Glucose Oligosaccharide and Long-Chain Glucomannan Feed Additives Induce Enhanced Activation of Intraepithelial NK Cells and Relative Abundance of Commensal Lactic Acid Bacteria in Broiler Chickens
Abstract
:1. Introduction
2. Materials and Methods
2.1. Overall Experimental Design
2.2. Screening of the Effect of Compounds on Activation of NK Cells and Macrophages In Vitro
2.2.1. NK Cell and T Cell Activation In Vitro and In Vivo as Assessed in the CD107 Assay
2.2.2. Assessment of the Effect of Compounds on the Phagocytic Activity of the Macrophage Cell Line HD11 In Vitro
2.2.3. NO Assay to Assess Activation of the Macrophage Cell Line HD11 In Vitro
2.3. Screening of the Effect of Compounds Following In Ovo Administration
2.4. Screening of the Effect of Compounds on Growth Performance In Vivo
2.5. In Vivo Supplementation of Glucose Oligosaccharide and Long-Chain Glucomannan and Their Modulatory Properties on Immune Cells and Microbiota Composition
2.5.1. Isolation of Immune Cells from the Intestine and the Spleen
2.5.2. Phenotypic Characterization of IELs and Splenic Leukocytes by Flow Cytometry
2.5.3. Microbiota Composition of Ileum and Ceca
2.6. Statistical Analysis
3. Results
3.1. Compounds Are Able to Induce Activation of NK Cells and Macrophages
3.2. In Ovo Administration of the Selected Compounds Showed No Adverse Effects on Embryonic Development and Hatching
3.3. No Negative Effects Were Observed on Performance Traits upon In Vivo Administration
3.4. Glucose Oligosaccharide and Long-Chain Glucomannan Significantly Increase Activation of Intraepithelial NK Cells Seven Days after In Vivo Supplementation
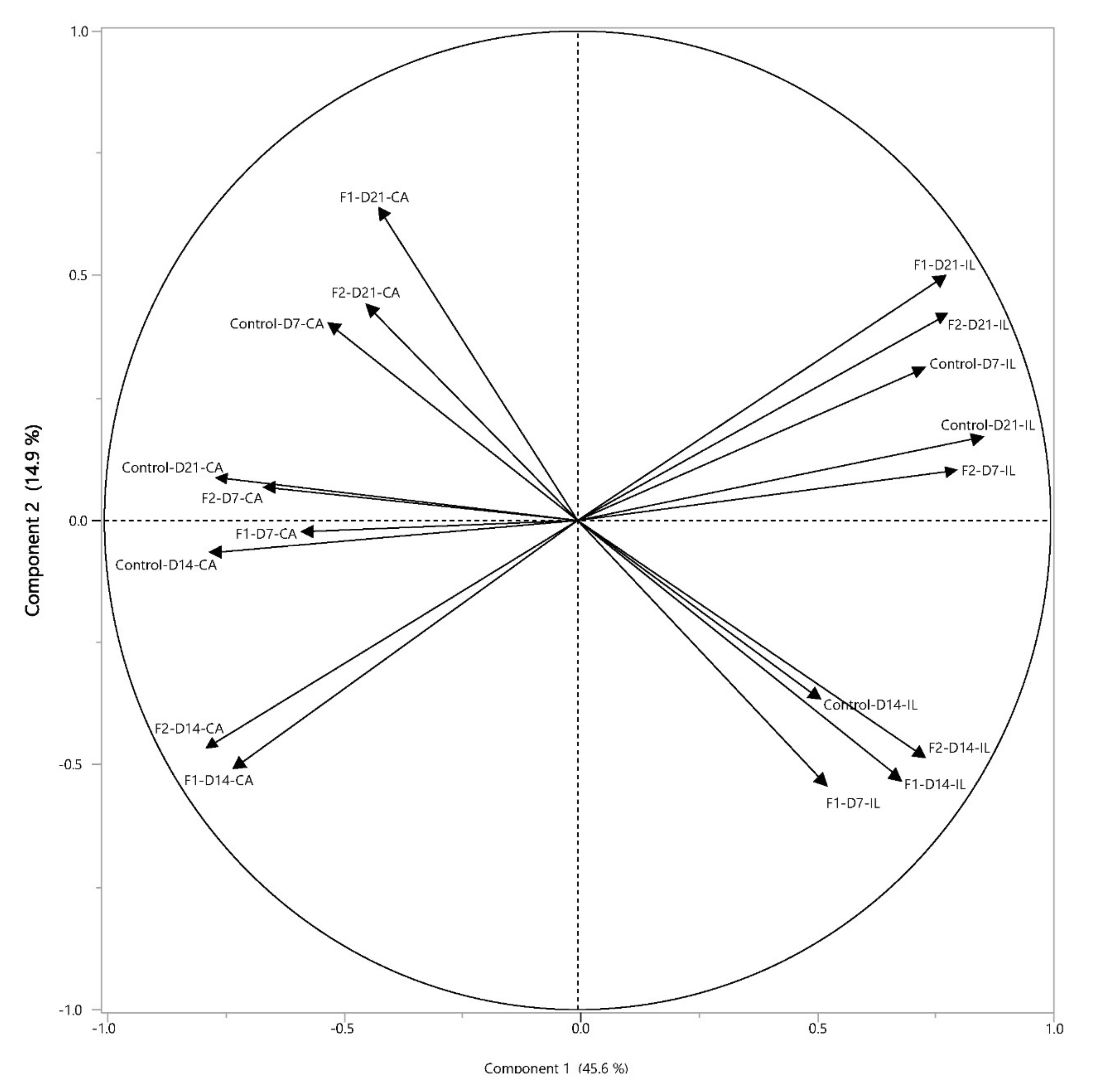
3.5. In Vivo Supplementation of Glucose Oligosaccharide and Long-Chain Glucomannan Led to Increased Relative Abundance of Lactobacillus Species in the Intestinal Microbiota
3.6. Positive Correlations between NK Cell Activation and Lactic Acid Bacteria upon In Vivo Supplementation by Glucose Oligosaccharide and Long-Chain Glucomannan
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Taha-Abdelaziz, K.; Hodgins, D.C.; Lammers, A.; Alkie, T.N.; Sharif, S. Effects of early feeding and dietary interventions on development of lymphoid organs and immune competence in neonatal chickens: A review. Vet. Immunol. Immunopathol. 2018, 201, 1–11. [Google Scholar] [CrossRef]
- Torok, V.A.; Allison, G.E.; Percy, N.J.; Ophel-Keller, K.; Hughes, R.J. Influence of antimicrobial feed additives on broiler commensal posthatch gut microbiota development and performance. Appl. Environ. Microbiol. 2011, 77, 3380–3390. [Google Scholar] [CrossRef] [Green Version]
- Schokker, D.; Jansman, A.J.M.; Veninga, G.; de Bruin, N.; Vastenhouw, S.A.; de Bree, F.M.; Bossers, A.; Rebel, J.M.J.; Smits, M.A. Perturbation of microbiota in one-day old broiler chickens with antibiotic for 24 h negatively affects intestinal immune development. BMC Genom. 2017, 18. [Google Scholar] [CrossRef] [Green Version]
- Broom, L.J.; Kogut, M.H. The role of the gut microbiome in shaping the immune system of chickens. Vet. Immunol. Immunopathol. 2018, 204, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Lei, F.; Zhu, L.; Li, S.; Wu, Z.; Zhang, R.; Gao, G.F.; Zhu, B.; Wang, X. Exposure of different bacterial inocula to newborn chicken affects gut microbiota development and ileum gene expression. ISME J. 2010, 4, 367–376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meijerink, N.; Kers, J.G.; Velkers, F.C.; van Haarlem, D.A.; Lamot, D.M.; de Oliveira, J.E.; Smidt, H.; Stegeman, J.A.; Rutten, V.; Jansen, P.M.G. Early Life Inoculation with Adult-Derived Microbiota Accelerates Maturation of Intestinal Microbiota and Enhances NK Cell Activation in Broiler Chickens. Front Vet. Sci. 2020, 7. [Google Scholar] [CrossRef]
