Effect of Honey and Syrup Diets Enriched with 1,3-1,6 β-Glucans on Honeybee Survival Rate and Phenoloxidase Activity (Apis mellifera L. 1758)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling and Honeybees Rearing
2.2. Melissopalynological Analysis
2.3. Survival Rate and Phenoloxidase Activity (PO)
2.4. Statistical Analysis
3. Results
3.1. Melissopalynological Analysis
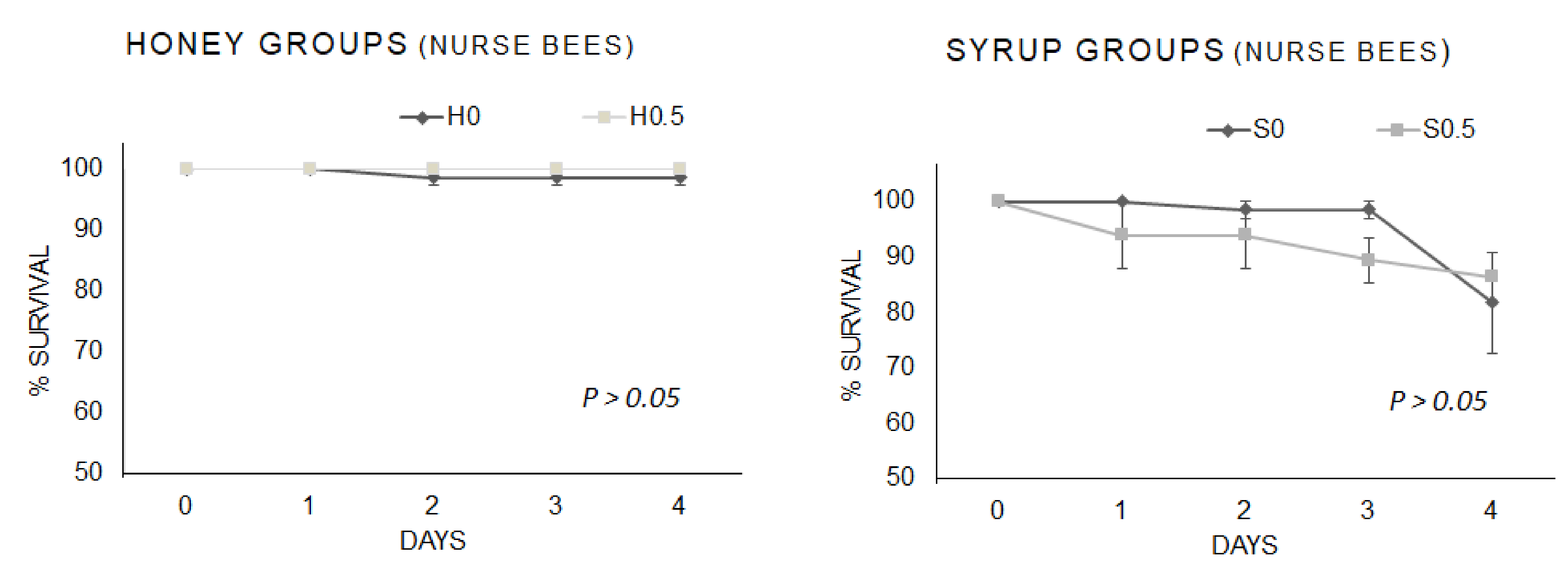
3.2. Survival Rate
3.2.1. Newly Emerged Bees Survival
3.2.2. Nurse Bees Survival
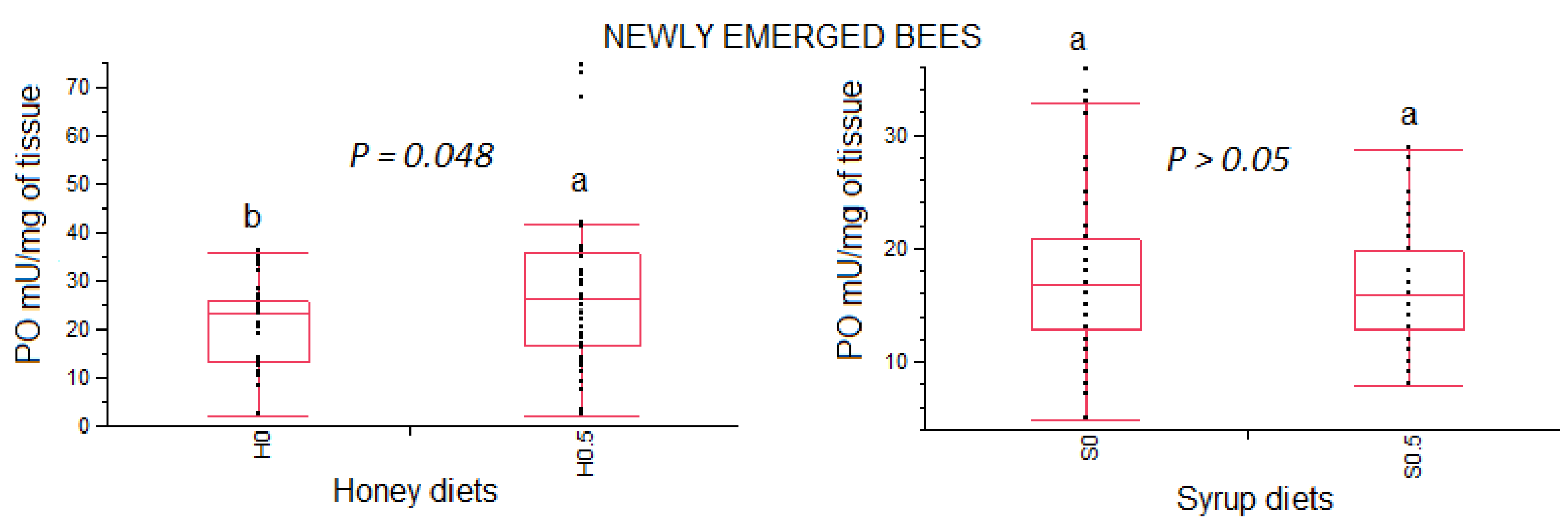
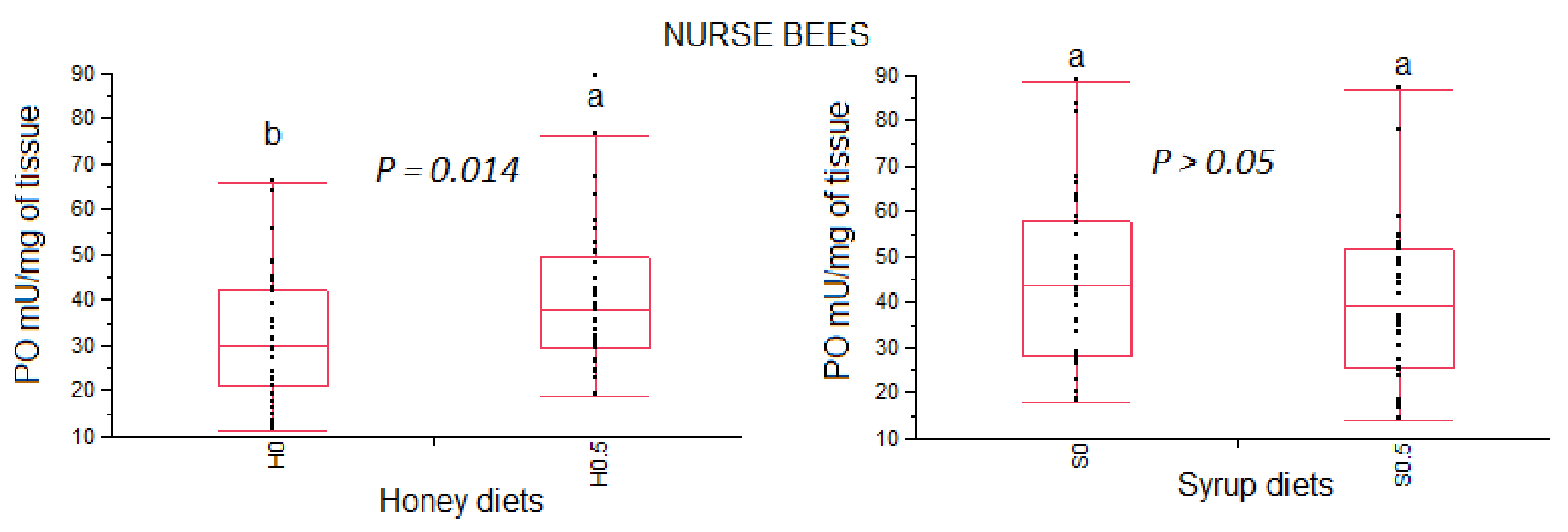
3.3. Phenoloxidase Activity
3.3.1. Phenoloxidase Activity in Newly Emerged Bees
3.3.2. Phenoloxidase Activity in Nurse Bees
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vásquez, A.; Forsgren, E.; Fries, I.; Paxton, R.J.; Flaberg, E.; Szekely, L.; Olofsson, T.C. Symbionts as major modulators of insect health: Lactic acid bacteria and honeybees. PLoS ONE 2012, 7, e33188. [Google Scholar] [CrossRef]
- Roussenova, N. Antibacterial activity of essential oils against the etiological agent of American Foulbrood disease (Paenibacillus larvae). BJVM 2011, 14, 17–24. [Google Scholar]
- Alaux, C.; Ducloz, F.; Crauser, D.; Le Conte, Y. Diet effects on honeybee immunocompetence. Biol. Lett. 2010, 6, 562–565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Pasquale, G.; Salignon, M.; Le Conte, Y.; Belzunces, L.P.; Decourtye, A.; Kretzschmar, A.; Suchail, S.; Brunet, J.L.; Alaux, C. Influence of pollen nutrition on honeybee health: Do pollen quality and diversity matter? PLoS ONE 2013, 8, e72016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DeGrandi-Hoffman, G.; Chen, Y. Nutrition, immunity and viral infections in honeybees. Curr. Opin. Insect Sci. 2015, 10, 170–176. [Google Scholar] [CrossRef] [Green Version]
- Bogo, G.; Bortolotti, L.; Sagona, S.; Felicioli, A.; Galloni, M.; Barberis, M.; Nepi, M. Effects of non-protein amino acids in nectar on bee survival and behavior. J. Chem. Ecol. 2019, 45, 278–285. [Google Scholar] [CrossRef]
