Report on the First African Swine Fever Case in Greece
Abstract
:1. Introduction
2. Case Presentation
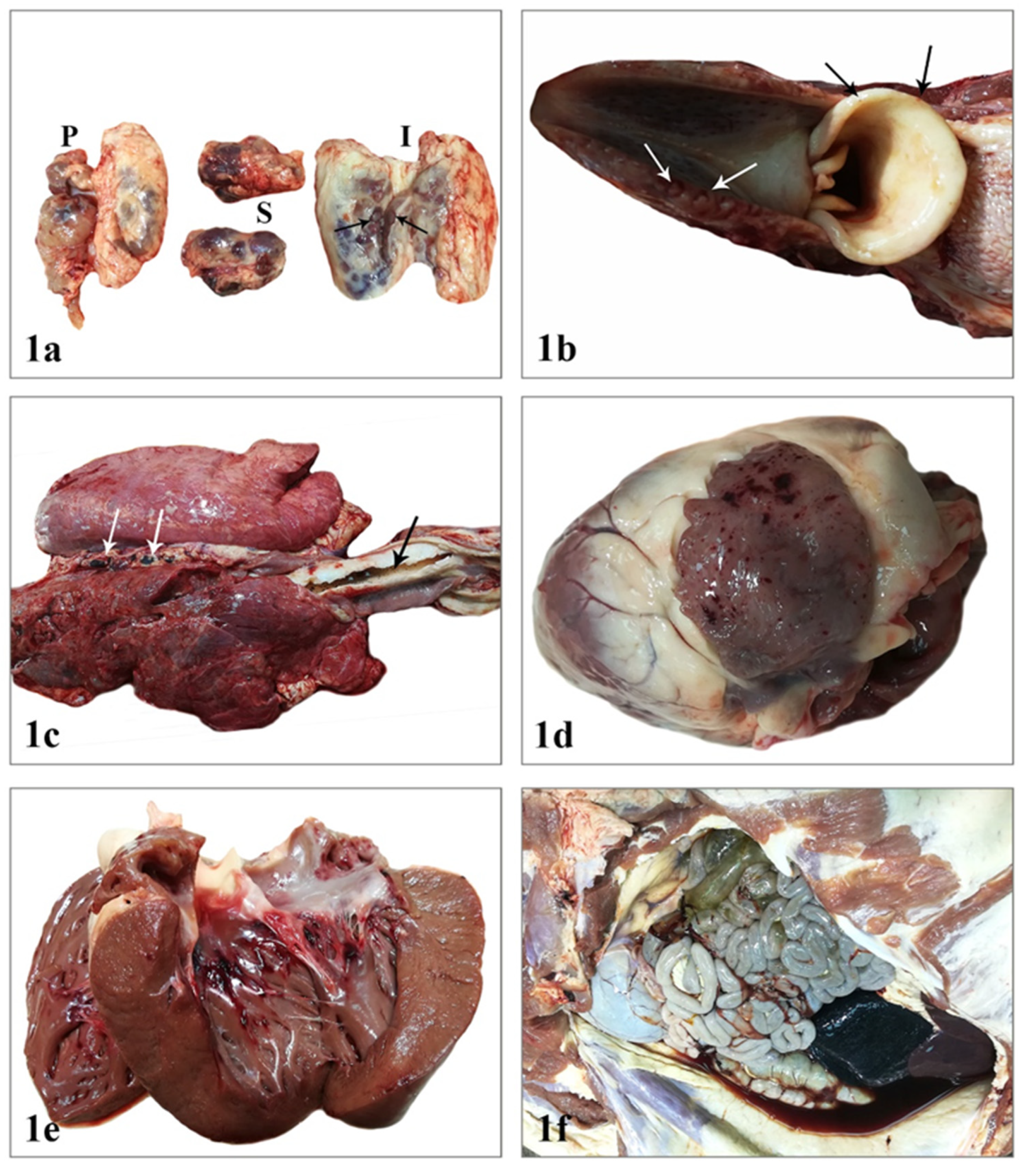
2.1. Postmortem Examination
2.2. Clinical Presentation in the Farm
2.3. Laboratory Investigation
2.4. Epidemiologic Results/Assessment
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kleiboeker, S.B.; Scoles, G.A. Pathogenesis of African swine fever virus in Ornithodoros ticks. Anim. Health Res. Rev. 2001, 2, 121–128. [Google Scholar]
- Mebus, C.A. African swine fever. Adv. Virus. Res. 1988, 35, 251–269. [Google Scholar]
- Anderson, E.C.; Hutchings, G.H.; Mukarati, N.; Wilkinson, P.J. African swine fever virus infection of the bushpig (Potamochoerus porcus) and its significance in the epidemiology of the disease. Vet. Microbiol. 1998, 62, 1–15. [Google Scholar] [PubMed]
- Penrith, M.L.; Vosloo, W. Review of African swine fever: Transmission, spread and control. J. S. Afr. Vet. Assoc. 2009, 80, 58–62. [Google Scholar]
- Guberti, V.; Khomenko, S.; Masiulis, M.; Kerba, S. African Swine Fever in Wild Boar Ecology and Biosecurity; FAO, OIE and EC: Rome, Italy, 2019; p. 8. [Google Scholar]
- Villeda, C.J.; Williams, S.M.; Wilkinson, P.J.; Viñuela, E. Haemostatic abnormalities in African swine fever a comparison of two virus strains of different virulence (Dominican Republic ‘78 and Malta ‘78). Arch. Virol. 1993, 130, 71–83. [Google Scholar] [PubMed]
- Rodriguez, F.; Fernandez, A.; Pérez, J.; Martin de las Mulas, J.; Sierra, M.A.; Jover, A. African swine fever: Morphopathology of a viral hemorrhagic disease. Vet. Rec. 1996, 139, 249–254. [Google Scholar]
- Robinson, W.F.; Robinson, N.A. Cardiovascular System. In Jubb, Kennedy and Palmer’s Pathology of Domestic Animals, 6th ed.; Maxie, M.G., Ed.; Elsevier: St. Louis, MO, USA, 2016; pp. 74–76. [Google Scholar]
- Beltrán-Alcrudo, D.; Arias, M.; Gallardo, C.; Kramer, S.; Penrith, M.L. African Swine Fever: Detection and Diagnosis–A Manual for Veterinarians; FAO: Rome, Italy, 2017; p. 7. [Google Scholar]
- Goatley, L.C.; Reis, A.L.; Portugal, R.; Goldswain, H.; Shimmon, G.L.; Hargreaves, Z.; Ho, C.S.; Montoya, M.; Sánchez-Cordón, P.J.; Taylor, G. A Pool of Eight Virally Vectored African Swine Fever Antigens Protect Pigs against Fatal Disease. Vaccines 2020, 8, 234. [Google Scholar]
- Sang, H.; Miller, G.; Lokhandwala, S.; Sangewar, N.; Waghela, S.D.; Bishop, R.P.; Mwangi, W. Progress Toward Development of Effective and Safe African Swine Fever Virus Vaccines. Front. Vet. Sci. 2020, 7, 84. [Google Scholar]