- Kogut, M.H. Impact of nutrition on the innate immune response to infection in poultry. J. Appl. Poult. Res. 2009, 18, 111–124. [Google Scholar] [CrossRef]
- Bar-Shira, E.; Sklan, D.; Friedman, A. Establishment of immune competence in the avian GALT during the immediate post-hatch period. Dev. Comp. Immunol. 2003, 27, 147–157. [Google Scholar] [CrossRef]
- Klasing, K.C.; Leshchinsky, T.V. Functions, costs and benefits of the immune system during development and growth. In Proceedings of the 22nd International Ornithological Congress, Durban, South Africa, 16–22 August 1998; Adams, N.J., Slotow, R.H., Eds.; Birdlife South Africa: Johannesburg, South Africa, 1999; pp. 2817–2835. [Google Scholar]
- Göbel, T.W.F.; Kaspers, B.; Stangassinger, M. NK and T cells constitute two major, functionally distinct intestinal epithelial lymphocyte subsets in the chicken. Int. Immunol. 2001, 13, 757–762. [Google Scholar] [CrossRef] [Green Version]
- Meijerink, N.; van Haarlem, D.A.; Velkers, F.C.; Stegeman, A.J.; Rutten, V.P.M.G.; Jansen, C.A. Analysis of chicken intestinal natural killer cells, a major IEL subset during embryonic and early life. Dev. Comp. Immunol. 2021, 114. [Google Scholar] [CrossRef]
- Lawn, A.M.; Rose, M.E.; Bradley, J.W.A.; Rennie, M.C. Lymphocytes of the intestinal mucosa of chickens. Cell Tissue Res. 1988, 251, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Lillehoj, H.S.; Trout, J.M. Avian gut-associated lymphoid tissues and intestinal immune responses to Eimeria parasites. Clin. Microbiol. Rev. 1996, 9, 349–360. [Google Scholar] [CrossRef] [PubMed]
- Swaggerty, C.L.; Callaway, T.R.; Kogut, M.H.; Piva, A.; Grilli, E. Modulation of the immune response to improve health and reduce foodborne pathogens in poultry. Microorganisms 2019, 7, 65. [Google Scholar] [CrossRef] [Green Version]
- Kidd, M.T. Nutritional modulation of immune function in broilers. Poult. Sci. 2004, 83, 650–657. [Google Scholar] [CrossRef]
- Abdolmaleki, M.; Yeap, S.K.; Tan, S.W.; Satharasinghe, D.A.; Bello, M.B.; Jahromi, M.Z.; Bejo, M.H.; Omar, A.R.; Ideris, A. Effects of newcastle disease virus infection on chicken intestinal intraepithelial natural killer cells. Front. Immunol. 2018, 9. [Google Scholar] [CrossRef]
- Jahromi, M.Z.; Bello, M.B.; Abdolmaleki, M.; Yeap, S.K.; Hair-Bejo, M.; Omar, A.R. Differential activation of intraepithelial lymphocyte-natural killer cells in chickens infected with very virulent and vaccine strains of infectious bursal disease virus. Dev. Comp. Immunol. 2018, 87, 116–123. [Google Scholar] [CrossRef]
- Jansen, C.A.; De Geus, E.D.; Van Haarlem, D.A.; Van De Haar, P.M.; Löndt, B.Z.; Graham, S.P.; Göbel, T.W.; Van Eden, W.; Brookes, S.M.; Vervelde, L. Differential lung NK cell responses in avian influenza virus infected chickens correlate with pathogenicity. Sci. Rep. 2013, 3. [Google Scholar] [CrossRef]
- Meijerink, N.; van den Biggelaar, R.H.; van Haarlem, D.A.; Stegeman, A.J.; Rutten, V.P.; Jansen, C.A. Contribution of innate and adaptive immune cells to the elimination of Salmonella enterica serotype Enteritidis infection in young broiler chickens. bioRxiv 2021. [Google Scholar] [CrossRef]
- Jansen, C.A.; van de Haar, P.M.; van Haarlem, D.; van Kooten, P.; de Wit, S.; van Eden, W.; Viertlböck, B.C.; Göbel, T.W.; Vervelde, L. Identification of new populations of chicken natural killer (NK) cells. Dev. Comp. Immunol. 2010, 34, 759–767. [Google Scholar] [CrossRef] [Green Version]
- De Geus, E.D.; Jansen, C.A.; Vervelde, L. Uptake of particulate antigens in a nonmammalian lung: Phenotypic and functional characterization of avian respiratory phagocytes using bacterial or viral antigens. J. Immunol. 2012, 188, 4516–4526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van den Biggelaar, R.H.G.A.; van Eden, W.; Rutten, V.P.M.G.; Jansen, C.A. Nitric oxide production and fc receptor-mediated phagocytosis as functional readouts of macrophage activity upon stimulation with inactivated poultry vaccines in vitro. Vaccines 2020, 8, 332. [Google Scholar] [CrossRef]
