Alterations in the Expression Profile of Serum miR-155, miR-223, miR-17, miR-200a, miR-205, as well as Levels of Interleukin 6, and Prostaglandins during Endometritis in Arabian Mares
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Ethical Approval for Use of Animals
2.3. Animals and Management
2.4. Blood and Endometrial Swabs Sampling
2.5. Cytological Examination
2.6. Microbial Culturing
2.7. Serum IL-6, PGF2α, and PGE2 Estimation
2.8. In Silico Analysis for the Selected Candidate miRNA
2.9. Serum miRNA Isolation, cDNA Synthesis, and Quantitative Real-Time PCR (qRT-PCR)
2.10. Statistical Analysis
3. Results
3.1. Cytological and Microbial Findings
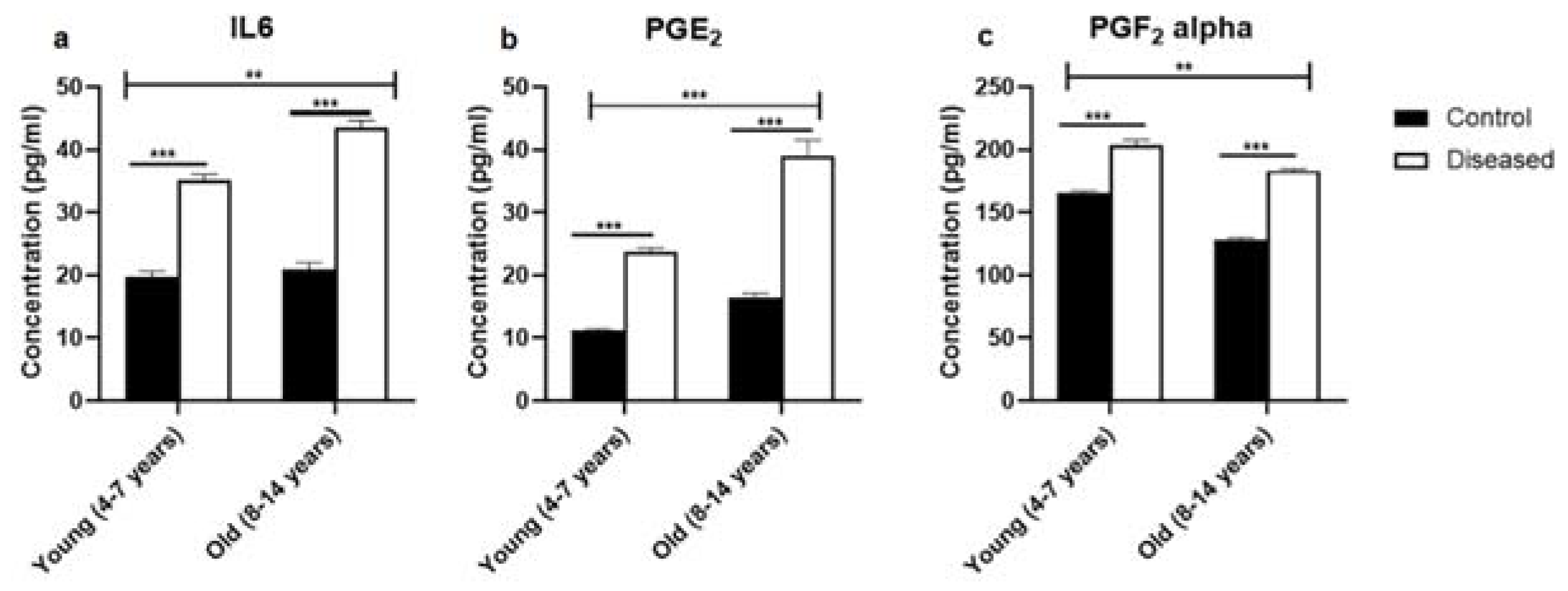
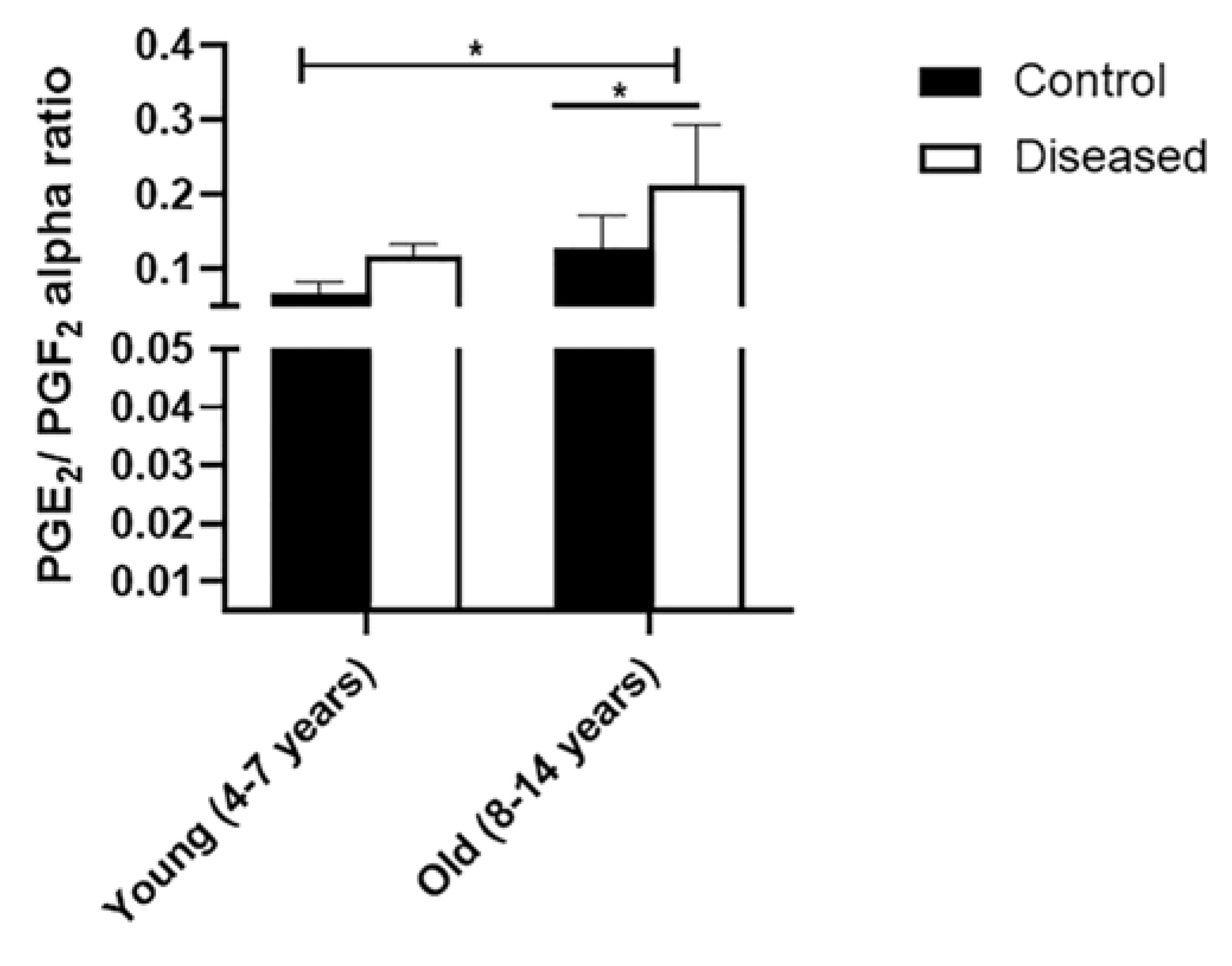
3.2. Dynamic Pattern of Serum IL-6, PGE2, and PGF2α in Mares with Endometritis (Diseased) Compared to Control Ones
3.3. Fold Regulation of Serum miRNA in Mares with Endometritis Compared to Normal Healthy Ones
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Canisso, I.F.; Segabinazzi, L.G.; Fedorka, C.E. Persistent Breeding-Induced Endometritis in Mares—A Multifaceted Challenge: From Clinical Aspects to Immunopathogenesis and Pathobiology. Int. J. Mol. Sci. 2020, 21, 1432. [Google Scholar] [CrossRef] [Green Version]
- Traub-Dargatz, J.L.; Salman, M.D.; Voss, J.L. Medical problems of adult horses, as ranked by equine practitioners. J. Am. Vet. Med. Assoc. 1991, 198, 1745–1747. [Google Scholar]
- Leblanc, M.M.; Causey, R.C. Clinical and Subclinical Endometritis in the Mare: Both Threats to Fertility. Reprod. Domest. Anim. 2009, 44, 10–22. [Google Scholar] [CrossRef]
- Mette, C.; Dooleweerdt, B.C.; Stine, J.; Miki, B.A.; Roenn, P.M.; Henrik, L.J. Evaluation of the systemic acute phase re-sponse and endometrial gene expression of serum amyloid A and pro- and anti-inflammatory cytokines in mares with ex-perimentally induced endometritis. Vet. Immunol. Immunopathol. 2010, 138, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Orsi, N.M.; Tribe, R.M. Cytokine Networks and the Regulation of Uterine Function in Pregnancy and Parturition. J. Neuroendocr. 2008, 20, 462–469. [Google Scholar] [CrossRef]
- Fumuso, E.; Giguere, S.; Wade, J.; Rogan, D.; Videla-Dorna, I.; Bowden, R.A. Endometrial IL-1beta, IL-6 and TNF-alpha, mRNA ex-pression in mares resistant or susceptible to post-breeding endometritis. Effects of estrous cycle, artificial insemination and immunomodulation. Vet. Immunol. Immunopathol. 2003, 96, 31–41. [Google Scholar] [CrossRef]
