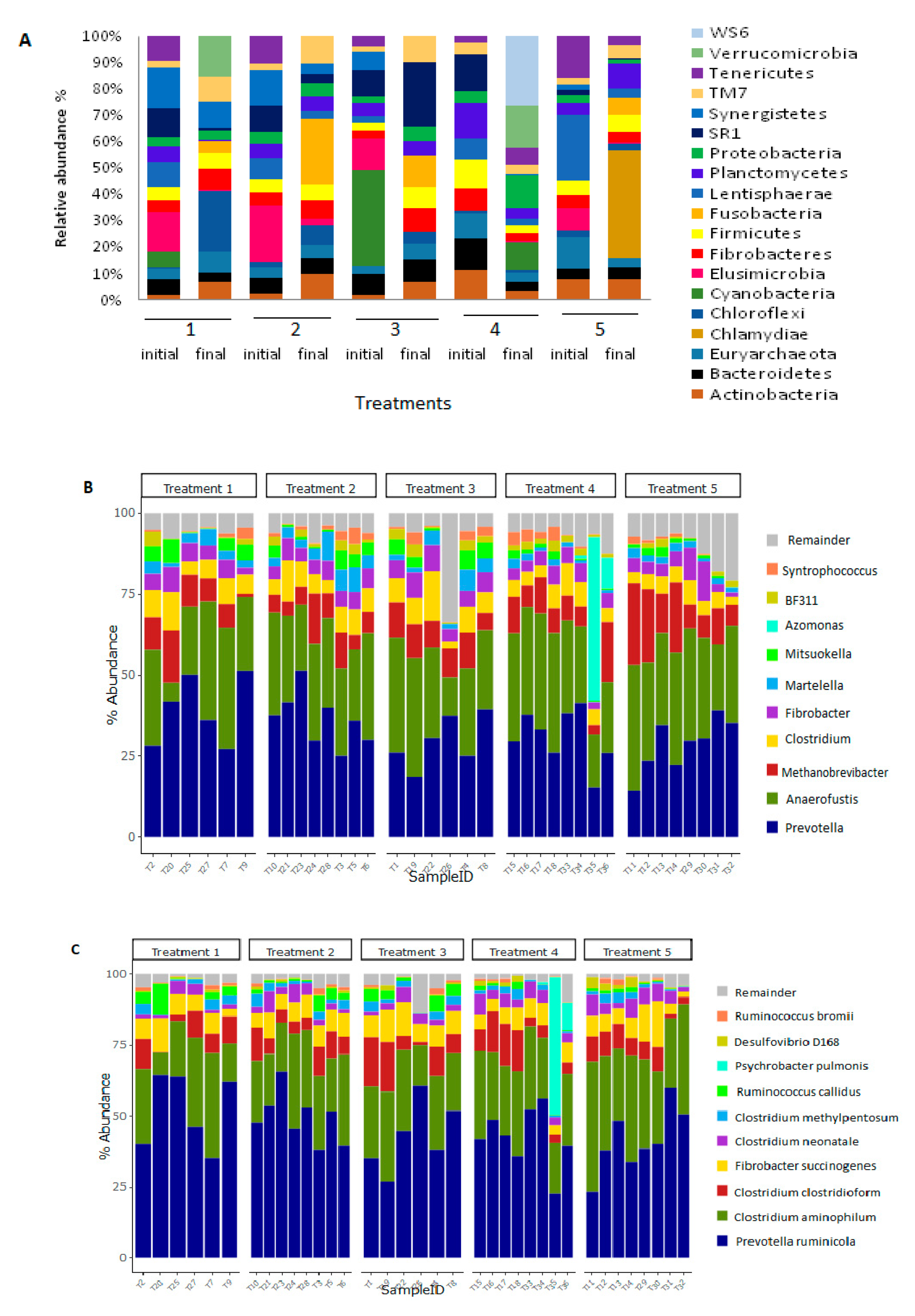
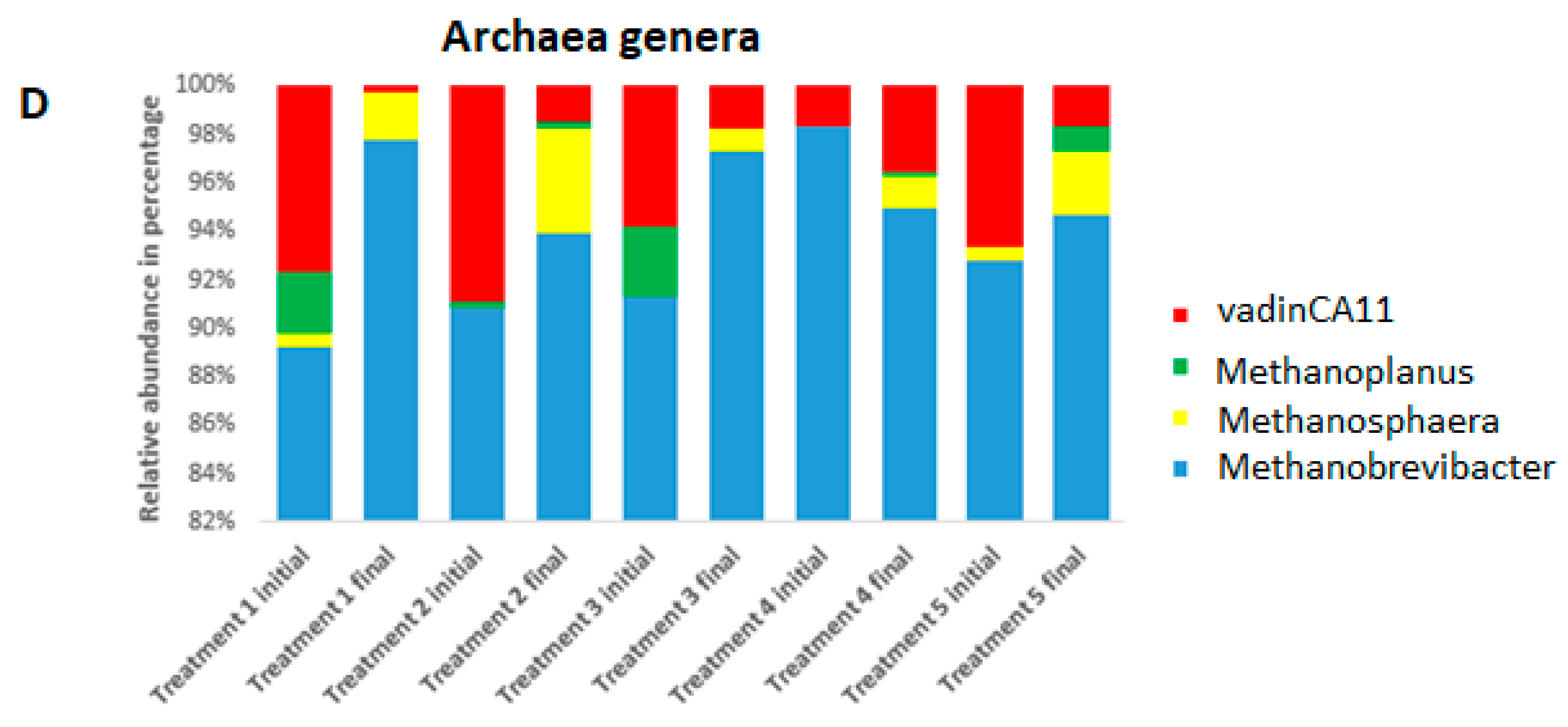
3.2. Bacterial and Archaeal Composition
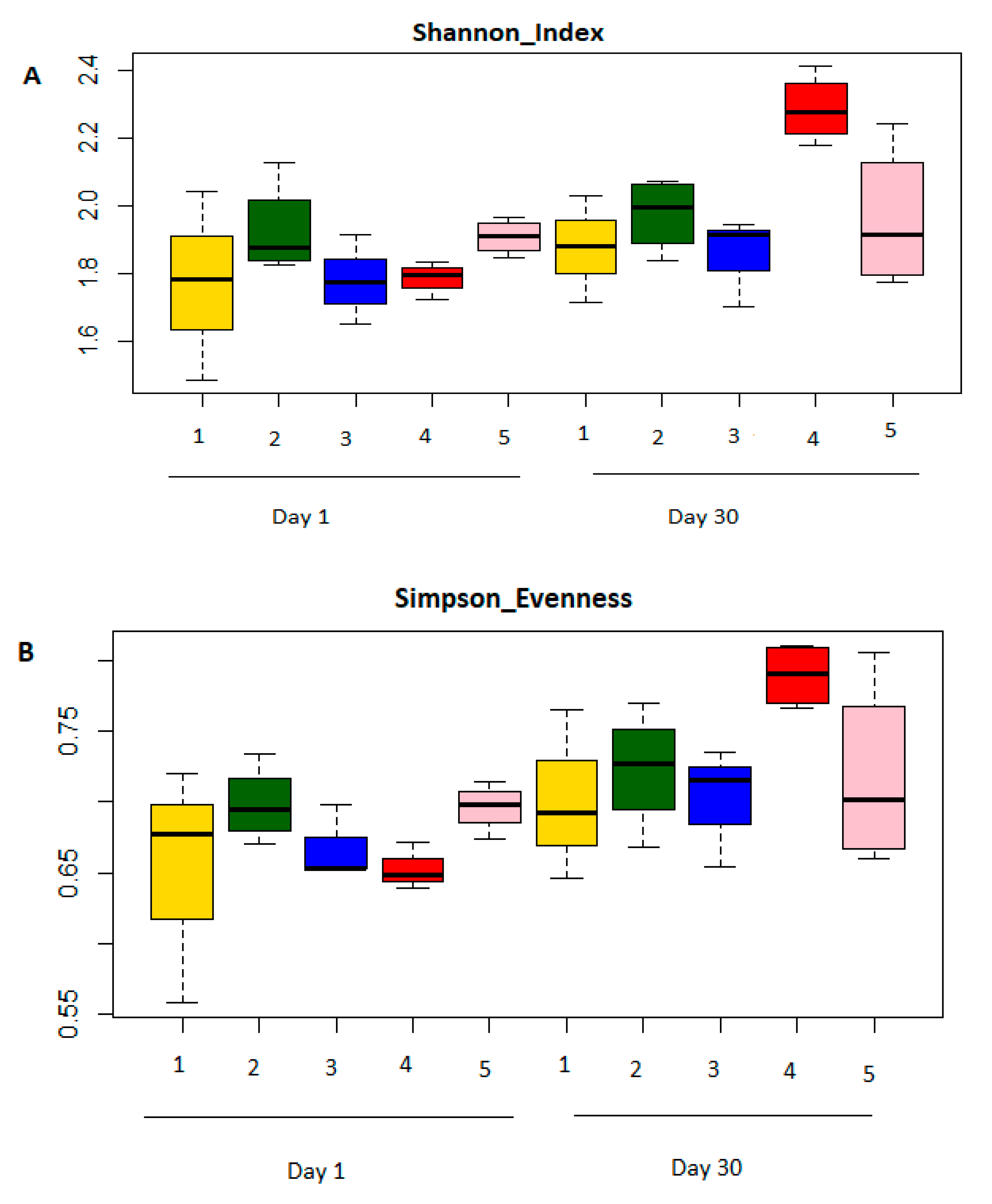
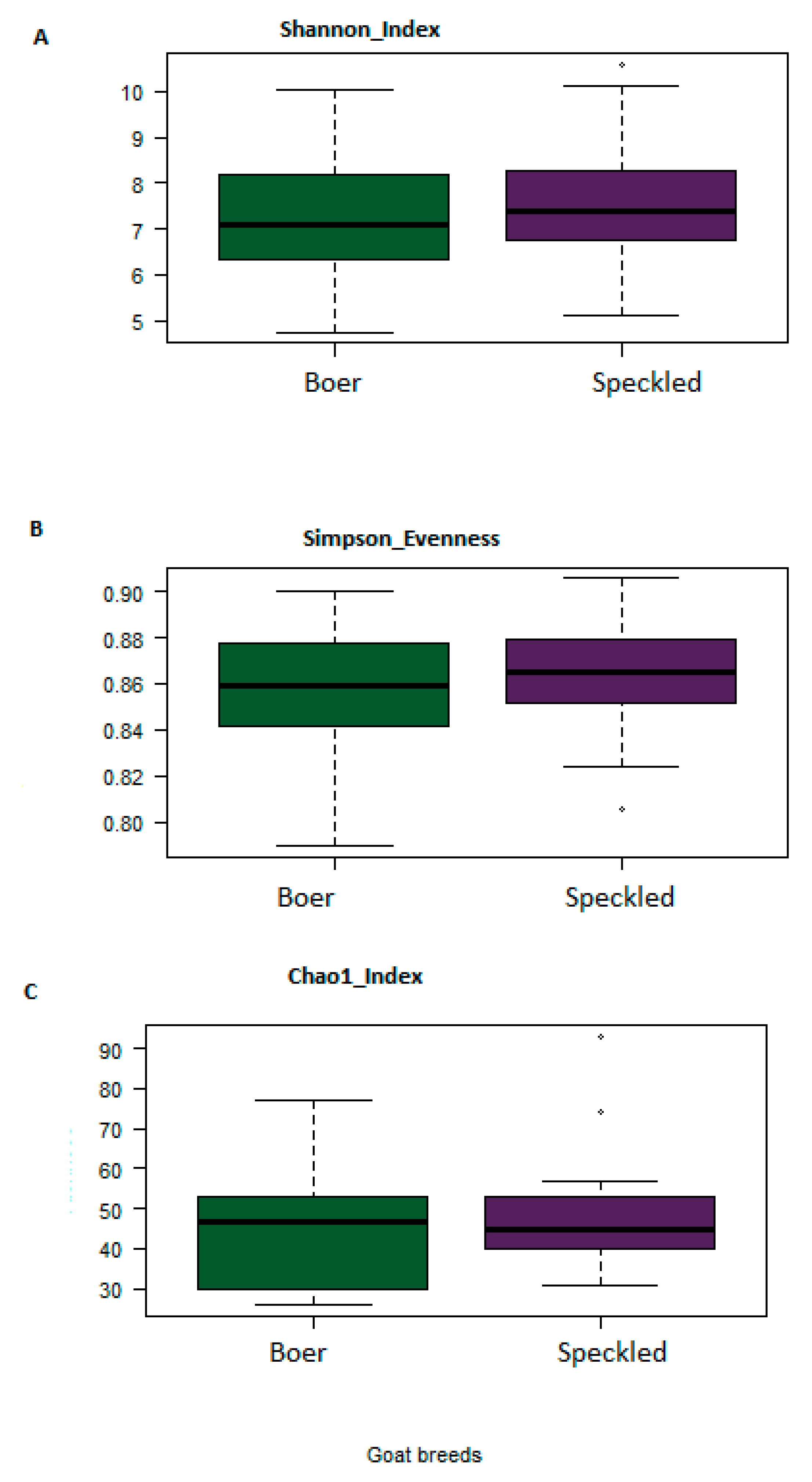
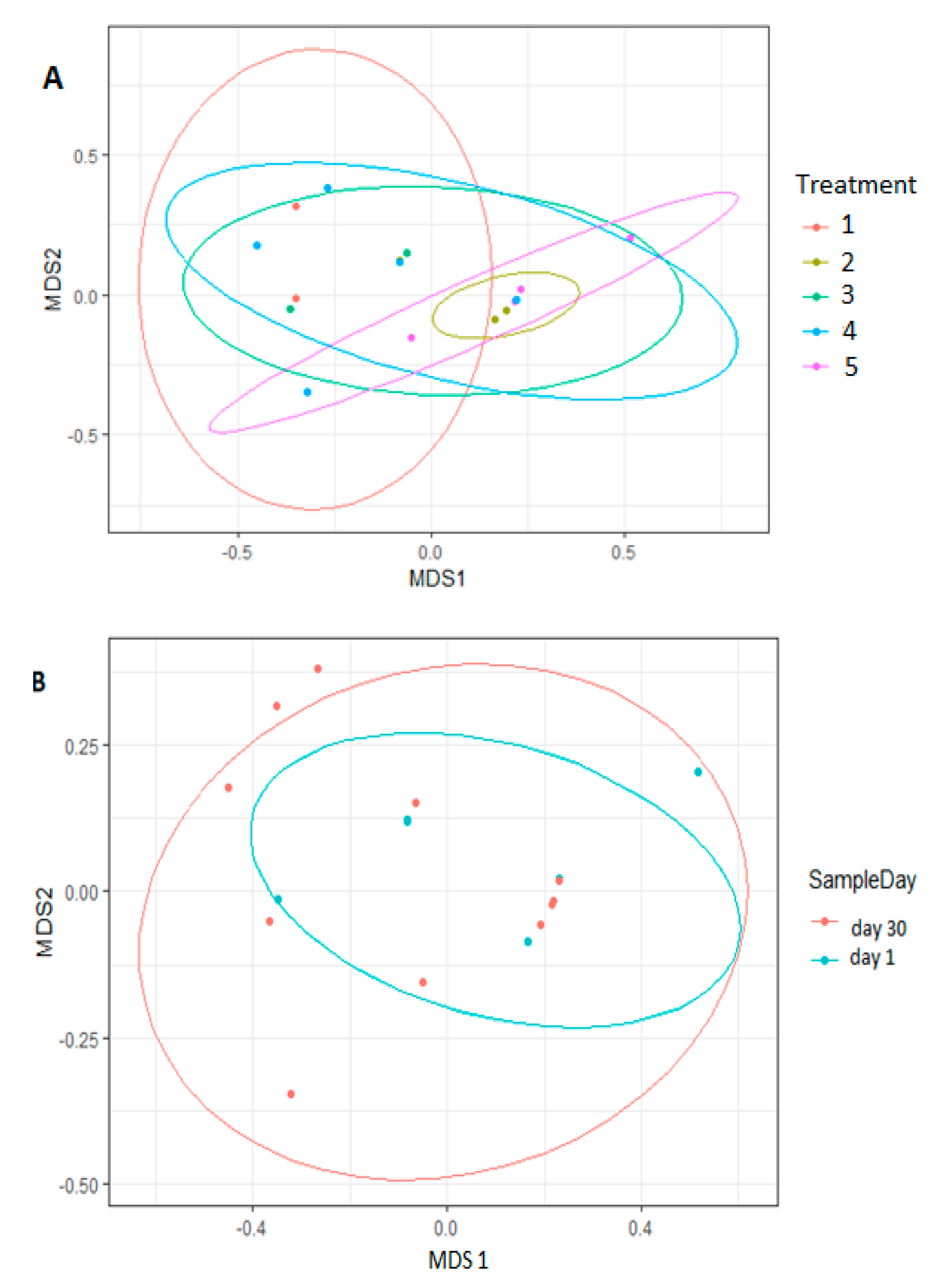
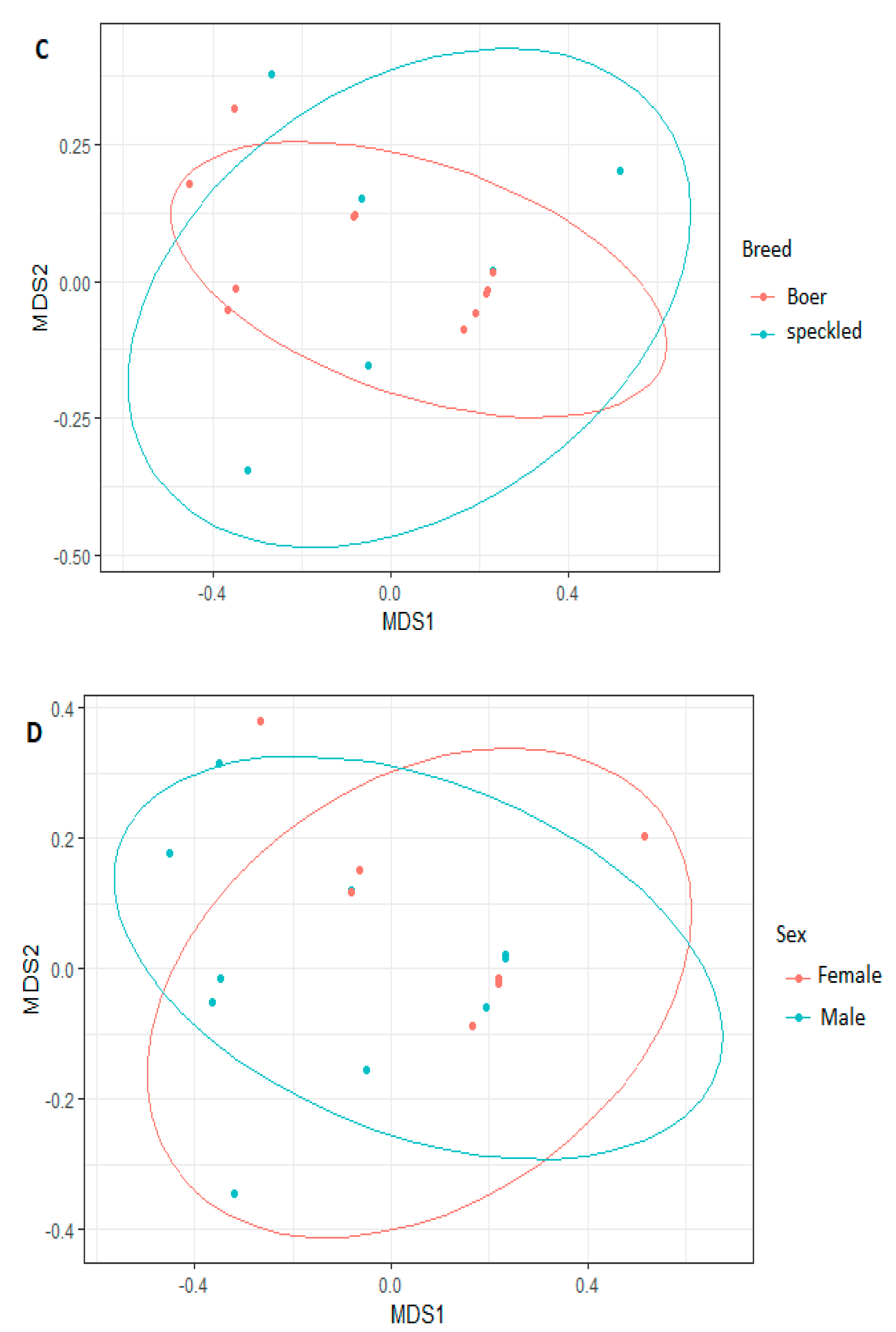
3.3. Comparison of Bacterial Diversity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Visser, C.; van Marle-Köster, E. The Development and Genetic Improvement of South African Goats. In IntechOpen; Goat Science: London, UK, 2018; pp. 20–36. [Google Scholar] [CrossRef] [Green Version]
- Mohlatlole, R.P.; Dzomba, E.F.; Muchadeyi, F.C. Addressing production challenges in goat production systems of South Africa: The genomics approach. Small Rumin. Res. 2015, 131, 43–49. [Google Scholar] [CrossRef]
- Mrode, R.; Tarekegn, G.M.; Mwacharo, J.M.; Djikeng, A. Invited review: Genomic selection for small ruminants in developed countries: How applicable for the rest of the world? Animal 2018, 12, 1333–1340. [Google Scholar] [CrossRef] [Green Version]
- Li, F.; Neves, A.L.; Ghoshal, B.; Guan, L.L. Symposium review: Mining metagenomic and metatranscriptomic data for clues about microbial metabolic functions in ruminants1. J. Dairy Sci. 2018, 101, 5605–5618. [Google Scholar] [CrossRef] [Green Version]