- Ariaans, M.P.; Matthijs, M.G.R.; van Haarlem, D.; van de Haar, P.; van Eck, J.H.H.; Hensen, E.J.; Vervelde, L. The role of phagocytic cells in enhanced susceptibility of broilers to colibacillosis after Infectious Bronchitis Virus infection. Vet. Immunol. Immunopathol. 2008, 123, 240–250. [Google Scholar] [CrossRef] [Green Version]
- Henkart, P.A. Lymphocyte-mediated cytotoxicity: Two pathways and multiple effector molecules. Immunity 1994, 1, 343–346. [Google Scholar] [CrossRef]
- Hirayama, D.; Iida, T.; Nakase, H. The phagocytic function of macrophage-enforcing innate immunity and tissue homeostasis. Int. J. Mol. Sci. 2018, 19, 92. [Google Scholar] [CrossRef] [Green Version]
- MacMicking, J.; Xie, Q.; Nathan, C. Nitric oxide and macrophage function. Annu. Rev. Immunol. 1997, 15. [Google Scholar] [CrossRef]
- Bar-Shira, E.; Friedman, A. Development and adaptations of innate immunity in the gastrointestinal tract of the newly hatched chick. Dev. Comp. Immunol. 2006, 30, 930–941. [Google Scholar] [CrossRef]
- Alizadeh, M.; Rogiewicz, A.; McMillan, E.; Rodriguez-Lecompte, J.C.; Patterson, R.; Slominski, B.A. Effect of yeast-derived products and distillers dried grains with solubles (DDGS) on growth performance and local innate immune response of broiler chickens challenged with Clostridium perfringens. Avian. Pathol. 2016, 45, 334–345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shashidhara, R.G.; Devegowda, G. Effect of dietary mannan oligosaccharide on broiler breeder production traits and immunity. Poult. Sci. 2003, 82, 1319–1325. [Google Scholar] [CrossRef] [PubMed]
- Baurhoo, B.; Ferket, P.; Ashwell, C.M.; de Oliviera, J.; Zhao, X. Cell walls of saccharomyces cerevisiae differentially modulated innate immunity and glucose metabolism during late systemic inflammation. PLoS ONE 2012, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roberts, T.; Wilson, J.; Guthrie, A.; Cookson, K.; Vancraeynest, D.; Schaeffer, J.; Moody, R.; Clark, S. New issues and science in broiler chicken intestinal health: Emerging technology and alternative interventions. J. Appl. Poult. Res. 2015, 24, 257–266. [Google Scholar] [CrossRef]
- Guo, F.C.; Savelkoul, H.F.J.; Kwakkel, R.P.; Williams, B.A.; Verstegen, M.W.A. Immunoactive, medicinal properties of mushroom and herb polysaccharides and their potential use in chicken diets. World’s Poult. Sci. J. 2003, 59, 427–440. [Google Scholar] [CrossRef]
- Ritz, B.W.; Nogusa, S.; Ackerman, E.A.; Gardner, E.M. Supplementation with active hexose correlated compound increases the innate immune response of young mice to primary influenza infection. J. Nutr. 2006, 136, 2868–2873. [Google Scholar] [CrossRef] [Green Version]
- Arreola, R.; Quintero-Fabián, S.; Lopez-Roa, R.I.; Flores-Gutierrez, E.O.; Reyes-Grajeda, J.P.; Carrera-Quintanar, L.; Ortuno-Sahagun, D. Immunomodulation and anti-inflammatory effects of garlic compounds. J. Immunol. Res. 2015, 2015. [Google Scholar] [CrossRef]
- Falchetti, R.; Fuggetta, M.P.; Lanzilli, G.; Tricarico, M.; Ravagnan, G. Effects of resveratrol on human immune cell function. Life Sci. 2001, 70, 81–96. [Google Scholar] [CrossRef]