- Christoffersen, M.; Woodward, E.; Bojesen, A.M.; Jacobsen, S.; Petersen, M.R.; Troedsson, M.H.; Lehn-Jensen, H. Inflammatory responses to induced infec-tious endometritis in mares resistant or susceptible to persistent endometritis. BMC Vet. Res. 2012, 8, 41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woodward, E.M.; Christoffersen, M.; Campos, J.; Betancourt, A.; Horohov, D.; Scoggin, K.E.; Squires, E.L.; Troedsson, M.H.T. Endometrial inflammatory markers of the early immune response in mares susceptible or resistant to persistent breeding-induced endometritis. Reproduction 2013, 145, 289–296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gabler, C.; Drillich, M.; Fischer, C.; Holder, C.; Heuwieser, W.; Einspanier, R. Endometrial expression of selected transcripts involved in prostaglandin synthesis in cows with endometritis. Theriogenology 2009, 71, 993–1004. [Google Scholar] [CrossRef]
- Canisso, I.F.; Stewart, J.; da Silva, M.A.C. Endometritis: Managing Persistent Post-Breeding Endometritis. Vet. Clin. N. Am. Equine Pract. 2016, 32, 465–480. [Google Scholar] [CrossRef]
- Atli, M.O.; Kurar, E.; Kayis, S.A.; Aslan, S.; Semacan, A.; Celik, S.; Guzeloglu, A.; Güzeloğlu, A. Evaluation of genes involved in prostaglandin action in equine endometrium during estrous cycle and early pregnancy. Anim. Reprod. Sci. 2010, 122, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, S.; Szóstek-Mioduchowska, A.; Skarzynski, D. Expression profiling of selected miRNAs in equine endometrium in response to LPS challenge in vitro: A new understanding of the inflammatory immune response. Vet. Immunol. Immunopathol. 2019, 209, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Nash, D.; Lane, E.; Herath, S.; Sheldon, I.M. ORIGINAL ARTICLE: Endometrial Explant Culture for Characterizing Equine Endometritis. Am. J. Reprod. Immunol. 2008, 59, 105–117. [Google Scholar] [CrossRef]
- Troedsson, M.H. Breeding-Induced Endometritis in Mares. Vet. Clin. N. Am. Equine Pract. 2006, 22, 705–712. [Google Scholar] [CrossRef]
- Sharma, A.; Shandilya, U.K.; Sullivan, T.; Naylor, D.; Canovas, A.; Mallard, B.A.; Karrow, N.A. Identification of Ovine Serum miRNAs Following Bacterial Lipopolysaccharide Challenge. Int. J. Mol. Sci. 2020, 21, 7920. [Google Scholar] [CrossRef] [PubMed]
- Dini, P.; Ali, H.E.-S.; Carossino, M.; Loux, S.C.; Esteller-Vico, A.; Scoggin, K.E.; Daels, P.; Ball, B.A. Expression Profile of the Chromosome 14 MicroRNA Cluster (C14MC) Ortholog in Equine Maternal Circulation throughout Pregnancy and Its Potential Implications. Int. J. Mol. Sci. 2019, 20, 6285. [Google Scholar] [CrossRef] [Green Version]