- Wu, K.; Liu, J.; Wang, L.; Fan, S.; Li, Z.; Li, Y.; Yi, L.; Ding, H.; Zhao, M.; Chen, J. Current State of Global African Swine Fever Vaccine Development under the Prevalence and Transmission of ASF in China. Vaccines 2020, 8, 531. [Google Scholar]
- Borca, M.V.; Ramirez-Medina, E.; Silva, E.; Vuono, E.; Rai, A.; Pruitt, S.; Espinoza, N.; Velazquez-Salinas, L.; Gay, C.G.; Gladue, D.P. ASFV-G-DI177L as an Effective Oral Nasal Vaccine against the Eurasia Strain of Africa Swine Fever. Viruses 2021, 13, 765. [Google Scholar]
- Sanchez-Vizcaino, J.M.; Mur, L.; Gomez-Villamandos, J.C.; Carrasco, L. An update on the epidemiology and pathology of African swine fever. J. Comp. Pathol. 2015, 152, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Walczak, M.; Żmudzki, J.; Mazur-Panasiuk, N.; Juszkiewicz, M.; Woźniakowski, G. Analysis of the Clinical Course of Experimental Infection with Highly Pathogenic African Swine Fever Strain, Isolated from an Outbreak in Poland. Aspects Related to the Disease Suspicion at the Farm Level. Pathogens 2020, 9, 237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan, I.C.; Hess, W.R. Virulence in African swine fever: Its measurement and implications. Am. J. Vet. Res. 1984, 45, 361–366. [Google Scholar] [PubMed]
- Salguero, F.J. Comparative Pathology and Pathogenesis of African Swine Fever Infection in Swine. Front. Vet. Sci. 2020, 7, 282. [Google Scholar] [CrossRef]
- Moulton, J.; Coggins, L. Comparison of lesions in acute and chronic African swine fever. Cornell. Vet. 1968, 58, 364–388. [Google Scholar]
- Mebus, C.A.; Dardiri, A.H. Additional characteristics of disease caused by the African swine fever viruses isolated from Brazil and the Dominican Republic. Proc. Annu. Meet. U. S. Anim. Health Assoc. 1979, 83, 227–239. [Google Scholar]
- Gomez-Villamandos, J.C.; Bautista, M.J.; Sanchez-Cordon, P.J.; Carrasco, L. Pathology of African swine fever: The role of monocyte-macrophage. Virus. Res. 2013, 173, 140–149. [Google Scholar] [CrossRef] [PubMed]
- McVicar, J.W. Quantitative aspects of the transmission of African swine fever. Am. J. Vet. Res. 1984, 45, 1535–1541. [Google Scholar] [PubMed]
- Pan, I.C. Spontaneously susceptible cells and cell culture methodologies for African swine fever virus. In African Swine Fever. Developments in Veterinary Virology; Becker, Y., Ed.; Springer: Boston, MA, USA, 1987; Volume 3, pp. 8–126. [Google Scholar]