- Leischner, C.; Burkard, M.; Pfeiffer, M.M.; Lauer, U.M.; Busch, C.; Venturelli, S. Nutritional immunology: Function of natural killer cells and their modulation by resveratrol for cancer prevention and treatment. Nutr. J. 2016, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ren, Y.; Yuan, C.; Deng, Y.; Kanagasabai, R.; Ninh, T.N.; Tu, V.T.; Chai, H.; Soejarto, D.D.; Fuchs, J.R.; Yalowich, J.C.; et al. Cytotoxic and natural killer cell stimulatory constituents of Phyllanthus songboiensis. Phytochemistry 2015, 111, 132–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheeja, K.; Kuttan, G. Modulation of natural killer cell activity, antibody-dependent cellular cytotoxicity, and antibody-dependent complement-mediated cytotoxicity by andrographolide in normal and Ehrlich ascites carcinoma-bearing mice. Integr. Cancer Ther. 2007, 6, 66–73. [Google Scholar] [CrossRef]
- Kilani-Jaziri, S.; Mustapha, N.; Mokdad-Bzeouich, I.; El Gueder, D.; Ghedira, K.; Ghedira-Chekir, L. Flavones induce immunomodulatory and anti-inflammatory effects by activating cellular anti-oxidant activity: A structure-activity relationship study. Tumor. Biol. 2016, 37, 6571–6579. [Google Scholar] [CrossRef] [PubMed]
- Murali, V.P.; Kuttan, G. Curculigoside augments cell-mediated immune responses in metastatic tumor-bearing animals. Immunopharmacol. Immunotoxicol. 2016, 38, 264–269. [Google Scholar] [CrossRef]
- Zhang, Y.; Song, T.T.; Cunnick, J.E.; Murphy, P.A.; Hendrich, S. Daidzein and genistein glucuronides in vitro are weakly estrogenic and activate human natural killer cells at nutritionally relevant concentrations. J. Nutr. 1999, 129, 399–405. [Google Scholar] [CrossRef] [Green Version]
- Hart, A.N.; Zaske, L.A.; Patterson, K.M.; Drapeau, C.; Jensen, G.S. Natural killer cell activation and modulation of chemokine receptor profile in vitro by an extract from the cyanophyta Aphanizomenon flos-aquae. J. Med. Food 2007, 10, 435–441. [Google Scholar] [CrossRef] [Green Version]
- Lessard, M.; Dupuis, M. Differential modulation of chicken lymphocyte blastogenesis and cytotoxic activity of natural killer cells in vitro by retinol, retinoic acid and beta-carotene. Nutr. Res. 1994, 14, 1201–1217. [Google Scholar] [CrossRef]
- Lai, H.; Chang, C.; Yang, C.; Hsu, Y.; Chen, C.; Lin, C.; Tsai, Y.; Huang, T.; Ojcius, D.M.; Tsai, Y.; et al. Activation of NK cell cytotoxicity by the natural compound 2,3-butanediol. J. Leukocyte Biol. 2012, 92, 807–814. [Google Scholar] [CrossRef] [Green Version]
- Grudzien, M.; Rapak, A. Effect of natural compounds on NK cell activation. J. Immunol. Res. 2018, 2018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nichenametla, S.N.; South, E.H.; Exon, J.H. Interaction of conjugated linoleic acid, sphingomyelin, and butyrate on formation of colonic aberrant crypt foci and immune functions in rats. J. Toxicol. Environ. Health Part A 2004, 67, 469–481. [Google Scholar] [CrossRef]
- Vannucci, L.; Fiserová, A.; Sadalapure, K.; Lindhorst, T.K.; Kuldová, M.; Rossmann, P.; Horváth, O.; Kren, V.; Krist, P.; Bezouska, K.; et al. Effects of N-acetyl-glucosamine-coated glycodendrimers as biological modulators in the B16F10 melanoma model in vivo. Int. J. Oncol. 2003, 23, 285–296. [Google Scholar] [CrossRef]
- Reddy, N.; Hernandez-Ilizaliturri, F.J.; Deeb, G.; Roth, M.; Vaughn, M.; Knight, J.; Wallace, P.; Czuczman, M.S. Immunomodulatory drugs stimulate natural killer-cell function, alter cytokine production by dendritic cells, and inhibit angiogenesis enhancing the anti-tumour activity of rituximab in vivo. Br. J. Haematol. 2008, 140, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.; Jha, R. Strategies to modulate the intestinal microbiota and their effects on nutrient utilization, performance, and health of poultry. J. Animal Sci. Biotechnol. 2019, 10. [Google Scholar] [CrossRef]
- Apajalahti, J.H.A.; Kettunen, A.; Bedford, M.R.; Holben, W.E. Percent G+C Profiling Accurately Reveals Diet-Related Differences in the Gastrointestinal Microbial Community of Broiler Chickens. Appl. Environ. Microbiol. 2001, 67, 5656–5667. [Google Scholar] [CrossRef] [Green Version]
