Surveillance for Babesia odocoilei in Hunter-Harvested Wild-Elk (Cervus elaphus canadensis) from Pennsylvania, USA (2016–2017)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Blood Collection and Storage
2.2. DNA Extraction and PCR Identification
2.3. Blood Smear Evaluation
2.4. Statistics
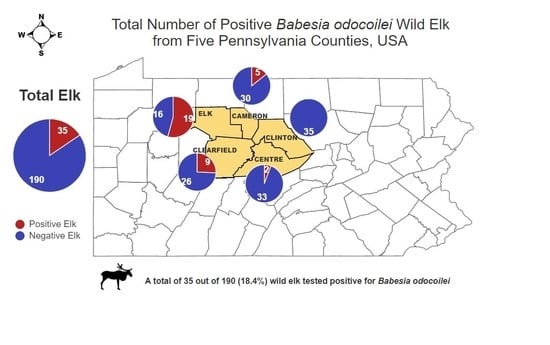
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Homer, M.J.; Aguilar-Delfin, I.; Telford, S.R.; Krause, P.J.; Persing, D.H. Babesiosis. Clin. Microbiol. Rev. 2000, 13, 451–469. [Google Scholar] [CrossRef] [PubMed]
- Yabsley, M.J. Natural History of Zoonotic Babesia: Role of Wildlife Reservoirs. Int. J. Parasitol. 2013, 6, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Criado-Fornelio, A.; Martinez-Marcos, A.; Buling-Saraña, A.; Barba-Carretero, J.C. Molecular Studies on Babesia, Theileria and Hepatozoon in Southern Europe: Part I. Epizootiological Aspects. Vet. Parasitol. 2003, 113, 189–201. [Google Scholar] [CrossRef]
- Milnes, E.L. Eco-Epidemiology and Treatment of Babesiosis in Cervids. Ph.D. Thesis, University of Guelph, Guelph, ON, Canada, 2018. [Google Scholar]
- Mathieu, A.; Pastor, A.R.; Berkvens, C.N.; Gara-Boivin, C.; Hébert, M.; Léveillé, A.N.; Barta, J.R.; Smith, D.A. Babesia odocoilei as a Cause of Mortality in Captive Cervids in Canada. Can. Vet. J. 2018, 59, 52–58. [Google Scholar] [PubMed]
- Emerson, H.R.; Wright, W.T. The Isolation of A Babesia In White-Tailed Deer. Bull. Wildl. Dis. Assoc. 1968, 4, 142–143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- CORRECTION Emerson, H.R.; Wright, W.T. The Isolation of A Babesia In White-Tailed Deer. Bull. Wildl. Dis. Assoc. 1970, 6, 519.
- Schoelkopf, L.; Hutchinson, C.E.; Bendele, K.G.; Goff, W.L.; Willette, M.; Rasmussen, J.M.; Holman, P.J. New Ruminant Hosts and Wider Geographic Range Identified for Babesia odocoilei. J. Wildl. Dis. 2005, 41, 683–690. [Google Scholar] [CrossRef] [Green Version]
- Gallatin, L.L.; Irizarry-Rovira, A.R.; Renninger, M.L.; Holman, P.J.; Wagner, G.G.; Sojka, J.E.; Christian, J.A. Babesia odocoilei Infection in Elk. J. Am. Vet. Med. Assoc. 2003, 223, 1027–1032. [Google Scholar] [CrossRef]
- Pattullo, K.M.; Wobeser, G.; Lockerbie, B.P.; Burgess, H.J. Babesia odocoilei Infection in a Saskatchewan Elk (Cervus elaphus canadensis) Herd. J. Vet. Diagn. Invest. 2013, 25, 535–540. [Google Scholar] [CrossRef]
- Goethert, H.K.; Iii, S.R.T. Enzootic Transmission of Babesia divergens Among Cottontail Rabbits on Nantucket Island, Massachusetts. Am. J. Trop. Med. Hyg. 2003, 69, 455–460. [Google Scholar] [CrossRef] [Green Version]
- Armstrong, P.M.; Katavolos, P.; Caporale, D.A.; Smith, R.P.; Spielman, A.; Telford, S.R., 3rd. Diversity of Babesia Infecting Deer Ticks (Ixodes dammini). Am. J. Trop. Med. Hyg. 1998, 58, 739–742. [Google Scholar] [CrossRef] [PubMed]
