Implementation of Mini-FLOTAC in Routine Diagnosis of Coccidia and Helminth Infections in Domestic and Exotic Birds
Abstract
1. Introduction
2. Materials and Methods
2.1. Bird Collections and Fecal Samplings
2.2. Coprological Techniques
2.2.1. Mini-FLOTAC
2.2.2. McMaster
2.2.3. Fecal Cultures
2.3. Statistical Analysis
3. Results
3.1. Epidemiological Results with Mini-FLOTAC in Domestic and Exotic Birds
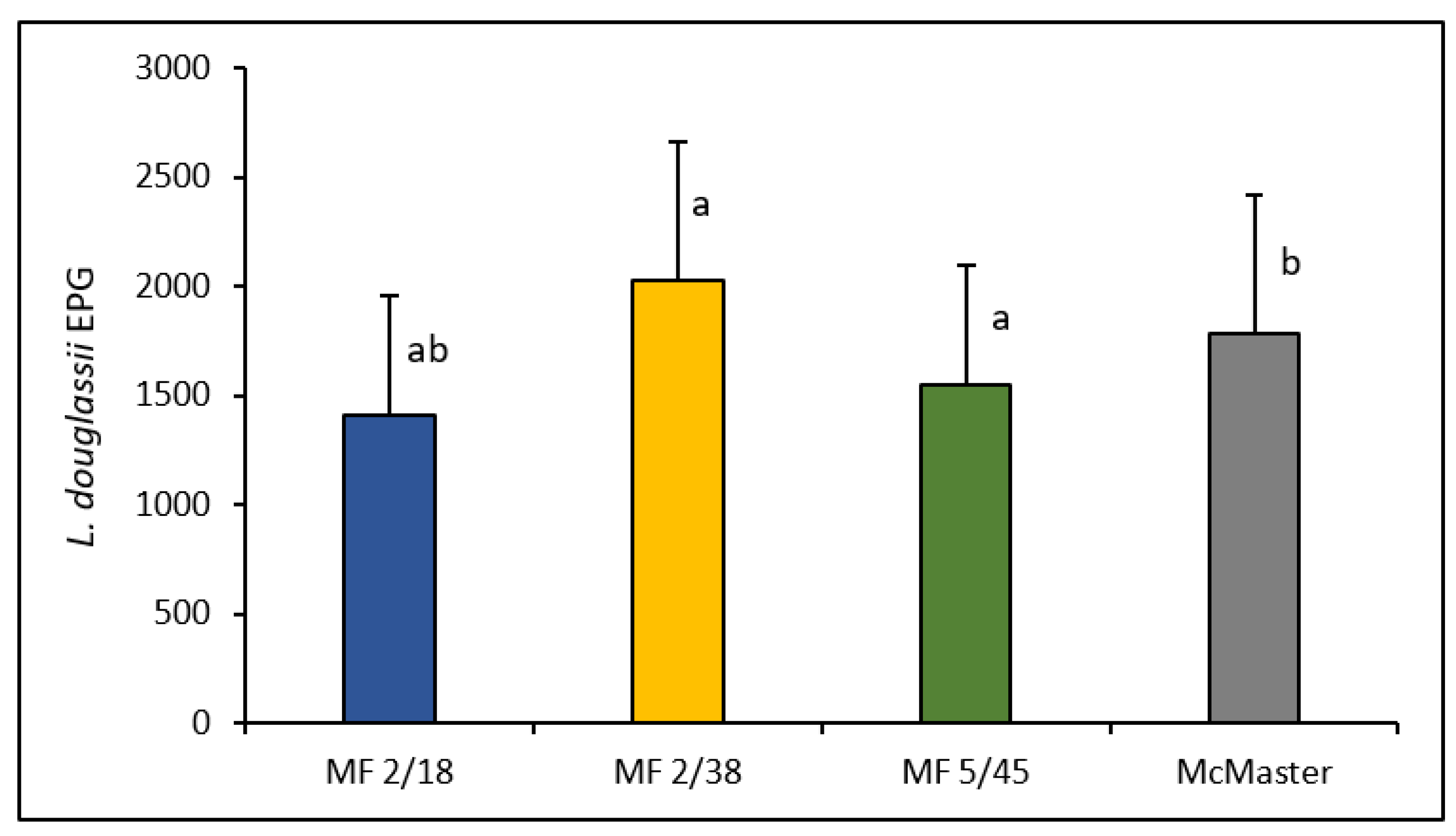
3.2. McMaster and Mini-FLOTAC Comparison
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Papini, R.; Girivetto, M.; Marangi, M.; Mancianti, F.; Giangaspero, A. Endoparasite infections in pet and zoo birds in Italy. Sci. World J. 2012, 2012, 253127. [Google Scholar] [CrossRef] [PubMed]
- Ederli, N.B.; Rodrigues de Oliveira, F.C. Gastrointestinal nematodes in ostriches, Struthio camelus, in different regions of the state of Rio de Janeiro, Brazil. Braz. J. Vet. Parasitol. 2015, 24, 168–173. [Google Scholar] [CrossRef][Green Version]
- Lozano, J.; Anaya, A.; Palomero Salinero, A.; Lux Hoppe, E.G.; Gomes, L.; Paz-Silva, A.; Teresa Rebelo, M.; Madeira de Carvalho, L. Gastrointestinal parasites of free-range chickens—A worldwide issue. Bull. UASVM Vet. Med. 2019, 76, 110–117. [Google Scholar] [CrossRef]
- Blake, D.P.; Knox, J.; Dehaeck, B.; Huntington, B.; Rathinam, T.; Ravipati, V.; Ayoade, S.; Gilbert, W.; Adebambo, A.O.; Jatau, I.D.; et al. Re-calculating the cost of coccidiosis in chickens. Vet. Res. 2020, 51, 115. [Google Scholar] [CrossRef]
- Carrera-Játiva, P.D.; Morgan, E.R.; Barrows, M.; Jiménez-Uzcátegui, G.; Tituaña, J.R.A. Free-ranging avifauna as a source of generalist parasites for captive birds in zoological settings: An overview of parasite records and potential for cross-transmission. J. Adv. Vet. Anim. Res. 2020, 7, 482–500. [Google Scholar] [CrossRef]
- Carrisosa, M.; Jin, S.; McCrea, B.A.; Macklin, K.S.; Dormitorio, T.; Hauck, R. Prevalence of select intestinal parasites in Alabama backyard poultry flocks. Animals 2021, 11, 939. [Google Scholar] [CrossRef] [PubMed]
- Titilincu, A.; Mircean, V.; Bejan, A.; Iovu, A.; Ungureanu, R.; Cozma, V. Prevalence of endoparasites in peacocks (Pavo cristatus). Sci. Parasitol. 2009, 10, 101–105. [Google Scholar]