- Felicioli, A.; Forzan, M.; Sagona, S.; D’Agostino, P.; Baido, D.; Fronte, B.; Mazzei, M. Effect of oral administration of 1, 3-1, 6 β-glucans in DWV naturally infected newly emerged bees (Apis mellifera L.). Vet. Sci. 2020, 7, 52. [Google Scholar] [CrossRef]
- Wilson, D.S.; Sober, E. Reviving the superorganism. J. Theor. Biol. 1989, 136, 337–356. [Google Scholar] [CrossRef]
- Brodschneider, R.; Crailsheim, K. Nutrition and health in honeybees. Apidologie 2010, 41, 278–294. [Google Scholar] [CrossRef]
- Rothembuhler, W.C. Behavior genetics of nest cleaning in honey bees. IV Responses of F1 and Backcross Generations to Disease-Killed Brood. Am. Zool. 1964, 4, 111–123. [Google Scholar] [CrossRef] [Green Version]
- Takenaka, T.; Ito, H.; Yatsunami, K.; Echigo, T. Changes of Glucose Oxidase Activity and Amount of Gluconic Acid Formation in the Hypopharyngeal Glands during the Lifespan of Honey Bee Workers (Apis mellifera L). Agric. Biol. Chem. 1990, 54, 2133–2134. [Google Scholar]
- Ohashi, K.; Natori, S.; Kubo, T. Expression of amylase and glucose oxidase in the hypopharyngeal gland with an age-dependent role change of the worker honeybee (Apis mellifera L.). Eur. J. Biochem 1999, 265, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Rowley, A.F.; Powell, A. Invertebrate Immune Systems–Specific, Quasi-Specific, or Nonspecific? J. Immunol. 2007, 179, 7209–7214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmid-Hempel, P. Evolutionary Ecology of Insect Immune Defenses. Annu. Rev. Entomol. 2005, 50, 529–551. [Google Scholar] [CrossRef]
- Cerenius, L.; Söderhäll, K. The prophenoloxidase-activating system in invertebrates. Immunol. Rev. 2004, 198, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Cerenius, L.; Lee, B.L.; Söderhäll, K. The proPO-system: Pros and cons for its role in invertebrate immunity. Trends Immunol. 2008, 29, 263–271. [Google Scholar] [CrossRef]
- Wu, K.; Han, F.; Yuan, Y.; Liu, Y.; Ling, E.; Wang, Q.; Huang, W. Effect of the insect phenoloxidase on the metabolism of l-DOPA. Arch. Insect Biochem. Physiol. 2018, 98, e21457. [Google Scholar] [CrossRef]