- Ameri, M.; Anderson, W.I.; Holman, P.J.; Palmer, G.W. Babesia odocoilei Infection in a North American Elk (Cervus elaphus canadensis). Comp. Clin. Pathol. 2012, 21, 363–365. [Google Scholar] [CrossRef]
- Puraite, I.; Rosef, O.; Radzijevskaja, J.; Lipatova, I.; Paulauskas, A. The First Detection of Species of Babesia Starcovici, 1893 in Moose, Alces alces (Linnaeus), in Norway. Folia Parasitol. (Praha) 2016, 63. [Google Scholar] [CrossRef] [Green Version]
- Teal, A.E.; Habura, A.; Ennis, J.; Keithly, J.S.; Madison-Antenucci, S. A New Real-Time PCR Assay for Improved Detection of the Parasite Babesia Microti. J. Clin. Microbiol. 2012, 50, 903–908. [Google Scholar] [CrossRef] [Green Version]
- Holman, P.J.; Bendele, K.G.; Schoelkopf, L.; Jones-Witthuhn, R.L.; Jones, S.O. Ribosomal RNA Analysis of Babesia odocoilei Isolates from Farmed Reindeer (Rangifer tarandus tarandus) and Elk (Cervus elaphus canadensis) in Wisconsin. Parasitol. Res. 2003, 91, 378–383. [Google Scholar] [CrossRef] [PubMed]
- Ndao, M.; Bandyayera, E.; Kokoskin, E.; Gyorkos, T.W.; MacLean, J.D.; Ward, B.J. Comparison of Blood Smear, Antigen Detection, and Nested-PCR Methods for Screening Refugees from Regions Where Malaria Is Endemic after a Malaria Outbreak in Quebec, Canada. J. Clin. Microbiol. 2004, 42, 2694–2700. [Google Scholar] [CrossRef] [Green Version]
- Aktas, M.; Altay, K.; Dumanli, N. Determination of Prevalence and Risk Factors for Infection with Babesia ovis in Small Ruminants from Turkey by Polymerase Chain Reaction. Parasitol. Res. 2007, 100, 797–802. [Google Scholar] [CrossRef]
- Ano, H.; Makimura, S.; Harasawa, R. Detection of Babesia Species from Infected Dog Blood by Polymerase Chain Reaction. J. Vet. Med. Sci. 2001, 63, 111–113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lockerbie, B.P.; Bollinger, T.K.; Burgess, H.J. Use of the Polymerase Chain Reaction Assay for the Detection of Babesia odocoilei 18S Ribosomal RNA in Formalin-Fixed Tissues. J. Vet. Diagn. Invest. 2014, 26, 538–541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aktaş, M.; Altay, K.; Dumanli, N. Development of a Polymerase Chain Reaction Method for Diagnosis of Babesia ovis Infection in Sheep and Goats. Vet. Parasitol. 2005, 133, 277–281. [Google Scholar] [CrossRef]
- Krause, P.J.; Telford, S.; Spielman, A.; Ryan, R.; Magera, J.; Rajan, T.V.; Christianson, D.; Alberghini, T.V.; Bow, L.; Persing, D. Comparison of PCR with Blood Smear and Inoculation of Small Animals for Diagnosis of Babesia microti Parasitemia. J. Clin. Microbiol. 1996, 34, 2791–2794. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holman, P.J.; Madeley, J.; Craig, T.M.; Allsopp, B.A.; Allsopp, M.T.E.P.; Petrini, K.R.; Waghela, S.D.; Wagner, G.G. Antigenic, Phenotypic and Molecular Characterization Confirms Babesia odocoilei Isolated from Three Cervids. J. Wildl. Dis. 2000, 36, 518–530. [Google Scholar] [CrossRef] [PubMed]