- Jaiswal, A.K.; Sudan, V.; Shanker, D.; Kumar, P. Endoparasitic infections in Indian peacocks (Pavo cristatus) of Veterinary College Campus, Mathura. J. Parasit. Dis. 2013, 37, 26–28. [Google Scholar] [CrossRef][Green Version]
- Prakashbabu, B.C.; Thenmozhi, V.; Limon, G.; Kundu, K.; Kumar, S.; Garg, R.; Clark, E.L.; Srinivasa Rao, A.S.R.; Raj, D.G.; Raman, M.; et al. Eimeria species occurrence varies between geographic regions and poultry production systems and may influence parasite genetic diversity. Vet. Parasitol. 2017, 233, 62–72. [Google Scholar] [CrossRef]
- Lolli, S.; Grilli, G.; Ferrari, L.; Ferrari, P.; Ferrante, V. Effect of range use on endo- and ectoparasite infestation in italian organic egg production. Ital. J. Anim. Sci. 2019, 18, 690–695. [Google Scholar] [CrossRef]
- Ilić, T.; Becskei, Z.; Gajić, B.; Özvegy, J.; Stepanović, P.; Nenadović, K.; Dimitrijević, S. Prevalence of endoparasitic infections of birds in zoo gardens in Serbia. Acta Parasitol. 2018, 63, 134–146. [Google Scholar] [CrossRef]
- Jansson, D.S.; Christensson, D. Gastrointestinala parasiter hos strutsfåglar i Sverige. Sven. Vet. Tidn. 2000, 52, 621–626. [Google Scholar]
- Ponce Gordo, F.; Herrera, S.; Castro, A.T.; García Durán, B.; Martínez Díaz, R.A. Parasites from farmed ostriches (Struthio camelus) and rheas (Rhea americana) in Europe. Vet. Parasitol. 2002, 107, 137–160. [Google Scholar] [CrossRef]
- McKenna, P.B. Libyostrongylus infections in ostriches—A brief review with particular reference to their detection in New Zealand. N. Z. Vet. J. 2005, 53, 267–270. [Google Scholar] [CrossRef] [PubMed]
- Kummrow, M.S. Ratites or Struthioniformes: Struthiones, Rheae, Cassuarii, Apteryges (Ostriches, Rheas, Emus, Cassowaries, and Kiwis), and Tinamiformes (Tinamous). In Fowler’s Zoo and Wild Animal Medicine; Eric Miller, R., Fowler, M.E., Eds.; Elsevier: Amsterdam, The Netherlands, 2015; Volume 8, pp. 75–82. [Google Scholar]
- Cringoli, G.; Maurelli, M.P.; Levecke, B.; Bosco, A.; Vercruysse, J.; Utzinger, J.; Rinaldi, L. The Mini-FLOTAC technique for the diagnosis of helminth and protozoan infections in humans and animals. Nat. Protoc. 2017, 12, 1723–1732. [Google Scholar] [CrossRef]
