Molecular Detection of Nosema spp. in Honey in Bulgaria
Abstract
:1. Introduction
2. Materials and Methods
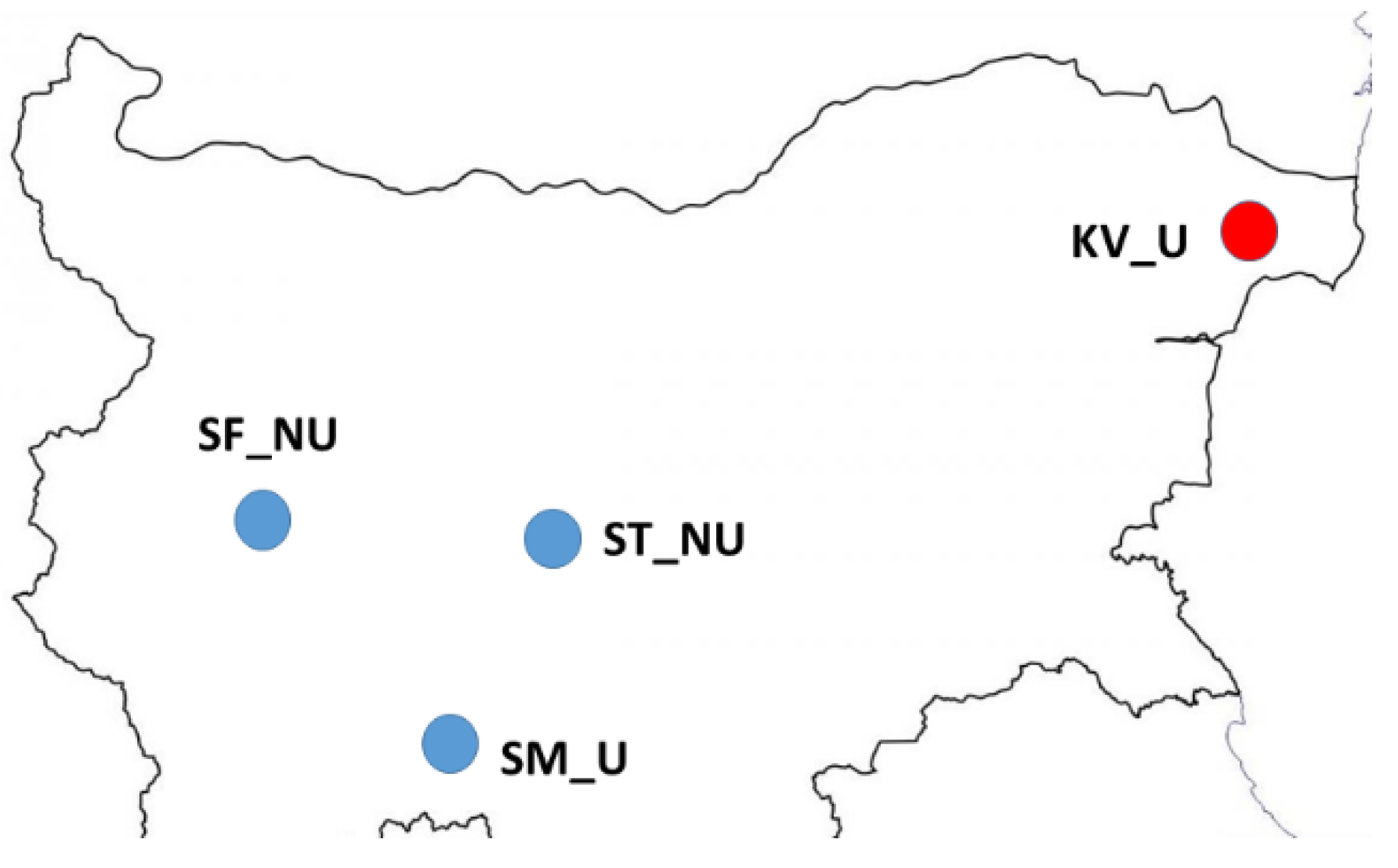
2.1. Honey Samples
2.2. DNA Extraction for Identification of eDNA from V. destructor, N. apis, and N. ceranae in Honey Samples, Using CTAB Protocol with a Spin Column
2.3. PCR Analysis
2.4. Sequence and Phylogenetic Analysis
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aizen, M.A.; Garibaldi, L.A.; Cunningham, S.A.; Klein, A.M. How much does agriculture depend on pollinators? Lessons from long-term trends in crop production. Ann. Bot. 2009, 103, 1579–1588. [Google Scholar] [CrossRef] [PubMed]
- Klatt, B.K.; Holzschuh, A.; Westphal, C.; Clough, Y.; Smit, I.; Pawelzik, E.; Tscharntke, T. Bee pollination improves crop quality, shelf life and commercial value. Proc. R. Soc. B 2014, 281, 20132440. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Suarez, J.M.; Tulipani, S.; Romandini, S.; Bertoli, E.; Battino, M. Contribution of Honey in Nutrition and Human Health: A Review. Med. J. Nutr. Metab. 2009, 3, 15–23. [Google Scholar] [CrossRef]
- Magrach, A.; González-Varo, J.P.; Boiffier, M.; Vilà, M.; Bartomeus, I. Honeybee Spillover Reshuffles Pollinator Diets and Affects Plant Reproductive Success. Nat. Ecol. Evol. 2017, 1, 1299–1307. [Google Scholar] [CrossRef]
- Goblirsch, M. Nosema ceranae disease of the honey bee (Apis mellifera). Apidologie 2018, 49, 131–150. [Google Scholar] [CrossRef] [Green Version]
- van Dooremalen, C.; Cornelissen, B.; Poleij-Hok-Ahin, C.; Blacquière, T. Single and interactive effects of Varroa destructor, Nosema spp., and imidacloprid on honey bee colonies (Apis mellifera). Ecosphere 2018, 9, e02378. [Google Scholar] [CrossRef] [Green Version]
- Grozinger, C.M.; Flenniken, M.L. Bee viruses: Ecology, pathogenicity, and impacts. Annu. Rev. Entomol. 2019, 64, 205–226. [Google Scholar] [CrossRef]
- Morfin, N.; Goodwin, P.H.; Guzman-Novoa, E. Interaction of field realistic doses of clothianidin and Varroa destructor parasitism on adult honey bee (Apis mellifera L.) health and neural gene expression, and antagonistic effects on differentially expressed genes. PLoS ONE 2020, 15, e0229030. [Google Scholar] [CrossRef] [Green Version]
- Gisder, S.; Möckel, N.; Linde, A.; Genersch, E. A cell culture model for Nosema ceranae and Nosema apis allows new insights into the life cycle of these important honey bee-pathogenic microsporidia. Environ. Microbiol. 2011, 13, 404–413. [Google Scholar] [CrossRef]
- Lamei, S.; Hu, Y.O.O.; Olofsson, T.C.; Andersson, A.F.; Forsgren, E.; Vásquez, A. Improvement of identification methods for honeybee specific Lactic Acid Bacteria; future approaches. PLoS ONE 2017, 12, e0174614. [Google Scholar] [CrossRef]
- Cameron, T.C.; Wiles, D.; Beddoe, T. Current Status of Loop-Mediated Isothermal Amplification Technologies for the Detection of Honey Bee Pathogens. Front. Vet. Sci. 2021, 8, 659683. [Google Scholar] [CrossRef]