- Schmid, M.R.; Brockmann, A.; Pirk, C.W.; Stanley, D.W.; Tautz, J. Adult honeybees (Apis mellifera L.) abandon hemocytic, but not phenoloxidase-based immunity. J. Insect Physiol. 2008, 54, 439–444. [Google Scholar] [CrossRef] [Green Version]
- Soltanian, S.; Stuyven, E.; Cox, E.; Sorgeloos, P.; Bossier, P. Beta-glucans as immunostimulant in vertebrates and invertebrates. Crit. Rev. Microbiol. 2009, 35, 109–138. [Google Scholar] [CrossRef]
- Akramienė, D.; Kondrotas, A.J.; Didžiapetrienė, J.; Kėvelaitis, E. Effects of beta-glucans on the immune system. Medicina 2007, 43, 597–606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Novak, M.; Vetvicka, V. Beta-glucans, history, and the present: Immunomodulatory aspects and mechanisms of action. J. Immunotoxicol. 2008, 5, 47–57. [Google Scholar] [CrossRef]
- Volman, J.J.; Ramakers, J.D.; Plat, J. Dietary modulation of immune function by β-glucans. Physiol. Behav. 2008, 94, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Mazzei, M.; Fronte, B.; Sagona, S.; Carrozza, M.L.; Forzan, M.; Pizzurro, F.; Bibbiani, C.; Miragliotta, V.; Abramo, F.; Millanta, F.; et al. Effect of 1,3-1,6 β-Glucan on Natural and Experimental Deformed Wing Virus Infection in Newly Emerged Honeybees (Apis mellifera ligustica). PLoS ONE 2016, 11, e0166297. [Google Scholar] [CrossRef] [PubMed]
- Richard, F.J.; Aubert, A.; Grozinger, C.M. Modulation of social interactions by immune stimulation in honey bee, Apis mellifera, workers. BMC Biol. 2008, 6, 50. [Google Scholar] [CrossRef] [PubMed]
- Mowlds, P.; Coates, C.; Renwick, J.; Kavanagh, K. Dose dependent cellular and humoral responses in Galleria mellonella larvae following β-glucan inoculation. Microbes Infect. 2010, 12, 146–153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Louveaux, J.; Maurizio, A.; Vorwohl, G. Methods of melissopalynology. Bee World 1978, 59, 139–157. [Google Scholar] [CrossRef]
- Ricciardelli-D’Albore, G.; Persano, L. Flora Apistica Italiana; Istituto Sperimentale per la Zoologia Agraria: Firenze, Italy, 1981; reprinted in Federazione Apicoltori Italiani: Roma, Italy, 1981. [Google Scholar]