- Torok, V.A.; Hughes, R.J.; Mikkelsen, L.L.; Perez-Maldonado, R.; Balding, K.; MacAlpine, R.; Percy, N.J.; Ophel-Keller, K. Identification and characterization of potential performance-related gut microbiotas in broiler chickens across various feeding trials. Appl. Environ. Microbiol. 2011, 77, 5868–5878. [Google Scholar] [CrossRef] [Green Version]
- Kogut, M.H. The effect of microbiome modulation on the intestinal health of poultry. Anim. Feed Sci. Technol. 2019, 250, 32–40. [Google Scholar] [CrossRef]
- Roto, S.M.; Rubinelli, P.M.; Ricke, S.C. An introduction to the avian gut microbiota and the effects of yeast-based prebiotic-type compounds as potential feed additives. Front. Vet. Sci. 2015, 2. [Google Scholar] [CrossRef] [Green Version]
- Zhu, N.; Wang, J.; Yu, L.; Zhang, Q.; Chen, K.; Liu, B. Modulation of growth performance and intestinal microbiota in chickens fed plant extracts or virginiamycin. Front. Microbiol. 2019, 10. [Google Scholar] [CrossRef]
- Göbel, T.W.F.; Chen, C.H.; Shrimpf, J.; Grossi, C.E.; Bernot, A.; Bucy, R.P.; Auffray, C.; Cooper, M.D. Characterization of avian natural killer cells and their intracellular CD3 protein complex. Eur. J. Immunol. 1994, 24, 1685–1691. [Google Scholar] [CrossRef] [Green Version]
- Beug, H.; von Kirchbach, A.; Döderlein, G.; Conscience, J.; Graf, T. Chicken hematopoietic cells transformed by seven strains of defective avian leukemia viruses display three distinct phenotypes of differentiation. Cell 1979, 18, 375–390. [Google Scholar] [CrossRef]
- Seliger, C.; Schaerer, B.; Kohn, M.; Pendl, H.; Weigend, S.; Kaspers, B.; Härtle, S. A rapid high-precision flow cytometry based technique for total white blood cell counting in chickens. Vet. Immunol. Immunopathol. 2012, 145, 86–99. [Google Scholar] [CrossRef]
- Van der Hoeven-Hangoor, E.; van der Vossen, J.M.B.M.; Schuren, F.H.J.; Verstegen, M.W.A.; de Oliveira, J.E.; Montijn, R.C.; Hendriks, W.H. Ileal microbiota composition of broilers fed various commercial diet compositions. Poult. Sci. 2013, 92, 2713–2723. [Google Scholar] [CrossRef] [PubMed]
- Druyan, S.; De Oliveira, J.E.; Ashwell, C.M. Focused microarrays as a method to evaluate subtle changes in gene expression. Poult. Sci. 2008, 87, 2418–2429. [Google Scholar] [CrossRef]
- De Oliveira, J.E.; Druyan, S.; Uni, Z.; Ashwell, C.M.; Ferket, P.R. Metabolic profiling of late-term turkey embryos by microarrays. Poult. Sci. 2013, 92, 1011–1028. [Google Scholar] [CrossRef] [PubMed]
- Yadgary, L.; Uni, Z. Yolk sac carbohydrate levels and gene expression of key gluconeogenic and glycogenic enzymes during chick embryonic development. Poult. Sci. 2012, 91, 444–453. [Google Scholar] [CrossRef]
- De Oliveira, J.E.; Uni, Z.; Ferket, P.R. Important metabolic pathways in poultry embryos prior to hatch. Worlds Poult. Sci. J. 2008, 64, 488–499. [Google Scholar] [CrossRef] [Green Version]
- Tako, E.; Ferket, P.R.; Uni, Z. Effects of in ovo feeding of carbohydrates and beta-hydroxy-beta-methylbutyrate on the development of chicken intestine. Poult. Sci. 2004, 83, 2023–2028. [Google Scholar] [CrossRef]
- Davani-Davari, D.; Negahdaripour, M.; Karimzadeh, I.; Seifan, M.; Mohkam, M.; Masoumi, S.J.; Berenjian, A.; Ghasemi, Y. Prebiotics: Definition, types, sources, mechanisms, and clinical applications. Foods 2019, 8, 92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Froebel, L.K.; Jalukar, S.; Lavergne, T.A.; Lee, J.T.; Duong, T. Administration of dietary prebiotics improves growth performance and reduces pathogen colonization in broiler chickens. Poult. Sci. 2019, 98, 6668–6676. [Google Scholar] [CrossRef] [PubMed]
- Abaza, I.M.; Shehata, M.A.; Shoieb, M.S.; Hassan, I.I. Evaluation of some natural feed additive in growing chicks diets. Int. J. Poult. Sci. 2008, 7, 872–879. [Google Scholar] [CrossRef] [Green Version]