- Unger, L.; Abril, C.; Gerber, V.; Jagannathan, V.; Koch, C.; Hamza, E. Diagnostic potential of three serum microRNAs as bi-omarkers for equine sarcoid disease in horses and donkeys. J. Vet. Intern. Med. 2021, 35, 610–619. [Google Scholar] [CrossRef]
- Bartel, D.P. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef] [Green Version]
- Bueno, M.J.; De Castro, I.P.; Malumbres, M. Control of cell proliferation pathways by microRNAs. Cell Cycle 2008, 7, 3143–3148. [Google Scholar] [CrossRef]
- Salilew-Wondim, D.; Ibrahim, S.; Gebremedhn, S.; Tesfaye, D.; Heppelmann, M.; Bollwein, H.; Pfarrer, C.; Tholen, E.; Neuhoff, C.; Schellander, K.; et al. Clinical and subclinical endometritis induced alterations in bovine endometrial transcriptome and miRNome profile. BMC Genom. 2016, 17, 1–21. [Google Scholar] [CrossRef] [Green Version]
- Gebremedhn, S.; Ali, A.; Hossain, M.; Hoelker, M.; Salilew-Wondim, D.; Anthony, R.V.; Tesfaye, D. MicroRNA-Mediated Gene Regulatory Mech-anisms in Mammalian Female Reproductive Health. Int. J. Mol. Sci. 2021, 22, 938. [Google Scholar] [CrossRef]
- Ibrahim, S.; Salilew-Wondim, D.; Rings, F.; Hoelker, M.; Neuhoff, C.; Tholen, E.; Looft, C.; Schellander, K.; Tesfaye, D. Expression pattern of inflammatory response genes and their regulatory micrornas in bovine oviductal cells in response to lipopolysaccharide: Implication for early em-bryonic development. PLoS ONE 2015, 10, e0119388. [Google Scholar] [CrossRef] [Green Version]
- Schulte, L.N.; Westermann, A.J.; Vogel, J. Differential activation and functional specialization of miR-146 and miR-155 in innate immune sensing. Nucleic Acids Res. 2012, 41, 542–553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kasimanickam, V.; Kastelic, J. Circulating cell-free mature microRNAs and their target gene prediction in bovine metritis. Sci. Rep. 2016, 6, 29509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carnevale, E.; Ginther, O. Relationships of age to uterine function and reproductive efficiency in mares. Theriogenology 1992, 37, 1101–1115. [Google Scholar] [CrossRef]
- Hurtgen, J.P. Pathogenesis and treatment of endometritis in the mare: A review. Theriogenology 2006, 66, 560–566. [Google Scholar] [CrossRef]