- Capasso, M.; Maurelli, M.P.; Ianniello, D.; Alves, L.C.; Amadesi, A.; Laricchiuta, P.; Silvestre, P.; Campolo, M.; Cringoli, G.; Rinaldi, L. Use of Mini-FLOTAC and Fill-FLOTAC for rapidly diagnosing parasitic infections in zoo mammals. Braz. J. Vet. Parasitol. 2019, 28, 168–171. [Google Scholar] [CrossRef] [PubMed]
- Maurelli, M.P.; Martins, O.M.; Morgan, E.R.; Charlier, J.; Cringoli, G.; Mateus, T.L.; Bacescu, B.; Chartier, C.; Claerebout, E.; De Waal, T.; et al. A Qualitative Market Analysis Applied to Mini-FLOTAC and Fill-FLOTAC for Diagnosis of Helminth Infections in Ruminants. Front. Vet. Sci. 2020, 7, 580649. [Google Scholar] [CrossRef] [PubMed]
- Bortoluzzi, C.; Paras, K.L.; Applegate, T.J.; Verocai, G.G. Comparison between McMaster and Mini-FLOTAC methods for the enumeration of Eimeria maxima oocysts in poultry excreta. Vet. Parasitol. 2018, 254, 21–25. [Google Scholar] [CrossRef]
- Coker, S.M.; Pomroy, W.E.; Howe, L.; McInnes, K.; Vallee, E.; Morgan, K.J. Comparing the Mini-FLOTAC and centrifugal faecal flotation for the detection of coccidia (Eimeria spp.) in kiwi (Apteryx mantelli). Parasitol. Res. 2020, 119, 4287–4290. [Google Scholar] [CrossRef]
- Daş, G.; Klauser, S.; Stehr, M.; Tuchscherer, A.; Metges, C.C. Accuracy and precision of McMaster and Mini-FLOTAC egg counting techniques using egg-spiked faeces of chickens and two different flotation fluids. Vet. Parasitol. 2020, 283, 109158. [Google Scholar] [CrossRef]
- Lozano, J.; Anaya, A.; Rinaldi, L.; Cringoli, G.; Gomes, L.; Oliveira, M.; Paz-Silva, A.; Teresa Rebelo, M.; Madeira de Carvalho, L. Diagnosis of coccidiosis by Eimeria spp. in free-range chickens using Mini-FLOTAC and McMaster techniques—Preliminary results. Sci. Parasitol. 2021, 22, 13–18. [Google Scholar]
- Zajac, A.M.; Conboy, G.A. Fecal Exam Procedures. In Veterinary Clinical Parasitology, 8th ed.; Zajac, A.M., Conboy, G.A., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2012; pp. 4–15. [Google Scholar]
- Gordon, H.; Whitlock, H.V. A new technique for counting nematode eggs in sheep faeces. J. Coun. Sci. Industr. Res. 1939, 12, 50–52. [Google Scholar]
- Cringoli, G.; Rinaldi, L.; Maurelli, M.P.; Utzinger, J. FLOTAC: New multivalent techniques for qualitative and quantitative copromicroscopic diagnosis of parasites in animals and humans. Nat. Protoc. 2010, 5, 503–515. [Google Scholar] [CrossRef]