- Al-Khalaifah, H. Benefits of probiotics and/or prebiotics for antibiotic-reduced poultry. Poult. Sci. 2018, 97, 3807–3815. [Google Scholar] [CrossRef] [PubMed]
- Arsenault, R.J.; Lee, J.T.; Latham, R.; Carter, B.; Kogut, M.H. Changes in immune and metabolic gut response in broilers fed β-mannanase in β-mannan-containing diets. Poult. Sci. 2017, 96, 4307–4316. [Google Scholar] [CrossRef]
- Świątkiewicz, S.; Arczewska-Włosek, A.; Józefiak, D. Immunomodulatory efficacy of yeast cell products in poultry: A current review. World’s Poult. Sci. J. 2014, 70, 57–68. [Google Scholar] [CrossRef]
- Doke, S.K.; Dhawale, S.C. Alternatives to animal testing: A review. Saudi Pharm. J. 2015, 23, 223–229. [Google Scholar] [CrossRef] [Green Version]
- Byrne, K.A.; Loving, C.L.; McGill, J.L. Innate Immunomodulation in Food Animals: Evidence for Trained Immunity? Front. Immunol. 2020, 11. [Google Scholar] [CrossRef]
- Netea, M.G.; Domínguez-Andrés, J.; Barreiro, L.B.; Chavakis, T.; Divangahi, M.; Fuchs, E.; Joosten, L.A.B.; van der Meer, J.W.M.; Mhlanga, M.M.; Mulder, W.J.M.; et al. Defining trained immunity and its role in health and disease. Nat. Rev. Immunol. 2020, 20, 375–388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kleinnijenhuis, J.; Quintin, J.; Preijers, F.; Joosten, L.A.B.; Jacobs, C.; Xavier, R.J.; van der Meer, J.W.M.; van Crevel, R.; Netea, M.G. BCG-induced trained immunity in NK cells: Role for non-specific protection to infection. Clin. Immunol. 2014, 155, 213–219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, Y.; Ali, R.A.; Qureshi, M.A. The influence of β-glucan on immune responses in broiler chicks. Immunopharmacol. Immunotoxicol. 2003, 25, 461–472. [Google Scholar] [CrossRef] [PubMed]
- Avci, F.Y.; Li, X.; Tsuji, M.; Kasper, D.L. Carbohydrates and T cells: A sweet twosome. Semin. Immunol. 2013, 25, 146–151. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Sheng, Z.; Qiu, S.; Yang, H.; Jia, J.; Wang, J.; Jiang, C. Purification, characterization and in vitro and in vivo immune enhancement of polysaccharides from mulberry leaves. PLoS ONE 2019, 14. [Google Scholar] [CrossRef]
- Kallon, S.; Li, X.; Ji, J.; Chen, C.; Xi, Q.; Chang, S.; Xue, C.; Ma, J.; Xie, Q.; Zhang, Y. Astragalus polysaccharide enhances immunity and inhibits H9N2 avian influenza virus in vitro and in vivo. J. Animal Sci. Biotechnol. 2013, 4. [Google Scholar] [CrossRef] [Green Version]
- Teng, P.; Kim, W.K. Review: Roles of prebiotics in intestinal ecosystem of broilers. Front. Vet. Sci. 2018, 5. [Google Scholar] [CrossRef]
- Saminathan, M.; Sieo, C.C.; Kalavathy, R.; Abdullah, N.; Ho, Y.W. Effect of prebiotic oligosaccharides on growth of Lactobacillus strains used as a probiotic for chickens. Afr. J. Microbiol. Res. 2011, 5, 57–64. [Google Scholar]
- Flint, H.J.; Duncan, S.H.; Scott, K.P.; Louis, P. Links between diet, gut microbiota composition and gut metabolism. Proc. Nutr. Soc. 2014, 760. [Google Scholar] [CrossRef] [Green Version]
- Rinttilä, T.; Apajalahti, J. Intestinal microbiota and metabolites-Implications for broiler chicken health and performance. J. Appl. Poult. Res. 2013, 22, 647–658. [Google Scholar] [CrossRef]
- Martín, R.; Miquel, S.; Benevides, L.; Bridonneau, C.; Robert, V.; Hudault, S.; Chain, F.; Berteau, O.; Azevedo, V.; Chatel, J.M.; et al. Functional characterization of novel Faecalibacterium prausnitzii strains isolated from healthy volunteers: A step forward in the use of F. prausnitzii as a next-generation probiotic. Front. Microbiol. 2017, 8. [Google Scholar] [CrossRef]
- Frank, D.N.; St. Amand, A.L.; Feldman, R.A.; Boedeker, E.C.; Harpaz, N.; Pace, N.R. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc. Natl. Acad. Sci. USA 2007, 104, 13780–13785. [Google Scholar] [CrossRef] [Green Version]