- Langda, S.; Zhang, C.; Zhang, K.; Gui, B.; Ji, D.; Deji, C.; Cuoji, A.; Wang, X.; Wu, Y. Diversity and composition of rumen bacteria, fungi, and protozoa in goats and sheep living in the same high-altitude pasture. Animals 2020, 10, 186. [Google Scholar] [CrossRef] [Green Version]
- Markowiak, P.; Ślizewska, K. The role of probiotics, prebiotics and synbiotics in animal nutrition. Gut Pathog. 2018, 10, 1–20. [Google Scholar] [CrossRef]
- Bahari, M. A Review on the Consumption of Probiotics in Feeding Young Ruminants. Approaches Poultry Dairy Vet. Sci. 2017, 1, 28–30. [Google Scholar] [CrossRef] [Green Version]
- Luton, P.E.; Wayne, J.M.; Sharp, R.J.; Riley, P.W. The mcrA gene as an alternative to 16S rRNA in the phylogenetic analysis of methanogen populations in landfill. Microbiology 2002, 148, 3521–3530. [Google Scholar] [CrossRef] [Green Version]
- Henderson, G.; Cox, F.; Ganesh, S.; Jonker, A.; Young, W.; Global Rumen Census Collaborators; Janssen, P.H. Rumen microbial community composition varies with diet and host, but a core microbiome is found across a wide geographical range. Sci. Rep. 2015, 5. [Google Scholar] [CrossRef]