- de Amorim, M.D.; Gartley, C.J.; Foster, R.A.; Hill, A.; Scholtz, E.L.; Hayes, A.; Chenier, T.S. Comparison of Clinical Signs, Endometrial Culture, En-dometrial Cytology, Uterine Low-Volume Lavage, and Uterine Biopsy and Combinations in the Diagnosis of Equine Endo-metritis. J. Equine Vet. Sci. 2016, 44, 54–61. [Google Scholar] [CrossRef]
- Dascanio, J.J. Uterine Culture Collection: Swab/Brush. Equine Reproductive Procedures. Equine Reprod. Proced. 2014, 28, 41–43. [Google Scholar]
- Bohn, A.A.; Ferris, R.A.; McCue, P.M. Comparison of equine endometrial cytology samples collected with uterine swab, uterine brush, and low-volume lavage from healthy mares. Vet. Clin. Pathol. 2014, 43, 594–600. [Google Scholar] [CrossRef]
- Aguilar, J.; Hanks, M.; Shaw, D.J.; Else, R.; Watson, E. Importance of using guarded techniques for the preparation of endo-metrial cytology smears in mares. Theriogenology 2006, 66, 423–430. [Google Scholar] [CrossRef]
- Nielsen, J.M. Endometritis in the mare: A diagnostic study comparing cultures from swab and biopsy. Theriogenology 2005, 64, 510–518. [Google Scholar] [CrossRef]
- Yu, J.; Chen, J.; Yang, H.; Chen, S.; Wang, Z. Overexpression of miR200a3p promoted inflammation in sepsisinduced brain injury through ROSinduced NLRP3. Int. J. Mol. Med. 2019, 44, 1811–1823. [Google Scholar] [PubMed]
- Mi, S.; Zhang, J.; Zhang, W.; Huang, R.S. Circulating MicroRNAs as Biomarkers for Inflammatory Diseases. MicroRNA 2013, 2, 64–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andersen, C.L.; Jensen, J.L.; Orntoft, T.F. Normalization of real-time quantitative reverse transcription-PCR data: A mod-el-based variance estimation approach to identify genes suited for normalization, applied to bladder and colon cancer data sets. Cancer Res. 2004, 64, 5245–5250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, S.; Fernald, R.D. Comprehensive Algorithm for Quantitative Real-Time Polymerase Chain Reaction. J. Comput. Biol. 2005, 12, 1047–1064. [Google Scholar] [CrossRef] [PubMed]
- Woodward, E.; Christoffersen, M.; Campos, J.; Squires, E.; Troedsson, M. Susceptibility to persistent breeding-induced endometritis in the mare: Relationship to endometrial biopsy score and age, and variations between seasons. Theriogenology 2012, 78, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Kasimanickam, R.K.; Kasimanickam, V.R.; Olsen, J.R.; Jeffress, E.J.; Moore, D.A.; Kastelic, J.P. Associations among serum pro- and an-ti-inflammatory cytokines, metabolic mediators, body condition, and uterine disease in postpartum dairy cows. Reprod. Biol. Endocrinol. 2013, 11, 103. [Google Scholar] [CrossRef] [Green Version]