- Faas, M.M.; Liu, Y.; Borghuis, T.; van Loo-Bouwman, C.A.; Harmsen, H.; de Vos, P. Microbiota Induced Changes in the Immune Response in Pregnant Mice. Front. Immunol. 2020, 10. [Google Scholar] [CrossRef] [Green Version]
- Clarke, T.B.; Davis, K.M.; Lysenko, E.S.; Zhou, A.Y.; Yu, Y.; Weiser, J.N. Recognition of peptidoglycan from the microbiota by Nod1 enhances systemic innate immunity. Nat. Med. 2010, 16, 228–231. [Google Scholar] [CrossRef] [Green Version]
- Macpherson, A.J.; Harris, N.L. Interactions between commensal intestinal bacteria and the immune system. Nat. Rev. Immunol. 2004, 4, 478–485. [Google Scholar] [CrossRef] [PubMed]
- Aziz, N.; Bonavida, B. Activation of natural killer cells by probiotics. Forum. Immunopathol. Dis. Ther. 2016, 7, 41–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferlazzo, G.; Rizzello, V.; Bonaccorsi, I.; Dongarr, M.L.; Fink, L.N. Role of natural killer and dendritic cell crosstalk in immunomodulation by commensal bacteria probiotics. J. Biomed. Biotechnol. 2011, 2011. [Google Scholar] [CrossRef] [Green Version]
- Hessle, C.; Andersson, B.; Wold, A.E. Gram-positive bacteria are potent inducers of monocytic interleukin-12 (IL-12) while gram-negative bacteria preferentially stimulate IL-10 production. Infect. Immun. 2000, 68, 3581–3586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trinchieri, G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat. Rev. Immunol. 2003, 3, 133–146. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, D.; Krempels, R.; Ryfe, J.; Weitzman, K.; Stephenson, D.; Gigley, J.P. NK cells in mucosal defense against infection. BioMed. Res. Int. 2014, 2014. [Google Scholar] [CrossRef]
- Brownlie, R.; Zhu, J.; Allan, B.; Mutwiri, G.K.; Babiuk, L.A.; Potter, A.; Griebel, P. Chicken TLR21 acts as a functional homologue to mammalian TLR9 in the recognition of CpG oligodeoxynucleotides. Mol. Immunol. 2009, 46, 3163–3170. [Google Scholar] [CrossRef] [PubMed]
- Levy, M.; Blacher, E.; Elinav, E. Microbiome, metabolites and host immunity. Curr. Opin. Microbiol. 2017, 35, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, H.; Thaiss, C.A.; Levy, M.; Elinav, E. The cross talk between microbiota and the immune system: Metabolites take center stage. Curr. Opin. Immunol. 2014, 30, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Skelly, A.N.; Sato, Y.; Kearney, S.; Honda, K. Mining the microbiota for microbial and metabolite-based immunotherapies. Nat. Rev. Immunol. 2019, 19, 305–323. [Google Scholar] [CrossRef]
- Esch, B.C.A.M.V.; Porbahaie, M.; Abbring, S.; Garssen, J.; Potaczek, D.P.; Savelkoul, H.F.J.; Neerven, R.J.J.V. The Impact of Milk and Its Components on Epigenetic Programming of Immune Function in Early Life and Beyond: Implications for Allergy and Asthma. Front. Immunol. 2020, 11. [Google Scholar] [CrossRef]






| Cell Population | Primary Antibody (Mouse-Anti-Chicken) | Clone/Isotype | Secondary Antibody |
|---|---|---|---|
| Peripheral blood cells | CD45-PE 1 | LT40/IgM | - |
| Bu-1-FITC 1 | AV20/IgG1 | - | |
| CD3-PB 1 | CT-3/IgG1 | - | |
| CD4-APC 1 | CT-4/IgG1 | - | |
| CD8α-PE/Cy5 1 | CT-8/IgG1 | - | |
| NK cells | CD45-FITC 1 | LT40/IgM | - |
| CD3-APC 1 | CT3/IgG1 | - | |
| IL-2Rα-UNL 2 | 28–4/IgG3 | Goat-anti-mouse-IgG3-PE 1 | |
| 20E5-BIOT 2 | IgG1 | Streptavidin (SA)-PercP 5 | |
| T cells | CD3-PE 1 | CT3/IgG1 | - |
| CD4-APC 1 | CT4/IgG1 | - | |
| TCRγδ-FITC 1 | TCR-1/IgG1 | - | |
| CD8α-UNL 1 | EP72/IgG2b | Goat-anti-mouse-IgG2b-APC/Cy7 1 | |
| CD8β-BIOT 1 | EP42/IgG2a | SA-PercP5 | |
| Activation of NK and T cells | |||
| in vitro CD107 | CD107a-APC 3 | LEP-100 I 5G10/IgG1 | - |
| CD41/61-FITC 4 | 11C3/IgG1 | - | |
| CD3-PE 1 | CT3/IgG1 | - | |
| in vivo CD107 | CD107a-APC 3 | LEP-100 I 5G10/IgG1 | - |
| CD41/61-FITC 4 | 11C3/IgG1 | - | |
| CD3-PE 1 | CT3/IgG1 | - | |
| CD8α-UNL 1 | EP72/IgG2b | Goat-anti-mouse-IgG2b-Alexa Fluor (AF) 790 6 | |