- Grünberg, W.; Constable, N. Function and Dysfunction of the Ruminant Forestomach Diseases of the Alimentary Tract—Ruminant. In Current Veterinary Therapy; Elsevier Inc.: Amsterdam, The Netherlands, 2009; pp. 12–19. [Google Scholar] [CrossRef]
- Maake, T.W.; Adeleke, M.; Aiyegoro, O.A. Effect of lactic acid bacteria administered as feed supplement on the weight gain and ruminal pH in two South African goat breeds. Trans. R. Soc. S. Afr. 2021. [Google Scholar] [CrossRef]
- Carberry, C.A.; Waters, S.M.; Kenny, D.A.; Creevey, C.J. Rumen methanogenic genotypes differ in abundance according to host residual feed intake phenotype and diet type. Appl. Environ. Microbiol. 2014, 80, 586–594. [Google Scholar] [CrossRef] [Green Version]
- Jiao, J.; Huang, J.; Zhou, C.; Tan, Z. Taxonomic identification of ruminal epithelial bacterial diversity during rumen development in goats. Appl. Environ. Microbiol. 2015, 81, 3502–3509. [Google Scholar] [CrossRef] [Green Version]
- Lei, Z.; Zhang, K.; Li, C.; Jiao, T.; Wu, J.; Wei, Y.; Tian, K.; Li, C.; Tang, D.; Davis, D.I.; et al. Ruminal metagenomic analyses of goat data reveals potential functional microbiota by supplementation with essential oil-cobalt complexes. BMC Microbiol. 2019, 19. [Google Scholar] [CrossRef] [Green Version]
- Khalid, F.M.; Shahzad, A.M.; Sarwar, M.; Rehman, A.; Sharif, M.; Mukhtar, N. Probiotics and lamb performance: A review. Afr. J. Agric. Res. 2011, 6, 5198–5203. [Google Scholar] [CrossRef]