- Garrido-Bailón, E.; Higes, M.; Martínez-Salvador, A.; Antúnez, K.; Botías, C.; Meana, A.; Prieto, L.; Martín-Hernández, R. The prevalence of the honeybee brood pathogens Ascosphaera apis, Paenibacillus larvae and Melissococcus plutonius in Spanish apiaries determined with a new multiplex PCR assay. Microb. Biotechnol. 2013, 6, 731–739. [Google Scholar] [CrossRef]
- Arismendi, N.; Bruna, A.; Zapata, N.; Vargas, M. PCR-specific detection of recently described Lotmaria passim (Trypanosomatidae) in Chilean apiaries. J. Invertebr. Pathol. 2016, 134, 1–5. [Google Scholar] [CrossRef]
- Milićević, V.; Radojičić, S.; Kureljušić, J.; Šekler, M.; Nešić, K.; Veljović, L.; Maksimović Zorić, J.; Radosavljević, V. Molecular detection of black queen cell virus and Kashmir bee virus in honey. AMB Expr. 2018, 8, 128. [Google Scholar] [CrossRef] [PubMed]
- Bakonyi, T.; Derakhshifar, I.; Grabensteiner, E.; Nowotny, N. Development and evaluation of PCR assays for the detection of Paenibacillus larvae in honey samples: Comparison with isolation and biochemical characterization. Appl. Environ. Microbiol. 2003, 69, 1504–1510. [Google Scholar] [CrossRef] [Green Version]
- Giersch, T.; Berg, T.; Galea, F.; Hornitzky, M. Nosemaceranae infects honey bees (Apis mellifera) and contaminates honey in Australia. Apidologie 2009, 40, 117–123. [Google Scholar] [CrossRef] [Green Version]
- Ribani, A.; Utzeri, V.J.; Taurisano, V.; Fontanesi, L. Honey as a source of environmental DNA for the detection and monitoring of honey bee pathogens and parasites. Vet. Sci. 2020, 7, 113. [Google Scholar] [CrossRef]
- Ribani, A.; Utzeri, V.J.; Taurisano, V.; Galuppi, R.; Fontanesi, L. Analysis of honey environmental DNA indicates that the honey bee (Apis mellifera L.) trypanosome parasite Lotmaria passim is widespread in the apiaries of the North of Italy. J. Invertebr. Pathol. 2021, 184, 107628. [Google Scholar] [CrossRef] [PubMed]
- Thomsen, P.F.; Willerslev, E. Environmental DNA–An emerging tool in conservation for monitoring past and present biodiversity. Biol. Conserv. 2015, 183, 4–18. [Google Scholar] [CrossRef]
- Nevers, M.B.; Byappanahalli, M.N.; Morris, C.C.; Shively, D.; Przybyla-Kelly, K.; Spoljaric, A.M.; Dickey, J.; Roseman, E.F. Environmental DNA (eDNA): A tool for quantifying the abundant but elusive round goby (Neogobius melanostomus). PLoS ONE 2018, 13, e0191720. [Google Scholar] [CrossRef] [PubMed]
- Ruppert, K.M.; Kline, R.J.; Rahman, M.S. Past, present, and future perspectives of Environmental DNA (eDNA) metabarcoding: A systematic review in methods, monitoring, and applications of global eDNA. Glob. Ecol. Conserv. 2019, 17, e00547. [Google Scholar] [CrossRef]
- Bargańska, Ż.; Ślebioda, M.; Namieśnik, J. Honey bees and their products: Bioindicators of environmental contamination. Crit. Rev. Environ. Sci. Technol. 2016, 46, 235–248. [Google Scholar] [CrossRef]