- Ederli, N.B.; Bonadiman, S.F.; Almeida de Moraes Neto, A.H.; DaMatta, R.A.; Santos, C.P. Mixed infection by Libyostrongylus douglassii and L. dentatus (Nematoda: Trichostrongylidae) in Struthio camelus (Ratites: Struthioniformes) from Brazil with further morphological characterization of adults. Vet. Parasitol. 2008, 151, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Ederli, N.B.; Rodrigues de Oliveira, F.C. Comparative morphology of the species of Libyostrongylus and Codiostomum, parasites from ostriches, Struthio camelus, with a identification key to the species. Braz. J. Vet. Parasitol. 2014, 23, 291–300. [Google Scholar] [CrossRef][Green Version]
- López-Osorio, S.; Chaparro-Gutiérrez, J.J.; Gómez-Osorio, L.M. Overview of poultry Eimeria life cycle and host-parasite interactions. Front. Vet. Sci. 2020, 7, 384. [Google Scholar] [CrossRef]
- Shamim, A.; Hassan, M.U.; Yousaf, A.; Iqbal, M.F.; Zafar, M.A.; Siddique, R.M.; Abubakar, M. Occurrence and identification of Eimeria species in broiler rearing under traditional system. J. Anim. Sci. Technol. 2015, 57, 41. [Google Scholar] [CrossRef] [PubMed]
- Kaboudi, K.; Umar, S.; Munir, M.T. Prevalence of coccidiosis in free-range chicken in Sidi Thabet, Tunisia. Scientifica 2016, 2016. [Google Scholar] [CrossRef]
- Thapa, S.; Hinrichsen, L.K.; Brenninkmeyer, C.; Gunnarsson, S.; Heerkens, J.L.T.; Verwer, C.; Niebuhr, K.; Willett, A.; Grilli, G.; Thamsborg, S.M.; et al. Prevalence and magnitude of helminth infections in organic laying hens (Gallus gallus domesticus) across Europe. Vet. Parasitol. 2015, 214, 118–124. [Google Scholar] [CrossRef]
- Saraiva, D.J.; Campina, A.C.C.; Gonçalves, F.C.S.; Melo-Viegas, D.; Santos, A.C.G.; Nogueira, R.M.S.; Costa, A.P. Gastrointestinal parasites in free-range chicken raised under extensive system from the northeast of Brazil. Braz. J. Poult. Sci. 2020, 23, 1–4. [Google Scholar] [CrossRef]
- Yazwinski, T.A.; Tucker, C.A. Nematodes and Acanthocephalans. In Diseases of Poultry, 12th ed.; Saif, Y.M., Ed.; Blackwell Publishing: Hoboken, NJ, USA, 2008; pp. 1025–1056. [Google Scholar]