| Age/Intestinal Segment | Control vs. F1 | Control vs. F2 | F1 vs. F2 |
|---|---|---|---|
| 7 days/ileum | C: Clostridium bartletii 1 and 2, Lactobacillus jenseni, Bifidobacterium 2, Enterococcus sp.; F1: Lactobacillus reuteri 1, Lactobacillus 3; | C: Clostridium bartletii 1 and 2; F2: Lactobacillus crispatus 2, Bifidobacterium 2; | F1: Lactobacillus 3, Lactobacillus reuteri 1; F2: Lactobacillus crispatus 2, Lactobacillus crispatus 3, Lactobacillus jenseni, Enterococcus sp.; |
| 7 days/ceca | C: Lachnospiraceae Incertae Sedis 6, Dorea formicigenerans, Fusobacterium group 2, Sanguibacter; F1: Lactobacillus reuteri 1, Lactobacillus 3, Lachnospiraceae Incertae Sedis 4, Ruminococcus sp.; | C: Ruminococcus sp.; F2: E. coli 1, E. coli 2, Serratia marcescens; | F1: Ruminococcus Incertae Sedis siraeum, Ruminococcaceae unclassified, Lactobacillus 3, Lactobacillus reuteri 1; F2: Faecalibacterium 1, Lachnospiraceae Incertae Sedis 6, Lachnospiraceae unclassified, Dorea formicigenerans, Serratia marcescens, Bifidobacterium gallinarum; |
| 14 days/ileum | C: Lachnospiraceae Incertae Sedis 2, Bacteroides uncult, Listeria; F1: Lactobacillus reuteri 1, Lactobacillus 3; | C: Lachnospiraceae Incertae Sedis 2, Bacteroides uncult, Clostridium bartletii 2; F2: none | None |
| 14 days/ceca | C: Ruminococcus unclassified, Clostridiales unclassified, Incertae Sedis Xlll unclassified; F1: Lactobacillus sp., Lactobacillus gasseri 2, Lactobacillus crispatus 3; | C: Salmonella 1, Parabacteroides 2, Rikenellaceae Alistipes 1, Salinococcus, Clostridiales unclassified, Incertae Sedis Xlll unclassified; F2: Lachnospiraceae Incertae Sedis 3; | F1: Lachnospiraceae Incertae Sedis 11; F2: Campylobacter jejuni; |
| 21 days/ileum | None | C: Lactobacillus crispatus 2, Bifidobacterium 2, Enterococcus hirae; F2: Lactobacillus panis; | None |
| 21 days/ceca | C: Lachnospiraceae unclassified, Ruminococcus Incertae Sedis; F1: none | C: Lachnospiraceae unclassified, Ruminococcus Incertae Sedis, Citrobacter; F2: Bacteria unclassified; | F1: Agreia; F2: none |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meijerink, N.; de Oliveira, J.E.; van Haarlem, D.A.; Hosotani, G.; Lamot, D.M.; Stegeman, J.A.; Rutten, V.P.M.G.; Jansen, C.A. Glucose Oligosaccharide and Long-Chain Glucomannan Feed Additives Induce Enhanced Activation of Intraepithelial NK Cells and Relative Abundance of Commensal Lactic Acid Bacteria in Broiler Chickens. Vet. Sci. 2021, 8, 110. https://doi.org/10.3390/vetsci8060110
Meijerink N, de Oliveira JE, van Haarlem DA, Hosotani G, Lamot DM, Stegeman JA, Rutten VPMG, Jansen CA. Glucose Oligosaccharide and Long-Chain Glucomannan Feed Additives Induce Enhanced Activation of Intraepithelial NK Cells and Relative Abundance of Commensal Lactic Acid Bacteria in Broiler Chickens. Veterinary Sciences. 2021; 8(6):110. https://doi.org/10.3390/vetsci8060110
Chicago/Turabian StyleMeijerink, Nathalie, Jean E. de Oliveira, Daphne A. van Haarlem, Guilherme Hosotani, David M. Lamot, J. Arjan Stegeman, Victor P. M. G. Rutten, and Christine A. Jansen. 2021. "Glucose Oligosaccharide and Long-Chain Glucomannan Feed Additives Induce Enhanced Activation of Intraepithelial NK Cells and Relative Abundance of Commensal Lactic Acid Bacteria in Broiler Chickens" Veterinary Sciences 8, no. 6: 110. https://doi.org/10.3390/vetsci8060110
APA StyleMeijerink, N., de Oliveira, J. E., van Haarlem, D. A., Hosotani, G., Lamot, D. M., Stegeman, J. A., Rutten, V. P. M. G., & Jansen, C. A. (2021). Glucose Oligosaccharide and Long-Chain Glucomannan Feed Additives Induce Enhanced Activation of Intraepithelial NK Cells and Relative Abundance of Commensal Lactic Acid Bacteria in Broiler Chickens. Veterinary Sciences, 8(6), 110. https://doi.org/10.3390/vetsci8060110