- Girotti, S.; Ghini, S.; Ferri, E.; Bolelli, L.; Colombo, R.; Serra, G.; Porrini, C.; Sangiorgi, S. Bioindicators and biomonitoring: Honeybees and hive products as pollution impact assessment tools for the Mediterranean area. Euro-Mediterr. J. Environ. Integr. 2020, 5, 62. [Google Scholar] [CrossRef]
- Hawkins, J.; de Vere, N.; Griffith, A.; Ford, C.R.; Allainguillaume, J.; Hegarty, M.J.; Baillie, L.; Adams-Groom, B. Using DNA metabarcoding to identify the floral composition of honey: A new tool for investigating honey bee foraging preferences. PLoS ONE 2015, 10, e0134735. [Google Scholar] [CrossRef] [Green Version]
- Utzeri, V.J.; Ribani, A.; Schiavo, G.; Bertolini, F.; Bovo, S.; Fontanesi, L. Application of next generation semiconductor based sequencing to detect the botanical composition of monofloral, polyfloral and honeydew honey. Food Control 2018, 86, 342–349. [Google Scholar] [CrossRef]
- Balkanska, R.; Stefanova, K.; Stoikova–Grigorova, R. Main honey botanical components and techniques for identification: A review. J. Apic. Res. 2020, 59, 852–861. [Google Scholar] [CrossRef]
- Olivieri, C.; Marota, I.; Rollo, F.; Luciani, S. Tracking plant, fungal, and bacterial DNA in honey specimens. J. Forensic Sci. 2012, 57, 222–227. [Google Scholar] [CrossRef]
- Forsgren, E.; Laugen, A.T. Prognostic value of using bee and hive debris samples for the detection of American foulbrood disease in honey bee colonies. Apidologie 2014, 45, 10–20. [Google Scholar] [CrossRef] [Green Version]
- Revainera, P.D.; Quintana, S.; de Landa, G.F.; Iza, C.G.; Olivera, E.; Fuentes, G.; Plischuk, S.; Medici, S.; Ruffinengo, S.; Marcangelli, J.; et al. Molecular detection of bee pathogens in honey. J. Insects Food Feed 2020, 6, 467–474. [Google Scholar] [CrossRef]
- Soares, S.; Grazina, L.; Costa, J.; Amaral, J.S.; Oliveira, M.B.P.P.; Mafra, I. Botanical authentication of Lavender (Lavandula spp.) honey by a novel DNA-barcoding approach coupled to high resolution melting analysis. Food Control 2018, 86, 367–373. [Google Scholar] [CrossRef] [Green Version]
- Lucek, K.; Galli, A.; Gurten, S.; Hohmann, N.; Maccagni, A.; Patsiou, T.; Willi, Y. Metabarcoding of honey to assess differences in plant-pollinator interactions between urban and non-urban sites. Apidologie 2019, 50, 317–329. [Google Scholar] [CrossRef]
- Soares, S.; Amaral, J.S.; Oliveira, M.B.P.P.; Mafra, I. Improving DNA isolation from honey for the botanical origin identification. Food Control 2015, 48, 130–136. [Google Scholar] [CrossRef] [Green Version]
- Utzeri, V.J.; Ribani, A.; Fontanesi, L. Authentication of honey based on a DNA method to dierentiate Apis mellifera subspecies: Application to Sicilian honey bee (A. m. siciliana) and Iberian honey bee (A. m. iberiensis) honeys. Food Control 2018, 91, 294–301. [Google Scholar] [CrossRef]