- Sierra, M.A.; Carrasco, L.; Gómez-Villamandos, J.C.; Martín de las Muías, J.; Mendez, A.; Jover, A. Pulmonary intravascular macrophages in lungs of pigs inoculated with African swine fever virus of differing virulence. J. Comp. Pathol. 1990, 102, 323–334. [Google Scholar] [CrossRef]
- Carrasco, L.; de Lara, F.C.; Gσmez-Villamandos, J.C.; Bautista, M.J.; Villeda, C.J.; Wilkinson, P.J.; Sierra, M.A. The pathogenic role of pulmonary intravascular macrophages in acute African swine fever. Res. Vet. Sci. 1996, 61, 193–198. [Google Scholar] [CrossRef]
- Gomez-Villamandos, J.C.; Hervas, J.; Mindez, A.; Carrasco, L.; Martιn de las Mulas, J.; Villeda, C.J.; Wilkinson, P.J.; Sierra, M.A. Ultrastructural study of the renaltubular system in acute experimental African swine fever: Virus replication in glomerular mesangial cells and in the collecting ducts. Arch. Virol. 1995, 140, 581–589. [Google Scholar] [CrossRef]
- Hervás, J.; Gómez-Villamandos, J.C.; Méndz, A.; Carrasco, L.; Sierra, M.A. The lesional changes and pathogenesis in the kidney in African swine fever. Vet. Res. Commun. 1996, 20, 285–299. [Google Scholar] [CrossRef]
- Carrasco, L.; Bautista, M.J.; Gomez-Villamandos, J.C.; Martin de las Mulas, J.; Chacon, L.F.; Wilkinson, P.J.; Sierra, M.A. Development of microscopic lesions in splenic cords of pigs infected with African swine fever virus. Vet. Res. 1997, 28, 93–99. [Google Scholar] [PubMed]
- Carrasco, L.; Chàcón-M de Lara, F.; Martin de Las Mulas, J.; Gomez-Villamandos, J.C.; Sierra, M.A.; Villeda, C.J.; Wilkinson, P.J. Ultrastructural changes related to the lymph node haemorrhages in acute African swine fever. Res. Vet. Sci. 1997, 62, 199–204. [Google Scholar] [CrossRef]
- Sánchez-Vizcaíno, J.M.; Arias, M. African swine fever. In Diseases of Swine, 10th ed.; Zimmerman, J.J., Karriker, L.A., Ramírez, A., Schwartz, K.J., Stevenson, G.W., Eds.; Wiley-Blackwell: Ames, IA, USA, 2012; pp. 396–404. [Google Scholar]
- Arias, M.; Martínez Escribano, J.A.; Rueda, A.; Sánchez-Vizcaíno, J.M. La peste porcina Africana. Med. Vet. 1986, 3, 333–359. [Google Scholar]
- Costard, S.; Mur, L.; Lubroth, J.; Sanchez-Vizcaıno, J.M.; Pfeiffer, D.U. Epidemiology of African swine fever virus. Virus Res. 2013, 173, 191–197. [Google Scholar] [CrossRef]
- Penrith, M.L. Current status of African swine fever. CABI Agric. Biosci. 2020, 1, 11. [Google Scholar] [CrossRef]