- Pak, D.; Jacobs, S.B.; Sakamoto, J.M. A 117-Year Retrospective Analysis of Pennsylvania Tick Community Dynamics. Parasit. Vectors 2019, 12, 189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calvente, E.; Pelletier, S.; Banfield, J.; Brown, J.; Chinnici, N. Prevalence of Winter Ticks (Dermacentor albipictus) in Hunter-Harvested Wild Elk (Cervus canadensis) from Pennsylvania, USA (2017–2018). Vet. Sci. 2020, 7, 177. [Google Scholar] [CrossRef] [PubMed]
- Holman, P.J.; Craig, T.M.; Crider, D.L.D.; Petrini, K.R.; Rhyan, J.; Wagner, G.G. Culture Isolation and Partial Characterization of a Babesia Sp. from a North American Elk (Cervus elaphus). J. Wildl. Dis. 1994, 30, 460–465. [Google Scholar] [CrossRef]
- Calvente, E.; Chinnici, N.; Brown, J.; Banfield, J.E.; Brooks, J.W.; Yabsley, M.J. Winter Tick (Dermacentor albipictus)-Associated Dermatitis in a Wild Elk (Cervus canadensis) in Pennsylvania, USA. J. Wildl. Dis. 2020, 56, 247–250. [Google Scholar] [CrossRef] [PubMed]
- Banfield, J.; Rosenbury, C. Elk Management in Pennsylvania a Five-Year Plan (2020–2025); Pennsylvania Game Commission: Harrisburg, PA, USA, 2020. [Google Scholar]
| Pennsylvania Elk Tested for Babesia odocoilei | |||||
|---|---|---|---|---|---|
| County Data | Gender Data | ||||
| County | Total Tested | Total (+) | Gender | Total Tested | Total (+) |
| Elk | 97 | 19.6% (19/97) | Female | 142 | 22.5% (32/142) |
| Clearfield | 37 | 24.3% (9/37) | |||
| Centre | 26 | 7.7% (2/26) | Male | 46 | 6.5% (3/46) |
| Cameron | 18 | 27.8% (5/18) | |||
| Clinton | 10 | 0% (0/10) | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calvente, E.J.; Steber, C.; Brown, J.; Brown, H.; Banfield, J.; Chinnici, N. Surveillance for Babesia odocoilei in Hunter-Harvested Wild-Elk (Cervus elaphus canadensis) from Pennsylvania, USA (2016–2017). Vet. Sci. 2021, 8, 94. https://doi.org/10.3390/vetsci8060094
Calvente EJ, Steber C, Brown J, Brown H, Banfield J, Chinnici N. Surveillance for Babesia odocoilei in Hunter-Harvested Wild-Elk (Cervus elaphus canadensis) from Pennsylvania, USA (2016–2017). Veterinary Sciences. 2021; 8(6):94. https://doi.org/10.3390/vetsci8060094
Chicago/Turabian StyleCalvente, Elizabeth Jean, Clay Steber, Justin Brown, Holly Brown, Jeremiah Banfield, and Nicole Chinnici. 2021. "Surveillance for Babesia odocoilei in Hunter-Harvested Wild-Elk (Cervus elaphus canadensis) from Pennsylvania, USA (2016–2017)" Veterinary Sciences 8, no. 6: 94. https://doi.org/10.3390/vetsci8060094
APA StyleCalvente, E. J., Steber, C., Brown, J., Brown, H., Banfield, J., & Chinnici, N. (2021). Surveillance for Babesia odocoilei in Hunter-Harvested Wild-Elk (Cervus elaphus canadensis) from Pennsylvania, USA (2016–2017). Veterinary Sciences, 8(6), 94. https://doi.org/10.3390/vetsci8060094