- Solignac, M.; Cornuet, J.M.; Vautrin, D.; Le Conte, Y.; Anderson, D.; Evans, J.; Cros-Arteil, S.; Navajas, M. The invasive Korea and Japan types of Varroa destructor, ectoparasitic mites of the Western honey bee (Apis mellifera), are two partly isolated clones. Proc. R. Soc. Lond. B Biol. Sci. 2005, 272, 411–419. [Google Scholar] [CrossRef] [Green Version]
- Navajas, M.; Anderson, D.L.; de Guzman, L.I.; Huang, Z.Y.; Clement, J.; Zhou, T.; Le Conte, Y. New Asian types of Varroa destructor: A potential new threat for world apiculture. Apidologie 2010, 41, 181–193. [Google Scholar] [CrossRef] [Green Version]
- Martín-Hernández, R.; Meana, A.; Prieto, L.; Salvador, A.M.; Garrido-Bailón, E.; Higes, M. Outcome of colonization of Apis mellifera by Nosema ceranae. Appl. Environ. Microbiol. 2007, 73, 6331–6338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruttner, F. Biogeography and Taxonomy of Honey Bees; Springer: Berlin/Heidelberg, Germany, 1988. [Google Scholar]
- Smyth, R.P.; Schlub, T.E.; Grimm, A.; Venturi, V.; Chopra, A.; Mallal, S.; Davenport, M.P.; Mak, J. Reducing chimera formation during PCR amplification to ensure accurate genotyping. Gene 2010, 469, 45–51. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Edgar, R.C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Schwartz, S.; Wagner, L.; Miller, W. A greedy algorithm for aligning DNA sequences. J. Comput. Biol. 2000, 7, 203–214. [Google Scholar] [CrossRef]
- Pelin, A.; Selman, M.; Aris-Brosou, S.; Farinelli, L.; Corradi, N. Genome analyses suggest the presence of polyploidy and recent human-driven expansions in eight global populations of the honeybee pathogen Nosema ceranae. Environ. Microbiol. 2015, 17, 4443–4458. [Google Scholar] [CrossRef] [PubMed]
- Basa, B.; Belay, W.; Tilahun, A.; Teshale, A. Review on medicinal value of honeybee products: Apitherapy. Adv. Biol. Res. 2016, 10, 236–247. [Google Scholar] [CrossRef]
- Jovetić, M.; Trifković, J.; Stanković, D.; Manojlović, D.; Milojković-Opsenica, D. Mineral content as a tool for the assessment of honey authenticity. J. AOAC Int. 2017, 100, 862–870. [Google Scholar] [CrossRef] [PubMed]
- Langlois, V.S.; Allison, M.J.; Bergman, L.C.; To, T.A.; Helbing, C.C. The need for robust qPCR-based eDNA detection assays in environmental monitoring and species inventories. Environ. DNA 2021, 3, 519–527. [Google Scholar] [CrossRef]
- Beng, K.C.; Corlett, R.T. Applications of environmental DNA (eDNA) in ecology and conservation: Opportunities, challenges and prospects. Biodivers. Conserv. 2020, 29, 2089–2121. [Google Scholar] [CrossRef]
- Ramsey, S.D.; Ochoa, R.; Bauchan, G.; Gulbronson, C.; Mowery, J.D.; Cohen, A.; Lim, D.; Joklik, J.; Cicero, J.M.; Ellis, J.D.; et al. Varroa destructor feeds primarily on honey bee fat body tissue and not hemolymph. Proc. Natl. Acad. Sci. USA 2019, 116, 1792–1801. [Google Scholar] [CrossRef] [Green Version]