- Hodges, D. The Pollen Loads of the Honey Bee: A Guide to Their Identification by Colour and Form; International Bee Research Association: London, UK, 1984; p. 114. [Google Scholar]
- Ricciardelli-D’Albore, G. Textbook of Melissopalynology; Apimondia Publishing House: Bucharest, Romania, 1997. [Google Scholar]
- Ricciardelli-D’Albore, G. Mediterranean Melis-Sopalynology; Istituto di Entomologia Agraria, Università degli Studi, Perugia: Perugia, Italy, 1998. [Google Scholar]
- Palmieri, N.; Grillenzoni, F.V.; Corvucci, F.; Biondi, C.; Bedini, G.; Floris, I. Guida allo Studio della Melissopalinologia; CREA-Centro di Ricerca Agricoltura e Ambiente, Università di Sassari-Dipartimento di Agraria, Studio Naturalistico-Il Pianeta Naturale, Associazione Apicoltori della Sardegna Apiaresos de Abarèe. Tipografia Gallizzi: Sassari, Italy, 2017. [Google Scholar]
- Colombo, R.; Marcazzan, G.L.; Sabatini, A.G.; Accorti, M.; Persano Oddo, L.; Piana, M.L.; Piazza, M.G.; Pulcini, P. Parte III Schede dei mieli uniflorali. In Conoscere il Miele, 2nd ed.; Avenue Media: Bologna-Milano, Italy, 2007; pp. 271–320. [Google Scholar]
- Oddo, L.P.; D’Albore, G.R. Nomenclatura melissopalinologica. Apicoltura 1989, 5, 63–72. [Google Scholar]
- SAS Institute. JMP Statistics and Graphics Guide; SAS Institute Inc.: Cary, NC, USA, 2008. [Google Scholar]
- Fluri, P.; Wille, H.; Gerig, L.; Lüscher, M. Juvenile hormone, vitellogenin and haemocyte composition in winter worker honeybees (Apis mellifera). Experientia 1977, 33, 1240–1241. [Google Scholar] [CrossRef]
- Dainat, B.; Evans, J.D.; Chen, Y.P.; Gauthier, L.; Neumann, P. Dead or alive: Deformed wing virus and Varroa destructor reduce the life span of winter honeybees. Appl. Environ. Microbiol. 2012, 78, 981–987. [Google Scholar] [CrossRef] [Green Version]
- Rylander, R.; Fogelmark, B.; McWilliam, A.; Currie, A. 1,3 β-D-glucan may contribute to pollen sensitivity. Clin. Exp. Immunol. 1999, 115, 383–384. [Google Scholar] [CrossRef]