- Nga, B.T.T.; Tran Anh Dao, B.; Nguyen Thi, L.; Osaki, M.; Kawashima, K.; Song, D.; Salguero, F.J.; Le, V.P. Clinical and Pathological Study of the First Outbreak Cases of African Swine Fever in Vietnam. Front. Vet. Sci. 2020, 7, 392. [Google Scholar] [CrossRef]
- Department for Environment, Food and Rural Affairs (DEFRA); Animal and Plant Health Agency (APHA); Advice Services-International Disease Monitoring. Updated Outbreak Assessment #08: African swine fever in Europe (Eastern Europe & Belgium). 31 Jan 20; Ref: VITT/1200 ASF in Europe (Eastern Europe & Belgium). Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/863212/asf-europe-update8.pdf (accessed on 15 February 2020).
- Hellenic Ministry of Rural Development and Food. African Swine Fever Current situation in Greece. In Proceedings of the Meeting of Standing Committee on Plants, Animals, Food and Feed-Section: “Animal health and Animal Welfare”, Brussels, Belgium, 13–14 February 2020; Available online: https://ec.europa.eu/food/system/files/2020-02/reg-com_ahw_20200213_asf_grc.pdf (accessed on 21 February 2021).
- Oura, C.A.L.; Arias, M. African swine fever (infection with African swine fever virus). In Manual of Diagnostic Tests and Vaccines for Terrestrial Animals 2021; OIE: Paris, France, 2021; pp. 1–15. [Google Scholar]
- Sanchez-Cordon, P.J.; Montoya, M.; Reis, A.L.; Dixon, L.K. African swine fever: A re-emerging viral disease threatening the global pig industry. Vet. J. 2018, 233, 41–48. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brellou, G.D.; Tassis, P.D.; Apostolopoulou, E.P.; Fortomaris, P.D.; Leontides, L.S.; Papadopoulos, G.A.; Tzika, E.D. Report on the First African Swine Fever Case in Greece. Vet. Sci. 2021, 8, 163. https://doi.org/10.3390/vetsci8080163
Brellou GD, Tassis PD, Apostolopoulou EP, Fortomaris PD, Leontides LS, Papadopoulos GA, Tzika ED. Report on the First African Swine Fever Case in Greece. Veterinary Sciences. 2021; 8(8):163. https://doi.org/10.3390/vetsci8080163
Chicago/Turabian StyleBrellou, Georgia D., Panagiotis D. Tassis, Emmanouela P. Apostolopoulou, Paschalis D. Fortomaris, Leonidas S. Leontides, Georgios A. Papadopoulos, and Eleni D. Tzika. 2021. "Report on the First African Swine Fever Case in Greece" Veterinary Sciences 8, no. 8: 163. https://doi.org/10.3390/vetsci8080163
APA StyleBrellou, G. D., Tassis, P. D., Apostolopoulou, E. P., Fortomaris, P. D., Leontides, L. S., Papadopoulos, G. A., & Tzika, E. D. (2021). Report on the First African Swine Fever Case in Greece. Veterinary Sciences, 8(8), 163. https://doi.org/10.3390/vetsci8080163