- Emsen, B.; Guzman-Novoa, E.; Hamiduzzaman, M.M.; Eccles, L.; Lacey, B.; Ruiz-Pérez, R.A.; Nasr, M. Higher prevalence and levels of Nosema ceranae than Nosema apis infections in Canadian honey bee colonies. Parasitol. Res. 2016, 115, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Pacini, A.; Mira, A.; Molineri, A.; Giacobino, A.; Bulacio Cagnolo, N.; Aignasse, A.; Zago, L.; Izaguirre, M.; Merke, J.; Orellano, E.; et al. Distribution and prevalence of Nosema apis and N. ceranae in temperate and subtropical eco-regions of Argentina. J. Invertebr. Pathol. 2016, 141, 34–37. [Google Scholar] [CrossRef]
- Giraudeau, M.; Mousel, M.; Earl, S.; McGraw, K. Parasites in the city: Degree of urbanization predicts poxvirus and coccidian infections in house finches (Haemorhous mexicanus). PLoS ONE 2014, 9, e86747. [Google Scholar] [CrossRef] [Green Version]
- Leong, M.; Kremen, C.; Roderick, G.K. Pollinator interactions with yellow starthistle (Centaurea solstitialis) across urban, agricultural, and natural landscapes. PLoS ONE 2014, 9, e86357. [Google Scholar] [CrossRef] [PubMed]
- Patz, J.A.; Daszak, P.; Tabor, G.M.; Aguirre, A.A.; Pearl, M.; Epstein, J.; Wolfe, N.D.; Kilpatrick, A.M.; Foufopoulos, J.; Molyneux, D.; et al. Unhealthy landscapes: Policy recommendations on land use change and infectious disease emergence. Environ. Health Perspect. 2004, 112, 1092–1098. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Youngsteadt, E.; Appler, R.H.; López-Uribe, M.M.; Tarpy, D.R.; Frank, S.D. Urbanization increases pathogen pressure on feral and managed honey bees. PLoS ONE 2015, 10, e0142031. [Google Scholar] [CrossRef] [Green Version]
- Decourtye, A.; Alaux, C.; Le Conte, Y.; Henry, M. Toward the protection of bees and pollination under global change: Present and future perspectives in a challenging applied science. Curr. Opin. Insect. Sci. 2019, 35, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Wakgari, M.; Yigezu, G. Honeybee keeping constraints and future prospects. Cogent Food Agric. 2021, 7, 1872192. [Google Scholar] [CrossRef]
- Brosi, B.J.; Delaplane, K.S.; Boots, M.; de Roode, J.C. Ecological and evolutionary approaches to managing honeybee disease. Nat. Ecol. Evol. 2017, 1, 1250–1262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steinhauer, N.; Kulhanek, K.; Antúnez, K.; Human, H.; Chantawannakul, P.; Chauzat, M.P. Drivers of colony losses. Curr. Opin. Insect Sci. 2018, 26, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Evans, J.D.; Zhou, L.; Boncristiani, H.; Kimura, K.; Xiao, T.; Litkowski, A.M.; Pettis, J.S. Asymmetrical coexistence of Nosema ceranae and Nosema apis in honey bees. J. Invertebr. Pathol. 2009, 101, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; St. Clair, A.L.; Dolezal, A.; Toth, A.L.; O’Neal, M. Honey bee (Hymenoptera: Apidea) pollen forage in a highly cultivated agroecosystem: Limited diet diversity and its relationship to virus resistance. J. Econ. Entomol. 2020, 113, 1062–1072. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.J.; Smart, M.D.; Anelli, C.M.; Sheppard, W.S. Honey bees (Apis mellifera) reared in brood combs containing high levels of pesticide residues exhibit increased susceptibility to Nosema (Microsporidia) infection. J. Invertebr. Pathol. 2012, 109, 326–329. [Google Scholar] [CrossRef]