- Mariño-González, G.A.; Ramírez-Hernández, A.; Cortés-Vecino, J.A. Libyostrongylus douglassii (Strongylida: Trichostrongylidae) in ostrich (Struthio camelus) farms from Colombia. Vet. Parasitol. 2017, 235, 53–56. [Google Scholar] [CrossRef]
- Eslami, A.; Rahmat, H.; Meshgi, B.; Ranjbar-Bahadori, S. Gastrointestinal parasites of ostrich (Struthio camelus domesticus) raised in Iran. Iran. J. Vet. Res. 2007, 8, 80–82. [Google Scholar]
- Barton, N.J.; Seward, D.A. Detection of Libyostrongylus douglassii in ostriches in Australia. Aust. Vet. J. 1993, 70, 31–32. [Google Scholar] [CrossRef] [PubMed]
- Button, C.; Barton, N.J.; Veale, P.I.; Overend, D.J. A survey of Libyostrongylus douglassii on ostrich farms in eastern Victoria. Aust. Vet. J. 1993, 70, 76. [Google Scholar] [CrossRef] [PubMed]
- More, S.J. The performance of farmed ostrich hens in eastern Australia. Prev. Vet. Med. 1996, 29, 107–120. [Google Scholar] [CrossRef]
- Mukaratirwa, S.; Cindzi, Z.M.; Maononga, D.B. Prevalence of Libyostrongylus douglassii in commercially reared ostriches in the highveld region of Zimbabwe. J. Helminthol. 2004, 78, 333–336. [Google Scholar] [CrossRef] [PubMed]
- Jansson, D.S.; Christensson, D.A.; Christensson, B.E. Winter survival in Sweden of L3-stage larvae of the ostrich wireworm Libyostrongylus douglassii. Vet. Parasitol. 2002, 106, 69–74. [Google Scholar] [CrossRef]


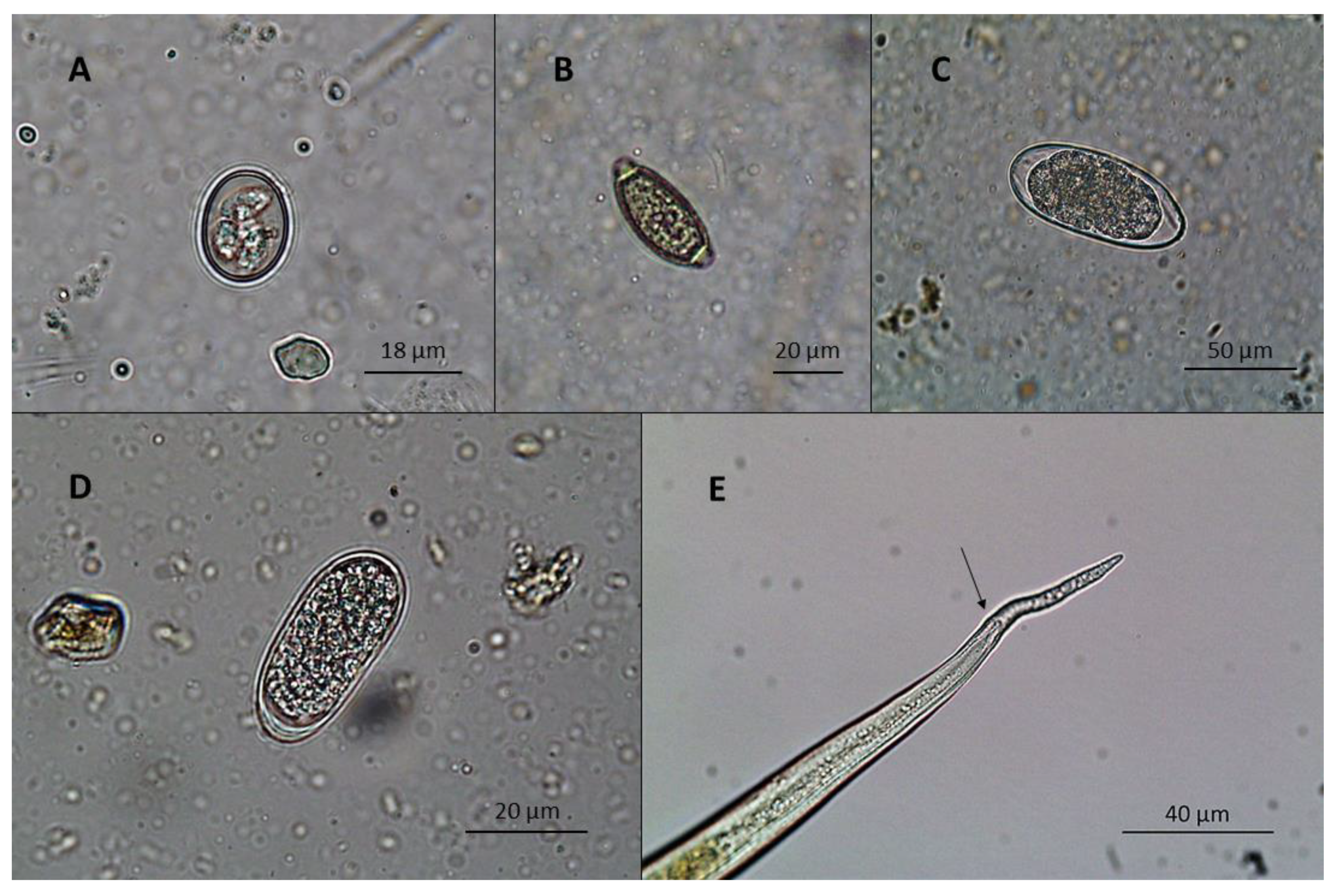
| Period | Collection | Species | No. of Birds | Age | Samples | Outdoor Area |
|---|---|---|---|---|---|---|
| October–December 2020 | Bird collection 1 (Lisbon) | Peacocks | 20 | 3 months–9 years | 29 | 9600 m2 |
| April 2021 | Bird collection 2 (Lisbon) | Peacocks | 40 | 3 months–19 years | 25 | 6000 m2 |
| September 2020–February 2021 | Bird collection 3 (Abrantes) | Ostriches | 2 | 4 years | 9 | 50,000 m2 |
| Emus | 6 | 7–14 years | 19 | |||
| Peacocks | 3 | 3–6 years | 14 | 6000 m2 | ||
| July–November 2020 | Poultry farm (Lourinhã) | Organic layers | 200 | 16 months | 46 | 1700 m2 |
| Collection | Bird Species | GI Parasites | OPG|EPG (Min–Max) | Prevalence (%) |
|---|---|---|---|---|
| Bird collection 1 | Peacocks | Eimeria spp. | 107 (0–750) | 29 |
| Helminths | 145 (0–2000) | 14 (Capillaria spp.) | ||
| 14 (S. pavonis) | ||||
| Bird collection 2 | Peacocks | Eimeria spp. | 502 (0–1800) | 25 |