- Khafipour, E.; Li, S.; Tun, H.M.; Derakhshani, H.; Moossavi, S.; Plaizier, J.C. Effects of grain feeding on microbiota in the digestive tract of cattle. Anim. Front. 2016, 6, 13–19. [Google Scholar] [CrossRef]
- Thomas, F.A.; Webb, M.; Ghimire, S.; Blair, A.; Olson, K.; Fenske, G.J.; Fonder, A.T.; Christopher-Hennings, J.; Brake, D.; Scaria, J. Metagenomic characterization of the effect of feed additives on the gut microbiome and antibiotic resistome of feedlot cattle. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef] [Green Version]
- Franzolin, R.; Dehority, B.A. the Role of pH on the Survival of Rumen Protozoa in Steers. Rumin. R. Bras. Zootec. 2010, 39, 2262–2267. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Xu, Q.; Kong, F.; Yang, Y.; Wu, D.; Mishra, S.; LiZhi, W. Exploring the goat rumen microbiome from seven days to two years. PLoS ONE 2016, 11, e0154354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cremonesi, P.; Conte, G.; Severgnini, M.; Turri, F.; Monni, A.; Capra, E.; Rapetti, L.; Colombini, S.; Chessa, S.; Battelli, G.; et al. Evaluation of the effects of different diets on microbiome diversity and fatty acid composition of rumen liquor in dairy goat. Animal 2018, 12, 1856–1866. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.; Deb, S.; Dey, A.; Somvanshi, S.; Singh, D.; Rathore, R.; Stiverson, J. 16S rDNA analysis of archaea indicates dominance of Methanobacterium and high abundance of Methanomassiliicoccaceae in rumen of Nili-Ravi buffalo. Anaerobe 2015, 35, 3–10. [Google Scholar] [CrossRef]
- Min, B.R.; Gurung, N.; Shange, R.; Solaiman, S.; Shange, R. Potential role of rumen microbiota in altering average daily gain and feed efficiency in meat goats fed simple and mixed pastures using bacterial tag-encoded FLX amplicon pyrosequencing1. J. Anim. Sci. 2019, 97, 3523–3534. [Google Scholar] [CrossRef]
- Sirohi, S.K.; Pandey, N.; Singh, B.; Puniya, A.K. Rumen methanogens: A review. Indian J. Microbiol. 2010, 50, 253–262. [Google Scholar] [CrossRef] [Green Version]
- Cersosimo, L.M.; Lachance, H.; St-Pierre, B.; Van Hoven, W.; Wright, A.-D.G. Examination of the Rumen Bacteria and Methanogenic Archaea of Wild Impalas (Aepycerosmelampusmelampus) from Pongola, South Africa. Microb. Ecol. 2015, 69, 577–585. [Google Scholar] [CrossRef] [Green Version]
- Iqbal, M.W.; Zhang, Q.; Yang, Y.; Li, L.; Zou, C.; Huang, C.; Lin, B. Comparative study of rumen fermentation and microbial community differences between water buffalo and Jersey cows under similar feeding conditions. J. Appl. Anim. Res. 2018, 46, 740–748. [Google Scholar] [CrossRef]