- Chen, Y.W.; Chung, W.P.; Wang, C.H.; Solter, L.F.; Huang, W.F. Nosema ceranae infection intensity highly correlates with temperature. J. Invertebr. Pathol. 2012, 111, 264–267. [Google Scholar] [CrossRef]
| Species | Primer Name | Primer Sequences (5′-3′) | Size in bp | Amplified Region | Reference |
|---|---|---|---|---|---|
| Apis mellifera | AM_Forward AM_Reverse | GGCAGAATAAGTGCATTG TTAATATGAATTAAGTGGGG | C lineage 85 | mtDNA coxI-coxII | [33] |
| Varroa destructor | 10KbCOIF1 6,5KbCOIR | CTTGTAATCATAAGGATATTGGAAC AATACCAGTGGGAACCGC | 929 | coxI | [34,35] |
| Varroa destructor | 10KbCytbF-1 10KbCytbPRIM | GCAGCTTTAGTGGATTTACCTAC CTACAGGACACGATCCCAAG | 985 | cytb | [34,35] |
| Nosemaapis | 321APIS-FOR 321APIS-REV | GGGGGCATGTCTTTGACGTACTATGTA GGGGGGCGTTTAAAATGGAAACAACTATG | 321 | SSU 16S rDNA | [36] |
| Nosemaceranae | 218MITOC-FOR 218MITOC-REV | CGGCGACGATGTGATATGAAAATATTAA CCCGGTCATTCTCAAACAAAAAACCG | 218 or 219 a | SSU 16S rDNA | [36] |
| N. ceranae GenBank Acc. no. | 6 | 6 | 6 | 6 | 6 | 6 | 6 |
| 1 | 1 | 1 | 1 | 1 | 2 | 2 | |
| 8 | 8 | 8 | 8 | 9 | 0 | 0 | |
| 6 | 6 | 7 | 8 | 6 | 2 | 3 | |
| 2 | 7 | 7 | 4 | 3 | 9 | 5 | |
| NW 020169312 reference | A | A | A | A | A | T | A |
| MG657260 Bulgaria | |||||||
| MW600361 Mexico | T | ||||||
| MN649205 UK | A | ||||||
| KC680638 Morocco | |||||||
| KC680637 Lebanon | |||||||
| MZ044978 Egypt | |||||||
| MW396669 Turkey | |||||||
| FJ227957 Argentina | |||||||
| DQ673615 Switzerland | |||||||
| DQ374656 Germany | |||||||
| DQ374655 France | |||||||
| LC493173 Japan | C | ||||||
| LC510232 Japan NCS48 | G | ||||||
| LC510233 Japan NCS49 | G | ||||||
| HM581509 Mexico | G | ||||||
| LC510243 Japan NCS59 | G | ||||||
| HM859897 Italy | G | ||||||
| HM802210 Mexico | G |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salkova, D.; Shumkova, R.; Balkanska, R.; Palova, N.; Neov, B.; Radoslavov, G.; Hristov, P. Molecular Detection of Nosema spp. in Honey in Bulgaria. Vet. Sci. 2022, 9, 10. https://doi.org/10.3390/vetsci9010010
Salkova D, Shumkova R, Balkanska R, Palova N, Neov B, Radoslavov G, Hristov P. Molecular Detection of Nosema spp. in Honey in Bulgaria. Veterinary Sciences. 2022; 9(1):10. https://doi.org/10.3390/vetsci9010010
Chicago/Turabian StyleSalkova, Delka, Rositsa Shumkova, Ralitsa Balkanska, Nadezhda Palova, Boyko Neov, Georgi Radoslavov, and Peter Hristov. 2022. "Molecular Detection of Nosema spp. in Honey in Bulgaria" Veterinary Sciences 9, no. 1: 10. https://doi.org/10.3390/vetsci9010010