- Nasreldin, N.; Ali, F.A.Z.; Abd-Elhafeez, H.H.; Hassan, M.; El-Zeftawy, M.; Senosy, W. Characterization of immunological, bio-chemical and inflammatory response of clinical and subclinical endometritis in ewes in the subtropics. Anim. Reprod. Sci. 2020, 219, 106541. [Google Scholar] [CrossRef]
- Diao, H.; Kohanawa, M. Endogenous Interleukin-6 Plays a Crucial Protective Role in Streptococcal Toxic Shock Syndrome via Suppression of Tumor Necrosis Factor Alpha Production. Infect. Immun. 2005, 73, 3745–3748. [Google Scholar] [CrossRef] [Green Version]
- Jones, S.A. Directing Transition from Innate to Acquired Immunity: Defining a Role for IL-6. J. Immunol. 2005, 175, 3463–3468. [Google Scholar] [CrossRef]
- Devi, Y.S.; DeVine, M.; DeKuiper, J.; Ferguson, S.; Fazleabas, A.T. Inhibition of IL-6 signaling pathway by curcumin in uter-ine decidual cells. PLoS ONE 2015, 10, e0125627. [Google Scholar] [CrossRef]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. IL-6 in Inflammation, Immunity, and Disease. Cold Spring Harb. Perspect. Biol. 2014, 6, a016295. [Google Scholar] [CrossRef]
- Ricciotti, E.; FitzGerald, G.A. Prostaglandins and inflammation. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 986–1000. [Google Scholar] [CrossRef]
- Katila, T. Onset and Duration of Uterine Inflammatory Response of Mares after Insemination with Fresh Semen. Biol. Reprod. 1995, 52, 515–517. [Google Scholar] [CrossRef]
- Pan, Q.; Chegini, N. MicroRNA Signature and Regulatory Functions in the Endometrium during Normal and Disease States. Semin. Reprod. Med. 2008, 26, 479–493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morris, L.H.; McCue, P.M.; Aurich, C. Equine endometritis: A review of challenges and new approaches. Reproduction 2020, 160, R95–R110. [Google Scholar] [CrossRef]
- Kaminska, B. MAPK signalling pathways as molecular targets for anti-inflammatory therapy—From molecular mechanisms to therapeutic benefits. Biochim. Biophys. Acta 2005, 1754, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Fesler, A.; Wang, H.; Ju, J. microRNA based prognostic biomarkers in pancreatic Cancer. Biomark. Res. 2018, 6, 1–5. [Google Scholar] [CrossRef]