- Ratti, R.P.; Botta, L.S.; Sakamoto, I.K.; Silva, E.L.; Varesche, M.B.A. Production of H2 from cellulose by rumen microorganisms: Effects of inocula pre-treatment and enzymatic hydrolysis. Biotechnol. Lett. 2014, 36, 537–546. [Google Scholar] [CrossRef] [PubMed]
- Shabana, I.I.; Albakri, N.N.; Bouqellah, N.A. Metagenomic investigation of faecal microbiota in sheep and goats of the same ages. J. Taibah Univ. Sci. 2021. [Google Scholar] [CrossRef]
- Bi, Y.; Zeng, S.; Zhang, R.; Diao, Q.; Tu, Y. Effects of dietary energy levels on rumen bacterial community composition in Holstein heifers under the same forage to concentrate ratio condition. BMC Microbiol. 2018, 18, 1–11. [Google Scholar] [CrossRef]
- Matthews, C.; Crispie, F.; Lewis, E.; Reid, M.; O’Toole, P.W.; Cotter, P.D. The rumen microbiome: A crucial consideration when optimising milk and meat production and nitrogen utilisation efficiency. Gut Microbes 2019, 10, 115–132. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Fernandez, G.; Denman, S.E.; Yang, C.; Cheung, J.; Mitsumori, M.; McSweeney, C. Methane inhibition alters the microbial community, hydrogen flow, and fermentation response in the rumen of cattle. Front. Microbiol. 2016, 7, 1–14. [Google Scholar] [CrossRef]