| Helminths | 66 (0–1000) | 8 (T. tenuis) | ||
| 4 (S. pavonis) | ||||
| Bird collection 3 | Ostriches | L. douglassii | 2731 (500–5700) | 100 |
| Emus | 60 (0–420) | 32 | ||
| Peacocks | Eimeria spp. | 9 (0–30) | 43 | |
| Poultry farm | Organic layers | Eimeria spp. | 24 (0–300) | 41 |
| Average Eimeria spp., Galliformes | 160 (0–1800) | 35 | ||
| Average Helminths, Peacocks | 70 (0–2000) | 15 | ||
| Average Helminths, Ratites | 1396 (0–5700) | 66 | ||
| Bird Groups | GI Parasites | Mini-FLOTAC EPG|OPG (Min–Max) | McMaster EPG | OPG (Min–Max) |
|---|---|---|---|
| Galliformes | Eimeria spp. | 160 (0–1800) | 188 (0–5100) |
| Peacocks (Collections 1 and 2) | Helminths | 105 (0–2000) | 16 (0–400) |
| Ratites | L. douglassii | 1396 (0–5700) | 1647 (0–10,000) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lozano, J.; Almeida, C.; Victório, A.C.; Melo, P.; Rodrigues, J.P.; Rinaldi, L.; Cringoli, G.; Gomes, L.; Oliveira, M.; Paz-Silva, A.; et al. Implementation of Mini-FLOTAC in Routine Diagnosis of Coccidia and Helminth Infections in Domestic and Exotic Birds. Vet. Sci. 2021, 8, 160. https://doi.org/10.3390/vetsci8080160
Lozano J, Almeida C, Victório AC, Melo P, Rodrigues JP, Rinaldi L, Cringoli G, Gomes L, Oliveira M, Paz-Silva A, et al. Implementation of Mini-FLOTAC in Routine Diagnosis of Coccidia and Helminth Infections in Domestic and Exotic Birds. Veterinary Sciences. 2021; 8(8):160. https://doi.org/10.3390/vetsci8080160
Chicago/Turabian StyleLozano, João, Cristina Almeida, Ana Cláudia Victório, Pedro Melo, João Paulo Rodrigues, Laura Rinaldi, Giuseppe Cringoli, Lídia Gomes, Manuela Oliveira, Adolfo Paz-Silva, and et al. 2021. "Implementation of Mini-FLOTAC in Routine Diagnosis of Coccidia and Helminth Infections in Domestic and Exotic Birds" Veterinary Sciences 8, no. 8: 160. https://doi.org/10.3390/vetsci8080160
APA StyleLozano, J., Almeida, C., Victório, A. C., Melo, P., Rodrigues, J. P., Rinaldi, L., Cringoli, G., Gomes, L., Oliveira, M., Paz-Silva, A., & Madeira de Carvalho, L. (2021). Implementation of Mini-FLOTAC in Routine Diagnosis of Coccidia and Helminth Infections in Domestic and Exotic Birds. Veterinary Sciences, 8(8), 160. https://doi.org/10.3390/vetsci8080160