- Hagedorn, H.H.; Moeller, F.E. The rate of pollen consumption by newly emerged honeybees. J. Apic. Res. 1967, 6, 159–162. [Google Scholar] [CrossRef]
- Sadd, B.M.; Schmid-Hempel, P. Insect immunity shows specificity in protection upon secondary pathogen exposure. Curr. Biol. 2006, 16, 1206–1210. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Pollen Species | Pollen Presence (%) | Pollen Species | Pollen Presence (%) |
|---|---|---|---|
| Acer pseudoplatanus gr | P | Palmae (Chamaerops f) | P |
| Actinidia | 1 | Potentilla/Fragaria f | 13 |
| Asparagus acutifolius gr | P | Prunus f | 1 |
| Brassica f | 1 | Quercus ilex gr | 25 |
| Castanea | 3 | Ranunculus repens gr | P |
| Eucalyptus | P | Robinia | 18 |
| Fraxinus ornus | 11 | Rubus f | 1 |
| Gleditsia | 1 | Salix | 3 |
| Graminaceae | 2 | Sambucus nigra | 4 |
| Hedera | 1 | Sedum f | 4 |
| Juncus | P | Trifolium repens gr | 1 |
| Lotus | P | Urticaceae/Moraceae | 1 |
| Mercurialis | P | Verbascum | 3 |
| Olea f | 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sagona, S.; Fronte, B.; Coppola, F.; Tafi, E.; Giusti, M.; Palego, L.; Betti, L.; Giannaccini, G.; Guglielminetti, L.; Felicioli, A. Effect of Honey and Syrup Diets Enriched with 1,3-1,6 β-Glucans on Honeybee Survival Rate and Phenoloxidase Activity (Apis mellifera L. 1758). Vet. Sci. 2021, 8, 130. https://doi.org/10.3390/vetsci8070130
Sagona S, Fronte B, Coppola F, Tafi E, Giusti M, Palego L, Betti L, Giannaccini G, Guglielminetti L, Felicioli A. Effect of Honey and Syrup Diets Enriched with 1,3-1,6 β-Glucans on Honeybee Survival Rate and Phenoloxidase Activity (Apis mellifera L. 1758). Veterinary Sciences. 2021; 8(7):130. https://doi.org/10.3390/vetsci8070130
Chicago/Turabian StyleSagona, Simona, Baldassare Fronte, Francesca Coppola, Elena Tafi, Matteo Giusti, Lionella Palego, Laura Betti, Gino Giannaccini, Lorenzo Guglielminetti, and Antonio Felicioli. 2021. "Effect of Honey and Syrup Diets Enriched with 1,3-1,6 β-Glucans on Honeybee Survival Rate and Phenoloxidase Activity (Apis mellifera L. 1758)" Veterinary Sciences 8, no. 7: 130. https://doi.org/10.3390/vetsci8070130