- Mani, S.; Aiyegoro, O.A.; Adeleke, M.A. Characterization of Rumen Microbiota of Two Sheep Breeds Supplemented With Direct-Fed Lactic Acid Bacteria. Front. Vet. Sci. 2021, 7, 570074. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Yang, Y.; Yan, H.; Wang, X.; Qu, L.; Chen, Y. Rumen bacterial diversity of 80 to 110-day- Old goats using 16s rRNAsequencing. PLoS ONE 2015. [Google Scholar] [CrossRef]
- Noel, S.J.; Olijhoek, D.W.; McLean, F.; Løvendahl, P.; Lund, P.; Højberg, O. Rumen and Fecal Microbial Community Structureof Holstein and Jersey Dairy Cows as Affected by Breed, Diet, and Residual Feed Intake. Animals 2019, 9, 498. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| Nutrients | g (kg) |
|---|---|
| Protein | 150 |
| Fat | 25 |
| Fibre | 110 |
| Calcium | 8 |
| Phosphorus | 2 |
| Urea | 1 |
| Chloride | 9 |
| Sodium | 9 |
| Magnesium | 1 |
| Potassium | 6 |
| Parameter | T1 | T2 | T3 | T4 | T5 | Boer | Speckled | p-Value |
|---|---|---|---|---|---|---|---|---|
| Initial pH | 6.99 ± 0.44 | 6.56 ± 0.42 | 7.12 ± 0.41 | 7.5 ± 0.45 | 7.19 ± 0.43 | 7.12 ± 0.42 | 7.12 ± 0.42 | 0.57 |