- Cosar, E.; Mamillapalli, R.; Ersoy, G.S.; Cho, S.; Seifer, B.; Taylor, H.S. Serum microRNAs as diagnostic markers of endometriosis: A comprehensive array-based analysis. Fertil. Steril. 2016, 106, 402–409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Condrat, C.E.; Thompson, D.C.; Barbu, M.G.; Bugnar, O.L.; Boboc, A.; Cretoiu, D.; Suciu, N.; Cretoiu, S.M.; Voinea, S.C. miRNAs as Biomarkers in Disease: Latest Findings Regarding Their Role in Diagnosis and Prognosis. Cells 2020, 9, 276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mori, M.A.; Ludwig, R.G.; Garcia-Martin, R.; Brandão, B.B.; Kahn, C.R. Extracellular miRNAs: From Biomarkers to Mediators of Physiology and Disease. Cell Metab. 2019, 30, 656–673. [Google Scholar] [CrossRef] [PubMed]
- Kamity, R.; Sharma, S.; Hanna, N. MicroRNA-Mediated Control of Inflammation and Tolerance in Pregnancy. Front. Immunol. 2019, 10, 718. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| miR Name | Accession Number | Mature miRNA Sequence |
|---|---|---|
| eca-miR-223 | MIMAT0013205 | UGUCAGUUUGUCAAAUACCCCA |
| eca-miR-155 | MIMAT0013182 | UUAAUGCUAAUCGUGAUAGGGGU |
| eca-miR-200a | MIMAT0012909 | UAACACUGUCUGGUAACGAUGU |
| eca-miR-17 | MIMAT0013084 | CAAAGUGCUUACAGUGCAGGUAG |
| eca-miR-205 | MIMAT0012962 | UCCUUCAUUCCACCGGAGUCUG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ibrahim, S.; Hedia, M.; Taqi, M.O.; Derbala, M.K.; Mahmoud, K.G.M.; Ahmed, Y.; Ismail, S.; El-Belely, M. Alterations in the Expression Profile of Serum miR-155, miR-223, miR-17, miR-200a, miR-205, as well as Levels of Interleukin 6, and Prostaglandins during Endometritis in Arabian Mares. Vet. Sci. 2021, 8, 98. https://doi.org/10.3390/vetsci8060098
Ibrahim S, Hedia M, Taqi MO, Derbala MK, Mahmoud KGM, Ahmed Y, Ismail S, El-Belely M. Alterations in the Expression Profile of Serum miR-155, miR-223, miR-17, miR-200a, miR-205, as well as Levels of Interleukin 6, and Prostaglandins during Endometritis in Arabian Mares. Veterinary Sciences. 2021; 8(6):98. https://doi.org/10.3390/vetsci8060098
Chicago/Turabian StyleIbrahim, Sally, Mohamed Hedia, Mohamed O. Taqi, Mohamed K. Derbala, Karima Gh. M. Mahmoud, Youssef Ahmed, Sayed Ismail, and Mohamed El-Belely. 2021. "Alterations in the Expression Profile of Serum miR-155, miR-223, miR-17, miR-200a, miR-205, as well as Levels of Interleukin 6, and Prostaglandins during Endometritis in Arabian Mares" Veterinary Sciences 8, no. 6: 98. https://doi.org/10.3390/vetsci8060098
APA StyleIbrahim, S., Hedia, M., Taqi, M. O., Derbala, M. K., Mahmoud, K. G. M., Ahmed, Y., Ismail, S., & El-Belely, M. (2021). Alterations in the Expression Profile of Serum miR-155, miR-223, miR-17, miR-200a, miR-205, as well as Levels of Interleukin 6, and Prostaglandins during Endometritis in Arabian Mares. Veterinary Sciences, 8(6), 98. https://doi.org/10.3390/vetsci8060098