| Final pH | 6.32 ± 0.41 | 6.37 ± 0.46 | 6.18 ± 0.52 | 6.4 ± 0.52 | 6.36 ± 0.56 | 6.80 ± 0.53 | 6.34 ± 0.55 | 0.0001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maake, T.W.; Aiyegoro, O.A.; Adeleke, M.A. Effects of Lactobacillus rhamnosus and Enterococcus faecalis Supplementation as Direct-Fed Microbials on Rumen Microbiota of Boer and Speckled Goat Breeds. Vet. Sci. 2021, 8, 103. https://doi.org/10.3390/vetsci8060103
Maake TW, Aiyegoro OA, Adeleke MA. Effects of Lactobacillus rhamnosus and Enterococcus faecalis Supplementation as Direct-Fed Microbials on Rumen Microbiota of Boer and Speckled Goat Breeds. Veterinary Sciences. 2021; 8(6):103. https://doi.org/10.3390/vetsci8060103
Chicago/Turabian StyleMaake, Takalani Whitney, Olayinka Ayobami Aiyegoro, and Matthew Adekunle Adeleke. 2021. "Effects of Lactobacillus rhamnosus and Enterococcus faecalis Supplementation as Direct-Fed Microbials on Rumen Microbiota of Boer and Speckled Goat Breeds" Veterinary Sciences 8, no. 6: 103. https://doi.org/10.3390/vetsci8060103
APA StyleMaake, T. W., Aiyegoro, O. A., & Adeleke, M. A. (2021). Effects of Lactobacillus rhamnosus and Enterococcus faecalis Supplementation as Direct-Fed Microbials on Rumen Microbiota of Boer and Speckled Goat Breeds. Veterinary Sciences, 8(6), 103. https://doi.org/10.3390/vetsci8060103






